MicroRNA-302a sensitizes testicular embryonal carcinoma cells to cisplatin-induced cell death
Abstract
Cisplatin is a commonly used chemotherapeutic agent for the treatment of several human malignancies, such as testicular germ cell tumors (TGCT). The toxic effects persist and those that are present long after chemotherapy affect the overall quality of life of patients. MicroRNAs (miRNAs) play important roles in the responses of cancer cells to chemotherapy and have been shown to modulate cell sensitivity to chemotherapeutic drugs. However, the relationship between miRNA expression and cisplatin sensitivity of TGCT has not been fully explored. In this study, the effects of miR-302a on cisplatin cytotoxicity in TGCT-derived cell line NTERA-2 (NT2) were evaluated. We found that expression levels of miR-302a were increased in cisplatin-treated NT2 cells. Up-regulation of miR-302a significantly increased the sensitivity of NT2 cells to cisplatin by enhancing cisplatin-induced G2/M phase arrest and the subsequent progression to apoptosis. MiR-302a also increased the killing effects of cisplatin by lowering the apoptotic threshold; the same result was also observed in another TGCT-derived cell line, NCCIT. Furthermore, miR-302a-enhanced cisplatin sensitivity was partially mediated through the down-regulation of p21 in NT2 cells. MiR-302a induced apoptosis was further enhanced by silencing of p53 in NT2 cells. p53 levels were inversely associated with the expression of Oct4, Sox2, and Nanog in response to cisplatin. Thus, targeting miR-302a may offer new therapeutic interventions in TGCT. J. Cell. Physiol. 228: 2294–2304, 2013. © 2013 Wiley Periodicals, Inc.
Testicular germ cell tumors (TGCT) are the most common solid tumors that affect adolescents and young men between the ages of 15 and 35 years. The incidence of TGCT has increased dramatically in recent years (Horwich et al., 2006). About 15–20% of patients (El-Helw and Coleman, 2005) who have advanced or recurrent testicular cancer may undergo treatment with very high doses of cis-diamminedichloroplatinum(II) (cisplatin or CDDP)-based chemotherapy, which is strongly cytotoxic and may produce more severe side effects including renal impairment, pulmonary toxicity, vascular disease, and loss of fertility (Beyer et al., 1997; Ishioka et al., 2008; Honecker et al., 2009; Pliarchopoulou and Pectasides, 2010). Thus, understanding the mechanism behind cisplatin sensitivity and exploring new treatment combinations may have implications for minimizing treatment complications, in particular for those patients with advanced or recurrent testicular cancer. Suggested mechanisms, including diminished drug exporters and cellular detoxification, low levels of DNA repair proteins, a high Bax/Bcl-2 ratio, microsatellite instability and cellular copper metabolism (Masters et al., 1993; Sark et al., 1995; Burger et al., 1998; Kuo et al., 2007; Honecker et al., 2009), have not been able to fully explain the enigma of cisplatin resistance of TGCT. It is noteworthy that, in contrast to many other malignancies, most TGCTs not only have a wild-type p53 but also express the p53 protein at higher than normal levels (di Pietro et al., 2005). Although p53 expression is increased sharply in cisplatin-treated cells, p21 (WAF1/CIP1) is normally expressed at low levels (Spierings et al., 2003; Spierings et al., 2004). Most strikingly, p21 is regarded as an important determining factor in the sensitivity of TGCT cells to cisplatin (Spierings et al., 2004).
A class of small non-coding RNAs, named microRNAs (miRNAs), has been shown to play a fundamental role in diverse biological and pathological processes through their targeting of genes (Ambros, 2004; Boehm and Slack, 2005; Kloosterman and Plasterk, 2006; Croce, 2009). p21, a target of miR-302 (Wu et al., 2010), is abundantly expressed in teratomas which always prevents sensitization or confers resistance to cisplatin treatment (Bartkova et al., 2000). In contrast, Palmer et al. (2010) observed that the miR-302 family is overexpressed in all malignant TGCTs, but not in benign GCTs (teratomas), suggesting that miR-302 clusters could be potential serum biomarkers of malignant germ cell tumors (Murray et al., 2011). Additionally, Lin et al. reported that overexpression of the miR-302 cluster caused cell death during reprogramming, indicating the anti-tumorigenicity effect of miR-302 (Lin et al., 2008, 2010). Consistent with the studies of Lin et al., Yadav et al. (2011) have also shown that miR-302 mediates ethanol-induced neuronal cell death. Recently, many studies have shown that miRNAs can modulate the sensitivity of cancer cells to anticancer drugs in substantial ways (Park et al., 2007; Si et al., 2007; Frank et al., 2010; Takeshita et al., 2010). However, whether the combination of cisplatin with miR-302a will produce a significant clinical improvement remains unknown.
In order to understand the mechanism of cisplatin sensitivity and explore new treatment combinations for minimizing treatment complications, the relationship between miR-302a and cisplatin sensitivity inTGCTs was identified in the pluripotent human embryonal carcinoma cell line NTERA-2 (NT2) by validating its target genes and its upstream regulatory factors.
Materials and Methods
Reagents
cis-Diamminedichloroplatinum(II) (Cisplatin or CDDP) was purchased from Sigma-Aldrich (Dorset, UK). Cisplatin was freshly dissolved in 0.9% (w/v) saline before each experiment at 1 mM, and then further diluted to the final concentrations. U0126 (MEK1/2 Inhibitor) was purchased from Cell Signaling Technology (Beverly, MA).
Cell culture
Both NT2 and NCCIT cells are derived from human embryonal carcinomas (Andrews, 1984; Teshima et al., 1988). NT2 and other cancer cell lines (DU145 and HepG2) were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (Life Technologies Inc., Grand Island, NY) and 1% antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin; Life Technologies). NCCIT cells were grown in RPMI-1640 medium supplemented with 10% (v/v) fetal bovine serum (Life Technologies) and 1% antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin; Life Technologies). Cells were incubated at 37°C in a humidified incubator with 5% carbon dioxide.
Plasmids and oligonucleotides
The pFUM3GW vector encoding all four native miR-302 family members was kindly provided by Dr. Dong Xiao (Cancer Research Institute, Southern Medical University, China).
Sequences with miRNA-302 family (miRNA-302a, miRNA-302b, miRNA-302c, miRNA-302d mimic, and miR-302a inhibitor), as well as scramble negative control (Mimic NC and Inhibitor NC), were chemically synthesized by the Shanghai Gene-Pharma Co (Shanghai, China). Small interfering RNA (siRNA) against p53 and p21 were obtained from Santa Cruz. siRNAs against Oct4, Sox2, and Nanog were obtained from the Shanghai Gene-Pharma Co. Plasmids were transfected with Fugene HD (Roche, Switzerland), whereas small RNAs were transfected with Lipofectamine™ RNAiMAX reagent (Invitrogen, Carlsbad, CA).
RNA isolation and real-time quantitative PCR
- Oct3/4-forward: 5′-TGGTCCGAGTGTGGTTCTGTAA-3′,
- Oct3/4-reverse: 5′-TGTGCATAGTCGCTGCTTGAT-3′,
- Sox2-forward: 5′-GCACATGAACGGCTGGAGCAACG-3′,
- Sox2-reverse: 5′-TGCTGCGAGTAGGACATGCTGTAGG-3′,
- Nanog-forward: 5′-CAAAGGCAAACAACCCACTT-3′,
- Nanog-reverse: 5′-TCTGCTGGAGGCTGAGGTAT-3′,
- pri-miR-302-forward: 5′-TTGCTGTGACATGACAAAAATAAGTG-3′,
- pri-miR-302-reverse: 5′-ACACAGTGTGGGCGTTAACG-3′,
- β-actin-forward: 5′-TGGCACCCAGCACAATGAA-3′,
- β-actin-reverse: 5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′.
Western blotting
Western blotting experiments were performed as previously described (Liang et al., 2011). The following antibodies were used for Western blotting analysis: anti-Caspase-3 (Cell Signaling Technology, Beverly, MA), anti-Caspase-8 (Cell Signaling), anti-Caspase-9 (Cell Signaling), anti-PARP (Cell Signaling), anti-p21 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-Sox2 (Abcam, Cambridge, MA), anti-Oct4 (Santa Cruz), anti-Nanog (Millipore, Billerica, MA), and anti-Actin (Abcam). Protein levels were normalized to actin and quantified using Tanon Gel image system (Tanon, Shanghai, China).
Cell proliferation and drug-sensitivity assay
For the cell proliferation assay, NT2 cells were plated at 3–5 × 103 cells/well in 96-well plates with five replicate wells for each condition. Forty-eight hours after transfection, 10 µl of Cell Counting Kit-8 (CCK-8) (Dojindo Laboratories, Kumamoto, Japan) was added to the medium and then incubated at 37°C for 2 h.
For drug sensitivity assays, NT2 cells were harvested during the exponential growth phase and seeded into 12-well plates at 40–60% confluence. The next day, cells were transfected with miR-302a mimic or mimic NC. After 24 h, cells were collected and reseeded into the 96-well plate at a density of 3–5 × 103 cells per well in 100 µl of culture medium. Cells were allowed to adhere overnight and then incubated with different doses of cisplatin, ranging from 0 to 20 µM, for an additional 72 h.
Both cell proliferation and drug-sensitivity were measured with CCK-8, as previously described (Yao et al., 2010). The numbers of viable cells were determined by detecting the absorbance at 450 nm using a 96-well format plate reader (ELX 800 universal microplate Reader; BioTek, Inc., Highland Park, IL).
Flow cytometric analysis (FACS)
For cell cycle detection, NT2 cells were harvested, washed in PBS and fixed in 70% ethanol overnight. Cells were then collected, washed in phosphate-buffered saline (PBS), incubated at 37°C for 10 min with PBS containing 100 µg/ml RNase A and 25 µg/ml propidium iodide (PI) (Sigma–Aldrich) and then analyzed by FACS.
For apoptosis detection, adherent and floating cells were harvested and analyzed according to the manufacturer's indicated protocol (Beijing Biosea Biotechnology Co., Ltd, Beijing, China). The lower left quadrant represented living cells, and was negative for FITC-Annexin V binding and for PI uptake. The upper left quadrant, which represented necrotic cells, was FITC-Annexin V negative and PI positive. The lower right quadrant represented early apoptotic cells, and was FITC-Annexin V positive and PI negative. The upper right quadrant, which represented late apoptotic cells, was positive for FITC-Annexin V binding and for PI uptake.
Cell cycle distribution and apoptosis were analyzed by FACScalibur flow cytometer (BD Biosciences, San Jose, CA). Data analysis was performed using the WinMDI software.
Statistics
Experiments in this study were repeated at least three times. Student's t-test was applied to examine the differences among variables. All data were shown as the means plus standard errors of the mean (SEM). P-values <0.05 were considered to be statistically significant.
Results
Dose- and time-dependent induction of miR-302a in NT2 cells exposed to CDDP
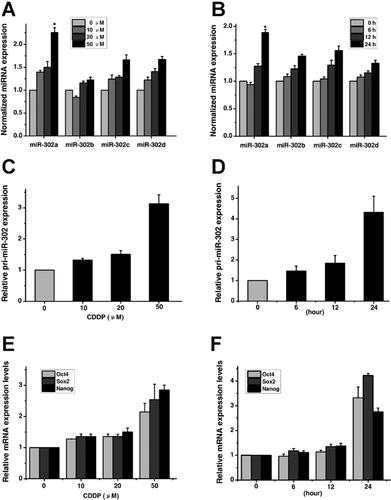
To determine whether variation of miR-302 levels in response to cisplatin could be a better determinant of sensitivity, we first investigated the expression level of miR-302a in NT2 cells exposed to cisplatin for different time periods or at different concentrations, by using TaqMan miRNA assays. The baseline expression levels of miR-302a were first examined in the NT2 and NCCIT cells (Fig. S1). As shown in Figure 1A,B, miR-302a was induced by cisplatin (CDDP) to a greater degree than other members. To determine whether the induction of miR-302a occurs at the transcriptional levels, the expression of primary miR-302 transcripts (pri-miR-302) and Oct4, Sox2, and Nanog (the transcription factors required for miR-302 expression) (Barroso-delJesus et al., 2008; Card et al., 2008; Marson et al., 2008; Stadler et al., 2010; Rosa and Brivanlou, 2011) in cisplatin-treated NT2 cells was examined by real-time quantitative PCR analysis. Consistent with the expression levels of mature miR-302a, the levels of pri-miR-302 (Fig. 1C,D), and Oct4, Sox2, and Nanog (Fig. 1E,F) were also shown to increase in a dose- and time-dependent manner under cisplatin treatment. Taken together, our results indicate that there is a correlation between the induction of Oct4, Sox2, Nanog and miR-302a in NT2 cells treated with cisplatin.
Up-regulation of miR-302a enhanced CDDP-induced apoptosis in NT2 cells
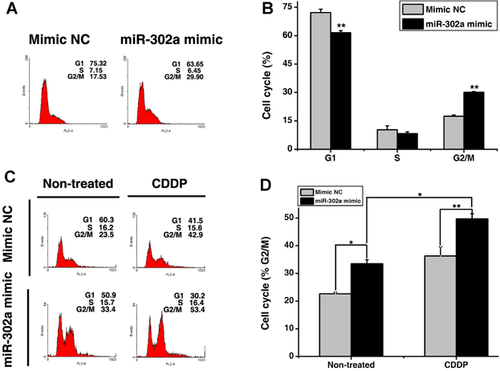
Next, the functional consequences of miR-302a on cell proliferation were evaluated in NT2 cells. As shown in Figure 2A, forced expression of miR-302a (Fig. S2A) at 100 nM significantly suppressed cell growth by nearly 30%, compared with mimic NC- or mock-transfected cells. To evaluate the effect of miR-302a on the viability of NT2 cells upon cisplatin treatment, NT2 cells were co-treated with miR-302a mimic and cisplatin. As shown in Figure 2B, the sensitivity to cisplatin in the miR-302a mimic-transfected NT2 cells was increased compared to mimic NC- or mock-transfected cells. We next explored whether the enhanced sensitivity shown by miR-302a was associated with an increase in cisplatin-induced apoptosis. As shown in Figure 2C–E, miR-302a mimic significantly increased cisplatin-induced apoptosis in NT2 cells. In addition, the activated caspase-3 (Fig. 2E), -8 (Fig. 2F), -9 (Fig. 2F), and cleaved PARP (Fig. 2E) were more obvious in miR-302a-transfected cells than in mimic NC-transfected cells after 50 µM cisplatin treatment for 24 h. In contrast, silencing of endogenous miR-302a (Fig. S2B) significantly reduced cisplatin-induced caspase-3 activation and PARP cleavage in NT2 cells (Fig. 2G). To further validate the role of miR-302a in NT2 cells, pFUM3GW, an miR-302 expression vector harboring human miR-302 and enhanced green fluorescent protein (EGFP) genes (Fig. 2H,I) was employed to examine the effect of miR-302s on cell apoptosis. Similarly, activated caspase-3, -8, and -9 and cleaved PARP were more profound in pFUM3GW-transfected cells than in control cells (Fig. 2J). These results suggested that miR-302a enhanced cisplatin-induced apoptosis was mediated via both mitochondrial and death-receptor-mediated pathways (Elmore, 2007).

MiR-302a increased CDDP-induced apoptosis by arresting cells in the G2/M phase
It has been shown that NT2 cells accumulated in the G2/M phase would subsequently progress to apoptosis when 10 µM cisplatin was applied (Mueller et al., 2006a). Note that in the absence of cisplatin, miR-302a had little effect on cell apoptosis (Fig. 2C,D). Therefore, we hypothesized that miR-302a-enhanced cisplatin-induced apoptosis may be due to cell cycle arrest in the G2/M phase. As shown in Figure 3A and B, miR-302a mimic caused an increase of cell population at the G2/M phase. In addition, co-treatment of miR-302a mimic and cisplatin resulted in more cells accumulated at the G2/M phase (Fig. 3C, D). The synergistic effect of miR-302a and cisplatin on the induction of G2/M cell cycle arrest indicates that miR-302a functions as a pro-apoptotic activator and triggers apoptosis in cisplatin-treated human testicular cancer cells.
MiR-302a increases the killing effects of cisplatin
Although cisplatin is a highly effective chemotherapeutic agent for treating various types of cancers, unfortunately, high doses of cisplatin are strongly cytotoxic and may produce severe side-effects. Therefore, low doses of chemotherapy drugs should be designed to minimize some of the side-effects. In this study, NT2 cells were co-treated with increasing amounts of cisplatin (ranging from 0 to 50 µM) and miR-302a mimic/mimic control for 24 h. As shown in Figure 4A, the cleavage of pro-caspase-3 and PARP could be detected in miR-302a-transfected NT2 cells in response to a low dose of cisplatin (20 µM). In contrast, these cleaved forms can be only detected in mimic NC-transfected cells after 50 µM cisplatin treatment. Furthermore, cells co-treated with cisplatin and miR-302a mimic displayed a much greater accumulation of cleaved PARP and activated caspase-3 than cells treated with equivalent doses of cisplatin (i.e., 50 µM) (Fig. 4A). These results suggest that overexpression of miR-302a in NT2 cells may lower the threshold for cisplatin-induced apoptosis.

We next examined whether the efficacy of the combination of miR-302a and cisplatin was a time-dependent response to cisplatin. As shown in Figure 4B, the cleavage of pro-caspase-3 was detectable as early as 6 h after cisplatin exposure in miR-302a-transfected cells. However, these cleaved forms were evident only after 24 h of cisplatin exposure in mimic NC-transfected cells. Likewise, the cleaved PARP was rapidly and robustly activated in miR-302a-transfected cells (Fig. 4B), suggesting faster apoptosis activation in the presence of the combination.
To assess the clinical relevance of the combination treatment, the transfected cells were treated with a lower concentration of cisplatin over a long exposure time. Firstly, the transfected cells were treated with 50 µM cisplatin for 2 h and then incubated in culture medium for a further 24 or 48 h, as described by Schweyer et al. (2004). As shown in Figure 4C, the apoptosis induced in miR-302a-transfected cells was more obvious than in mimic NC-transfected cells. Furthermore, the transfected cells were treated with a lower, more physiological concentration (ranging from 0 to 20 µM) for 48 h. As shown in Figure 4D, the cleaved PARP was more obvious in miR-302a-transfected cells than in mimic NC-transfected cells, indicating that miR-302a could enhance the effects of cisplatin at physiological concentrations.
To determine whether the synergic effect between miR-302a and cisplatin on apoptosis is a general phenomenon, or simply specific to TGCT-derived cells, DU145 (human prostate cancer cell lines), HepG2 (human hepatocellular liver carcinoma cell line), and another TGCT-derived cell NCCIT (pluripotent extragonadal germ cell tumor cell line) were used. DU145 and HepG2 were selected because both not only exhibit low sensitivity to cisplatin treatment but also have the dose-limiting toxicity of cisplatin (Zhang et al., 2001; Amantana et al., 2004). We ectopically expressed miR-302a in these cell lines and an apoptosis marker was determined. The results demonstrated that there is no synergic effect on apoptosis in DU145 (Fig. 4E) and HepG2 cells (Fig. 4F). Similar results were also observed in cells transfected with pFUM3GW (Fig. 4G,H). NCCIT cells were transfected with either mimic NC or miR-302a mimic for 24 h and then treated with 50 µM cisplatin for another 24 h. As shown in Figure 4I, the cleavage of the apoptosis marker was more obvious in miR-302a-transfected cells than in mimic NC-transfected cells in NCCIT cells. Similarly, a lower cisplatin concentration was used to clarify the effects of miR-302a in NCCIT cells. The transfected NCCIT cells were incubated with different concentrations of cisplatin ranging from 0 to 10 µM for 48 h; apoptosis was more evident in miR-302a-transfected cells (Fig. 4J). Altogether, these results indicated that the enhanced sensitivity to cisplatin by miR-302a was specific to TGCT-derived cells such as NT2 and NCCIT but not to other cancer cells, at least not in DU145 and HepG2.
Repression of p21 expression by miR-302a enhances the sensitivity of NT2 cells to cisplatin
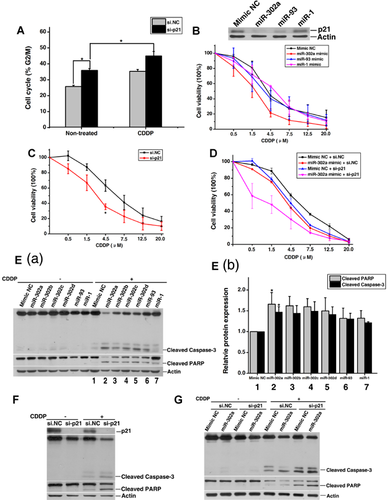
Given that p21 is one of the target genes of miR-302a (Wu et al., 2010), and our previous study indicated that silencing of p21 leads to G2/M phase arrest in NT2 cells (Lian et al., 2010), we next explored whether p21 induced phenotypes associated with miR-302a function. Firstly, we validated that knockdown of p21 (si-p21) in NT2 cells also induced a significant increase of G2/M phase cell population (Fig. 5A). Furthermore, co-treatment with si-p21 and cisplatin increased the already elevated G2/M phase cells (Fig. 5A). Secondly, both miR-302a (Fig. 5B) and si-p21 (Fig. 5C) enhanced the sensitivity of NT2 cells to cisplatin. As negative controls, miR-1 and miR-93 had no effect on cisplatin sensitivity (Fig. 5B). However, miR-93, rather than miR-1, down-regulated p21 expression (Ivanovska et al., 2008; Wu et al., 2010) (Fig. 5B). Furthermore, co-transfection of p21 siRNA with miR-302a mimic exhibited markedly increased sensitivity to cisplatin (Fig. 5D). Similarly, both miR-302a and p21 siRNA significantly promoted cisplatin-induced apoptosis in NT2 cells (Fig. 5E,F). In addition, NT2 cells co-transfected with p21 siRNA and miR-302a mimic had greater levels of apoptosis than cells transfected with miR-302a alone (Fig. 5G). We also found that apoptosis of miR-302a-transfected cells was more obvious compared with cells transfected with other members of miR-302 family or miR-1/miR-93 in response to cisplatin (Fig. 5E). Taken together, these results showed that the pro-apoptotic effect of miR-302a is mediated, at least partially, through the inhibition of p21 expression in NT2 cells.
p53 protects from cisplatin-induced apoptosis
As mentioned above, miR-302a was induced by cisplatin in testicular cancer cells. We next investigated how miR-302a itself is regulated in the context of cisplatin-treated NT2 cells. It has been shown that both the ERK pathway and p53 pathway participate in cisplatin-induced apoptosis in testicular cancer cells (Chresta et al., 1996; Schweyer et al., 2004). As shown in Figure 6A, there was no difference in pri-miR-302 expression between control and U0126 (an inhibitor of ERK pathway)-treated cells, suggesting that cisplatin-induced miR-302 expression was ERK-independent. On the other hand, si-p53 significantly increased cisplatin-induced levels of pri-miR-302 (Fig. 6B), mature miR-302a (Fig. 6C), and apoptosis (Fig. 6D).
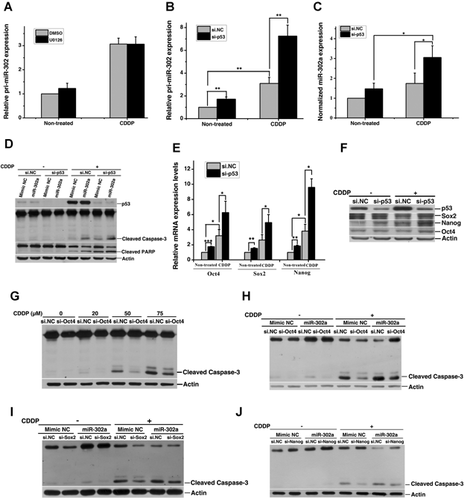
Since the accumulation of p53 could induce spontaneous differentiation of hESCs by suppressing the expression of Oct4 and Nanog (Qin et al., 2007), we questioned whether p53 affects the expression of Oct4, Sox2, and Nanog in cisplatin-treated NT2 cells. Our results revealed that silencing of p53 elevated both basal and cisplatin-induced mRNA (Fig. 6E) and protein (Fig. 6F) levels of Oct4, Sox2, and Nanog in NT2 cells. In addition, si-Oct4 (Fig. S2C) inhibited cisplatin-induced apoptosis (Fig. 6G) in NT2 cells, while miR-302a mimic could partially reverse the effect (Fig. 6H). Similar results were also observed in cells transfected with si-Sox2 and si-Nanog (Fig. S2D,E and 6). These data indicated that si-p53 enhanced apoptosis is partially mediated through a transcriptional regulation of miR-302a in NT2 cells.
Discussion
In this study, miR-302a was up-regulated by cisplatin in a dose- and time-dependent manner in NT2 cells. MiR-302a significantly enhanced cisplatin-induced apoptosis through down-regulating p21 expression in NT2 cells. Moreover, silencing of p53 further enhanced this effect by up-regulating the transcription factors Oct4, Sox2, and Nanog. Our data not only partially explain why TGCT confers exquisite sensitivity to cisplatin, but also propose that combined treatment with miR-302a and the conventional chemotherapeutic agents such as cisplatin could provide a new avenue for cancer patients.
Recently, many studies have shown that apart from oncogenic or tumor suppressor functions, some miRNAs could also regulate the cellular response to chemo- and radiotherapy (Zhao et al., 2008). Therefore, miRNAs could be potential biomarkers used for cancer diagnosis, prognosis, and personalized therapy (Zhao et al., 2008; Liang et al., 2010; Murray et al., 2011). Combined treatment with miRNAs and conventional chemotherapeutic agents such as cisplatin may provide a new approach to treating cancer (Park et al., 2007; Si et al., 2007; Frank et al., 2010; Takeshita et al., 2010). For example, Lin et al. (2008) have reported that miR-302, an important regulator of stem cell reprogramming (Card et al., 2008; Lin et al., 2008, 2010, 2011), induces apoptosis during reprogramming and in ethanol-stimulated neuron cells (Yadav et al., 2011). Furthermore, miR-302 inhibits the tumorigenicity of human pluripotent stem cells through suppressing the expression of CDK2 and CDK4/6 (Lin et al., 2010). In the present study, overexpression of miR-302a significantly increased chemosensitivity of cisplatin due to enhanced cisplatin-induced apoptosis in NT2 cells, while the inhibition of miR-302a partially abrogated cisplatin-induced apoptosis in NT2, which clearly establishes the ability of miR-302a to potentiate cisplatin effects. It has been shown that cellular miR-302 levels correlate with the induction of apoptosis in cells (Lin et al., 2010). We assumed that the high basal levels of miR-302 in testicular cancer patients make them more sensitive to cisplatin treatment. Thus, ectopic expression of miR-302a promoted basal and cisplatin-induced apoptosis in TGCT-derived cells such as NT2 and NCCIT. However, combined treatment with miR-302a and cisplatin did not result in similar antitumor effects in other cancer cell lines such as DU145 and HepG2. Recently, Bourguignon et al. (2012) have suggested that the induction of miR-302 in CSC like CD44v3high ALDH1high cells conferred the resistance of head and neck cancers to cisplatin. The results from the current study and that of Bourguignon et al. (2012) suggested that the enhanced cisplatin-induced apoptosis in miR-302a-overexpressing cells is specific to TGCT-derived cells, which partially explains the mechanism of cisplatin sensitivity in TGCT. Some men who had advanced or recurrent testicular cancer may undergo treatment with very high doses of chemotherapy (Beyer et al., 1997; Ishioka et al., 2008). However, these high doses of chemotherapy not only kill cancer cells but also have serious toxic effects (Pliarchopoulou and Pectasides, 2010). Thus, miR-302a can act as an enhancer of cisplatin in therapy of human testicular cancer.
MiRNAs are known to influence diverse biological processes and disease status through targeting genes (Lian et al., 2010; Yao et al., 2010; Yin et al., 2012). A direct target gene of miR-302a is p21 (Borgdorff et al., 2010), which is regarded as an important factor in the sensitivity of TGCT cells (Spierings et al., 2004). Here, miR-302a enhanced cisplatin-induced G2/M phase arrest through down-regulation of p21 expression and subsequently progressed to apoptosis. However, Scheel et al. mentioned that miR-302 targets tumor suppressor p63 in malignant testicular germ cell lines, indicating the ability of miR-302 to prevent apoptosis (Scheel et al., 2009). These seemingly contradictory studies suggest that other oncogenic miRNAs are involved in the development of TGCT by neutralizing the miR-302 pathway.
Unlike the low levels of p21 following cisplatin treatment, p53 levels are sharply induced by cisplatin in some TGCT cell lines (Spierings et al., 2004). Knockdown of p53 in H1 human embryonic stem cell lines or in mouse SSC line GSDG1 cells significantly elevated the expression of Oct4 and Nanog (Qin et al., 2007; Kuijk et al., 2010). In addition, p53 knockout mouse germ cells spontaneously formed embryonic-like stem cells (Kanatsu-Shinohara et al., 2004), whereas activation of p53 by DNA damage suppressed Nanog expression (Lin et al., 2005). Loss of Oct-3/4 expression in TGCT cells leads to a higher apoptotic threshold and cisplatin resistance (Mueller et al., 2006b). Persistent Oct4 expression is associated with TGCT (Looijenga et al., 2003; Jones et al., 2004; Cheng et al., 2007). MiR-302 is co-transcribed with the ESC-specific transcription factors Oct3/4, Sox2, and Nanog in a polycistronic manner (Card et al., 2008; Marson et al., 2008). Here, we demonstrated a synergistic effect between loss of p53 and miR-302a mimic in apoptosis of NT2 cells to cisplatin, through up-regulation of the expression levels of Oct4, Sox2, and Nanog. In addition, ectopic expression of miR-302a could partially reverse the anti-apoptotic effect induced by silencing of these transcription factors. Our data reveal a new regulatory pathway mediating cisplatin sensitivity in TGCT.
The relationship between p53 status and drug sensitivity is still controversial. Several lines of evidence have revealed that p53 down-regulation may confer cell resistance to cisplatin-induced apoptosis (Kerley-Hamilton et al., 2005; Gutekunst et al., 2011). For example, loss of p53 can rescue cells from apoptosis induced by cisplatin, indicating a close relationship between p53 protein levels and apoptotic rate (Gutekunst et al., 2011). However, the reason why cisplatin-resistant cells such as MCF-7 and A549 still have relatively high levels of p53 is not explained. Burger et al. (1997) observed that NCCIT (mutant p53) and S2 (no p53 protein) cells were readily triggered into apoptosis by cisplatin in comparison with 2102 EP cells (wild-type p53), indicating that p53 status did not correlate directly with the sensitivity of TGCT cells to cisplatin. Taken collectively, our results suggest that both the high levels of p53 and the specific cellular context (e.g., p53-mediated cisplatin-induced miR-302a and the transcriptional factors Oct4, Sox2, and Nanog expressions) determined the exquisite sensitivity of NT2 cells to cisplatin.
In the present study, we demonstrated for the first time that miR-302a plays a key role in cisplatin hypersensitivity in TGCT cells. Thus, miR-302a could be a potential strategy of hypersensitization of testicular cancer cells to chemotherapeutic agents.
Acknowledgments
We thank Dr. Dong Xiao from the Southern Medical University (Guangzhou, China) for the pFUM3GW vector. This work was supported by the following grants (to F.S.): the National Basic Research Program of China (2009CB941700); the National Natural Science Foundation of China (81125005); and the Chinese Academy of Sciences Knowledge Creative Program (KSCX2-EW-R-07).