RKIP: Much more than Raf Kinase inhibitory protein†
All authors have contributed in writing of this manuscript. Authors have no conflict of interest.
Abstract
From its discovery as a phosphatidylethanolamine-binding protein in bovine brain to its designation as a physiological inhibitor of Raf kinase protein, RKIP has emerged as a critical molecule for maintaining subdued, well-orchestrated cellular responses to stimuli. The disruption of RKIP in a wide range of pathologies, including cancer, Alzheimer's disease, and pancreatitis, makes it an exciting target for individualized therapy and disease-specific interventions. This review attempts to highlight recent advances in the RKIP field underscoring its potential role as a master modulator of many pivotal intracellular signaling cascades that control cellular growth, motility, apoptosis, genomic integrity, and therapeutic resistance. Specific biological and functional niches are highlighted to focus future research towards an enhanced understanding of the multiple roles of RKIP in health and disease. J. Cell. Physiol. 228: 1688–1702, 2013. © 2013 Wiley Periodicals, Inc.
The PEBP-1 protein was initially identified in bovine brain (Bernier and Jolles, 1984; Schoentgen et al., 1987, 1992) and rat spermatozoa (Banfield et al., 1998; Simister et al., 2002). PEBP-1 is a member of the evolutionary conserved phosphatidylethanolamine-binding protein (PEBP) superfamily, which contains more than 400 members from variety of species from bacteria to plants (Serre et al., 2001; Odabaei et al., 2004). In 1999, Yeung et al. used the yeast-2 hybrid screen to identify PEBP-1 as a RAF-1 binding protein that could inhibit MEK phosphorylation and activation. The authors named it Raf Kinase inhibitory protein (RKIP; Yeung et al., 1999). This landmark study was the first to elucidate a role for PEBP-1/RKIP in a pivotal cellular signaling cascade. Earlier data had demonstrated that PEBP-1/RKIP participates in diverse functions in different species, organisms, and cell types. It was reported that Tfs1p, an RKIP homolog in yeast, inhibits carboxypeptidase Y that acts as an inhibitor of yeast Ras GAP and consequently up regulates Ras (Bruun et al., 1998). RKIP expression has been detected in all mammalian tissues, such as brain, liver, stomach, spleen and muscle of human, cow, rat, and chicken; it is mainly localized to the cytoplasm and inner periplasmic membrane (Bollengier and Mahler, 1988; Seddiqi et al., 1994; Frayne et al., 1999; Vallee et al., 2001). RKIP is most highly expressed in the secretory product of testicular germ cells (Saunders et al., 1995; Seddiqi et al., 1996), brain oligodendrocytes, and specific cortical and hippocampal neuronal cell layers (Frayne et al., 1999; Seddiqi et al., 1996). RKIP is a precursor to the hippocampal neurostimulating peptide (HCNP), which is involved in neuronal differentiation and acts as a factor in acetylcholine synthesis (Seddiqi et al., 1994; Mitake et al., 1995, 1996a, b). RKIP-knockout mice were utilized to delineate the role of RKIP in the development of brain functions. Interestingly, this mouse strain showed a mild olfaction deficient condition at the age of 10 months, linking RKIP expression to olfactory system development (Theroux et al., 2007). In mice, RKIP was shown to modulate the photic entrainment of the suprachiasmatic nucleus circadian clock through the Raf-MEK-ERK pathway (Antoun et al., 2012). Photons can apparently induce RKIP phosphorylation at the S153 residue in the suprachiasmatic nucleus (Antoun et al., 2012). In nematodes, PEBP is one of the membranous secreted proteins conferring a protective role against the host immunological responses (Morgan et al., 2006). Drosophila PEBP homologs, of which at least seven isoforms are known, are expressed in olfactory cells (Rautureau et al., 2009). Interestingly, PEBP1 overexpression has been shown to protect Drosophila against bacterial infection by enhancing the release of immunity-related proteins in their hemolymph (Reumer et al., 2009), and in plants, RKIP was found to be associated with growth and differentiation, transforming plants from the vegetative to reproductive growth phases (Banfield and Brady, 2000). Furthermore, RKIP is reportedly involved in Cep290-mediated photoreceptor degeneration and retinopathy. The immunolocalization and accumulation of RKIP and its partner protein Cep290 prevented cilia formation in zebrafish and cultured cells (Murga-Zamalloa et al., 2011). Therefore, the diverse phylogenic conservation and functions attained by RKIP in different species and tissues/cell-types underscores its functional importance and potential cardinal roles in cellular signaling (Table 1).
| Protein names | Phosphatidylethanolamine-binding protein 1 |
| HCNP | |
| Neuropolypeptide h3 | |
| Prostatic-binding protein | |
| Raf kinase inhibitor protein | |
| Cleaved into: HCNP | |
| Gene names | PEBP1, PEBP, PBP |
| Epigenetics | EZH2-targeted inhibition of RKIP through SNAIL |
| CpG islands methylation | |
| Genomics | Chromosomal location: 12q24.23, Length: 9,728 bp |
| Gene Layout: 4 exons, 3 introns | |
| PEBP1 gene promoter: houses multiple CpG islands, E1 and E2-box, androgen response elements (ARE), p53 binding site | |
| Proteomics | Subcellular location: cytoplasm and occasionally nuclear |
| Length: 187 a.a | |
| Mass: 21–23 kDa | |
| Subunit: interacts with RAF-1 and enhanced by the phosphorylation of RAF-1 ‘S338,’ ‘S339,’ ‘Y340,’ and ‘Y341’ | |
| Functions | Scavenger protein. Binds nucleotides, opioids and phosphatidylethanolamine. |
| Inhibits the kinase activity of RAF-1 by inhibiting its activation and by dissociating the RAF-1/MEK complex and acting as a competitive inhibitor of MEK phosphorylation. | |
| Modulation of behavioral responses and circadian rhythms | |
| Organization of phospholipids in myelin sheath | |
| Memory and learning | |
| Endocrine factor in cardiac physiology | |
| Spermatogenesis | |
| Signaling pathways | MAPK |
| GPCR | |
| NFkB | |
| GSK3β | |
| Downstream effector molecules | RAF-1 |
| MEK | |
| ERK | |
| GRK2 | |
| TRAF6 | |
| TAK1 | |
| NIK | |
| IKK | |
| P38 | |
| GSK3β | |
| NRF2 | |
| KEAP 1 | |
| Aurora B | |
| Physiological behavior influenced by PEBP1 | Growth and differentiation |
| Proliferation | |
| Migration | |
| Motility | |
| Cell cycle | |
| Genomic stability | |
| Apoptosis | |
| Drug resistance | |
| Orthologs | Human, mouse, chicken, rat, fruit fly, dog, cow, chimpanzee, yeast, bacteria |
| Diseases associated with PEBP1 perturbation | Metastasis, Alzheimer's disease, diabetic neuropathy, prostate cancer, gastric cancer, melanoma, breast cancer, colorectal cancer, ovarian cancer, Gastrointestinal stromal tumors, hepatocellular carcinoma, nasopharyngeal carcinoma, lung cancer, gliomas |
| Expression in normal tissues | Protein: expressed in almost all tissues to variable extent |
| Neurons, neuroendocrine cells, liver, testes, prostate, glandular epithelia of breast, salivary glands and pancreas, kidney, bladder, endothelia of lymph and blood vessel, milk duct epithelial cells, primary melanocytes, blood plasma and urine, HEK-293 cell lines, bronchoalveolar lavage cells | |
| mRNA | |
| The highest mRNA expression levels were reported in the testis (epididymis, seminal vesicle), adrenal cortex, brain, thyroid, liver, thymus, bone marrow, heart, lung, prostate, pancreas, kidney, and spleen | |
| Useful links | General information |
| http://atlasgeneticsoncology.org/Genes/PEBP1ID44021ch12q24.html | |
| http://www.ncbi.nlm.nih.gov/sites/entrez?db=gene&term=5037 | |
| http://bioinf.umbc.edu/dmdm/gene_prot_page.php?search_type=protein&id=1352726 | |
| Tissue expression | |
| http://www.ebi.ac.uk/gxa/gene?gid=P30086 | |
| http://en.wikipedia.org/wiki/Phosphatidylethanolamine_binding_protein_1 | |
| Uniport Protein database | |
| http://www.uniprot.org/uniprot/P30086 |
RKIP: An inhibitory Modulator Protein
Especially since its discovery as the only physiological endogenous inhibitor of the Raf-MEK-ERK signaling pathway, research has primarily focused on elucidating the mechanism of RKIP actions (Yeung et al., 1999); The Raf-MEK-ERK is a highly conserved signaling pathway that regulates cell growth, differentiation, migration, and apoptosis (Hagan et al., 2006). Upon binding of a growth factor to the transmembrane receptor tyrosine kinases (RTKs), the MAPK signaling pathway subsequently activates nuclear transcription factors that influence many cellular functions (Zeng et al., 2008). The molecular mechanism by which RKIP exert its inhibitory effect on the Raf-MEK-ERK pathway was described by Yeung et al. (2000) who demonstrated that RKIP binds either to Raf-1 or MEK, which contain partially overlapping binding sites. It was further demonstrated that RKIP exerts its inhibitory effect on Raf-1 by inhibiting the phosphorylation of the N-region of Raf-1 by PAK kinases as well as residues S338 and T340/341 of Raf-1 by SRC kinases (Trakul et al., 2005). RKIP was also found to bind MEK and ERK, which subsequently causes Raf-1 to dissociate from MEK, thereby preventing its phosphorylation and activation by Raf-1. This process diminishes downstream ERK kinase signaling (Yeung et al., 2000). Moreover, RKIP has been shown to inhibit B-Raf (Park et al., 2005). Using the yeast-two hybrid system, Park et al. (2005) showed that RKIP physically interacts with B-Raf and antagonizes its kinase activity.
RKIP has been implicated in the control of G-protein coupled receptors (GPCR) signaling cascade (Lorenz et al., 2003). GPCRs comprise a large family of membrane receptors and are essential physiological determinants of neurotransmission, inflammation, and regulation of blood pressure (Ribas et al., 2007). G protein-coupled receptor kinase 2 (GRK-2) has been revealed as a primary negative feedback inhibitor of GPCRs. GRK2 phosphorylates activated receptors, which causes them to dissociate from G proteins; this process leads to GPCR internalization and ultimately the inhibition of GPCR signaling. RKIP was identified as a physiological inhibitor of GRK-2 (Lorenz et al., 2003); however, its inhibitory function is phosphorylation-dependent (Corbit et al., 2003; Lorenz et al., 2003). When a cell is stimulated by a growth factor, thereby stimulating protein kinase C (PKC; Corbit et al., 2003; Lorenz et al., 2003). The PKC isoenzymes -α,-βI, -βII, -γ, and atypical PKCζ—mediate the phosphorylation of RKIP at residue S153 (Corbit et al., 2003), allowing RKIP to dissociate from Raf-1. pRKIP then binds to GRK-2 and blocks its inhibitory activity. It was recently revealed that RKIP phosphorylation at S153 triggers its dimerization and that this process is required to switch RKIP from Raf-1 to GRK2 (Deiss et al., 2012). The relevance of RKIP dimers and their consequent binding specificity to other targets is still unclear.
Acting in a similar inhibitory fashion, RKIP controls nuclear factor κB (NF-κB) activation. In its inactive form, NF-κB is localized to the cytoplasm, where it is bound to inhibitory κB (IκB; Dyson and Komives, 2012). IκB phosphorylation by IκB kinase (IKK) induces IκB degradation. Moreover, NF-κB-inducing kinase (NIK) and transforming growth factor B-activated kinase-1 (TAK-1) activate IKK by phosphorylation. Consequently, NF-κB is released and translocates to the nucleus where it transcriptionally regulates genes involved in immunity, inflammation, cell proliferation, apoptosis, and cell migration (Karin et al., 2002). RKIP has been reported to inhibit NF-κB signaling by inhibiting the upstream kinases TAK1, NIK, and IKK (Yeung et al., 2001). This inhibition results in the downstream inhibition of anti-apoptotic gene products and sensitizes the cell to apoptosis-induced chemotherapy (Chatterjee et al., 2004). Because RKIP can interact with multiple core components of the NF-κB signaling pathway, RKIP may act as a scaffold protein, facilitating the assembly of a multi-component protein complex. A subsequent analysis demonstrated that RKIP also interacts with the upstream activators of IKK complex, namely TRAF6; therefore, promoting the ubiquitination of TAK1 in cancer cells (Tang et al., 2010). RKIP was recently shown to interact with the syntenin protein melanoma differentiation associated gene-9 (MDA-9). This interaction suppresses FAK via the activation of MDA-9 by SRC, which is required for metastatic progression in melanoma (Das et al., 2012). Therefore, RKIP may can be considered an MDA-9 inhibitor.
It is noteworthy that RKIP's inhibitory function is not an off/on switch; rather, it may function to modulate the signal intensities (Trakul and Rosner, 2005). Loss or reduction of RKIP expression does not initiate an “on” response; instead it enhances EGF and ERK activation several-folds. Conversely, RKIP overexpression reduces the amplitude of the EGF stimulation response.
RKIP: An Activator Protein
As an inhibitor, RKIP blocks the access of kinases to their substrates. However, an additional mode of RKIP action has recently been discovered. In a search for RKIP-binding protein partners using a protein array-based screen, we found that RKIP regulates Glycogen synthase kinase 3 (GSK3β) in two ways (Al-Mulla et al., 2011a). First, RKIP binds and maintaining GSK3β protein levels, and second, RKIP prevents GSK3β inhibitory phosphorylation. RKIP depletion induced an intense oxidative stress response that lead to the activation of p38 MAPK (Al-Mulla et al., 2011a), which inhibited GSK3β by phosphorylating its inhibitory T390 residue (Thornton et al., 2008). Thus, normal RKIP levels preserve GSK3β activity, while RKIP depletion reduces active GSK3β protein levels. Importantly, RKIP depletion resulted in the activation of downstream GSK3β targets, which resulted in the stabilization of cyclin D1; cyclin D1 induces cell cycle progression, and the expression of β-catenin, SNAIL and SLUG to promote the epithelial to mesenchymal transition (EMT) and invasion (Al-Mulla et al., 2011b). The method by which RKIP maintains a subdued oxidative stress response in cells is unclear, and it is not known how its depletion or reduced expression induces intense oxidative stress response. This specific knowledge niche requires urgent attention. Acting as an activator, albeit indirectly, RKIP has been shown to enhance and activate micro-RNA (mir) let-7 function (Dangi-Garimella et al., 2009). Specifically, by inhibiting the RAF-1/MEK/ERK pathway, c-Myc expression and the transcription of LIN28, which is a target of Myc activation, were reduced (Yun et al., 2011). LIN28 is essential for bone metastasis in aggressive breast cancer and it appears to inhibit the maturation of mir let-7. In turn, let-7 inhibits HMGA2; a chromatin remodeling protein that plays a role in the activation of pro-invasive and pro-metastatic genes involving Snail, CXCR4 and OPN and BACH, which activates the bone metastasis genes MMP1 and CXCR4 (Kang et al., 2003).
MAPK, NF-κB, GPCR, GSK3β, let-7, and the reactive oxygen species (ROS) signaling cascades are major pathways by which a cell transforms extracellular stimuli into distinct signals to maintain cellular integrity and homeostasis. Defects within any single molecule in these cascades may lead to drastic, improper cellular behavior in terms of growth, proliferation, cell division, migration, apoptosis, and genomic instability; overall, these defects may promote cancer development. Therefore, RKIP mediates the cross talking molecule between these vital cellular pathways and plays a cardinal modulatory role in which its downregulation or absence may deregulate these pivotal cellular processes (Fig. 1).
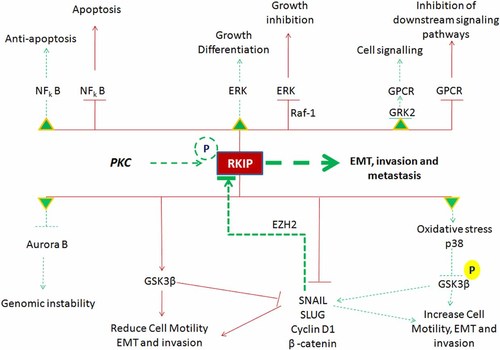
RKIP modulates cardinal cellular signaling pathways in normal cells. RKIP, under basal conditions, inhibits, Raf-1, NF-κB, and SNAIL and activates GSK3β (shown in burgundy colored lines and arrows). Upon phosphorylation of RKIP at S153, or loss/diminution these pathways, including the GPCRs are attenuated inducing a variety of pathological processes denoted by green triangles, broken green lines and arrows.
Lessons From RKIP Molecular Structure
The human RKIP molecule is 23 kDa, and the mature protein is composed of 186 amino acids (after the removal of the first methionine residue; Bernier and Jolles, 1984; Hochstrasser et al., 1992). RKIP has been delineated and published in several structures, including human (Banfield et al., 1998), bovine (Serre et al., 1998), bacteria (Serre et al., 2001), and plant (Banfield and Brady, 2000). Studies have revealed that RKIP tends to crystallize in two asymmetric molecules. The two molecules of RKIP (180 and 185 residues) have been modeled; however, its functional oligomeric and dimeric states were unknown (Banfield et al., 1998). Previous reports suggested that RKIP may exist as high-order oligomers and dimers (Schoentgen et al., 1987), which was supported by the observation of RKIP dimers in gel electrophoresis and fluorescence studies (Banfield et al., 1998). It was recently reported that after, S153 phosphorylation, RKIP dimerization, is an important step for the switch between RAF-1 and GRK2 binding (Deiss et al., 2012). Therefore, RKIP oligomerization is a key event that regulates its ability to modulate kinases and this process warrants further attention from the scientific community. The compact structure of RKIP is uniquely based on the 9-stranded β sheets and 4 α-helices that are folded in a special pattern that confers protein stability and gives RKIP unique properties. RKIP possesses a conserved ligand-binding pocket and several compounds that bind to RKIP as ligands have been identified by NMR and elution studies (Shemon et al., 2010; Tavel et al., 2012). The RKIP ligand-binding pocket is composed of 16 amino acid residues (Fig. 2A) and its has been shown to accommodate various nucleotides, including guanosine tri- and di-phosphate (GTP and GDP), flavin mononucleotide (FMN; Banfield et al., 1998; Tavel et al., 2012), phospholipids (Vallee et al., 2001) and many non-lipid organic compounds like locostatin (Zhu et al., 2005; Shemon et al., 2009). In fact, column-dependent purification of RKIP relies on this protein's ability to bind to nucleotides. Previous studies strongly agree that the nucleotides-binding property of RKIP is enhanced at lower pH values (Tavel et al., 2012). It is noteworthy that at neutral pH, which approximates normal physiological conditions, rat recombinant RKIP failed to bind to nucleotides; however, phospholipid binding was unaffected by a reasonable pH change (Shemon et al., 2010). Recent evidence, using human RKIP, reaffirmed that the RKIP ligand pocket can bind various nucleotides under near physiological conditions (Tavel et al., 2012). Although rat and human RKIP protein sequences share more than 87% amino acids similarity, these experiments suggest that the RKIP pocket may play different roles in different species or at different pH levels within the microenvironment of a cell. Therefore, additional species-specific structural and binding dynamic experiments should be conducted to illuminate this niche. The ability to alter the properties of the RKIP ligand pocket by altering the pH values may be relevant to the development of target-based therapeutics because cellular functions, such as migration, phagocytosis, intracellular oxidant generation (especially in neutrophils), and cancer cell cytotoxicity, are pH dependent (Roos and Boron, 1981; Serrano et al., 1996; Allen et al., 1997).
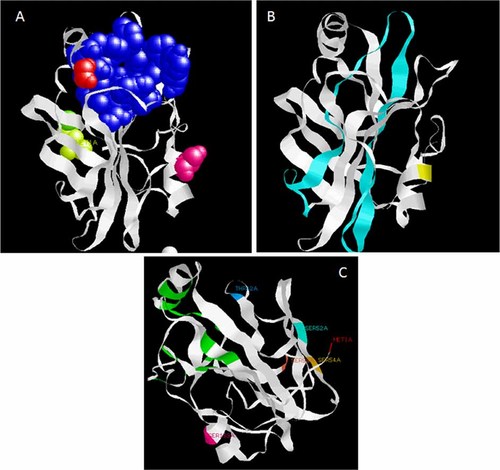
Structural ribbon models of human RKIP. A: Depicts the RKIP ligand-binding pocket, which is formed by 16 amino acid residues (D70, A73, P74, Y81, W84, H86, V107, G108, G110, P111, P112, H118, Y120, L180, Y181, and L184) shown as blue spheres. S153, which is phosphorylated by PKC is shown in purple. B: Shows amino acid residues 93–134, which form the Raf-1 binding domain in cyan and S153 residue in yellow for orientation. C: RKIP other phosphorylated sites, T42 in blue, S52 in cyan, S54 in beige and S99 in brown. For orientation purpose, the RKIP ligand pocket is shown in green, S153 in pink and M1 in red.
The RKIP ligand pocket and the other highly conserved amino acids depicted in Figure 2B have been found to significantly contribute to Raf-1 binding. The tri-phosphorylated and unphosphorylated forms of the Raf-1 protein (Shemon et al., 2010; Tavel et al., 2012) bind to RKIP. This binding of tri-phosphorylated Raf-1 (Park et al., 2006; Tavel et al., 2012) is tighter and involves the RKIP ligand pocket and the A73, S75, P74, and H86 residues. Moreover, residues K150, V151, and A152, which immediately precede S153 in α-helix H1 of human RKIP, are influenced by binding of the tri-phosphorylated Raf-1 peptide (Granovsky et al., 2009; Tavel et al., 2012). Mutations in the recombinant RKIP, namely P74L and H86A, have been shown to disrupt binding of RKIP to tri-phosphorylated Raf-1 (Granovsky et al., 2009). Interestingly, the binding of unphosphorylated Raf-1 to RKIP involves residue G110 at the bottom and A73 and Y81 residues at the edge of the protein but it does not involve additional residues within the RKIP ligand pocket (Shemon et al., 2009, 2010; Tavel et al., 2012).
The S153 residue of RKIP has gained tremendous attention because its phosphorylation by PKC dislodges it from Raf-1 binding by mere steric hindrance (Corbit et al., 2003). This process enhances its dimerization (Deiss et al., 2012) and shifts its affinity toward inhibiting GRK2, an inhibitor of the GPCRs including the β2-adrenergic receptor pathway (Lorenz et al., 2003). Therefore this process, activates both the Raf-MEK-ERK and GPCR pathways (Fig. 1). However, there are several questions that remain unanswered. For example, how do the unique topology, specific folding and conserved ligand-binding site of RKIP allow such a small protein to co-ordinate the binding of so many other fundamental signaling proteins (Raf-1, GRK2, NIK, TAK1, GSK3α, and β) or act as a scaffold in cellular signaling pathways? Where do these proteins bind on the RKIP molecule? Answers to these fundamental questions may be determined after examining the other sides of RKIP. Residues T42, S52, S54, and S99 have received very little attention from the research community (Fig. 2C). In a wide-proteomic screen for proteins modified by phosphorylation during mitosis, Dephoure et al. (2008) found that the T42 residue of RKIP was unphosphorylated in the G1 phase but was phosphorylated in mitosis, while S52 and S54 phosphorylation shifted in the opposite direction. These data may indicate the importance of these residues in RKIP regulatory functions. Moreover, preliminary data from our laboratories have identified S99 as a residue subjected to phosphorylation by ERK activation (unpublished data). The consequences of these post-translational modifications on RKIP functions and its binding to other proteins have not yet been discovered.
The Role of RKIP in Cell Cycle Control and Genomic Stability
Because ERK 1/2 signaling strongly affects the cell cycle, one can predict that RKIP exerts a significant impact on controlling the cell cycle. Several positive and negative regulators act in harmony during cell cycle phases to ensure faithful DNA transmission from the mother cell into two daughter cells. However, molecular deregulation would affect the harmonic interaction and as a consequence, it may influence the cell division process, leading to genomic instability and cancer development. In a study by Eves and colleagues RKIP was found to be associated with centrosomes and kinetochores in cultured mammalian cells, thus regulating the spindle checkpoint (Eves et al., 2006; Eves and Rosner, 2010). The authors reported that RKIP-depleted cells rapidly transit to anaphase and display a defective spindle checkpoint. RKIP depletion and Raf-1 hyper-activation reduced the localization (Eves et al., 2006) and abrogated the kinase activity of Aurora B, a conserved kinase that plays a pivotal role during chromosomal alignment, cell division, and spindle checkpoints (Adams et al., 2001). During pro-metaphase, Aurora B, and chromosomal passenger proteins, reside at the inner centromeres, thereby controlling the interaction between microtubules and kinetochores (Eves et al., 2006). The authors have also demonstrated that RKIP deficient cells displayed decreased localization of phosphorylated Aurora B to kinetochores and inhibited its kinase activity due to the hyperactivation of the MAPK pathway; this process then led to chromosomal abnormalities in cultured cells. Comparative genomic hybridization and allelotyping were used to determine the correlation between RKIP expression and chromosomal loss in colorectal tumor samples. CRC tumors lacking or weakly expressing RKIP displayed more significant chromosomal loss and consequently genomic instability than RKIP expressing cancers (Al-Mulla et al., 2008). Consistent with the observation of Eves et al., we reported that RKIP depleted cells exerted a shorter transition time from nuclear envelope breakdown to anaphase. We also observed the downregulation of Aurora B and G2/M checkpoint molecules. Together, these changes induced the cells to be highly proliferative due to the faster completion of cell cycle phases (Al-Mulla et al., 2011b). These data suggest that RKIP influences the cell proliferation rate by modulating cell cycle kinetics (namely the G1/S and G2/M transitions). Moreover, RKIP overexpression reduced cell growth and proliferation compared with uninduced cells in an RKIP-dependent, manner, suggesting that RKIP may also act as a tumor suppressor. Whole transcriptome profiling experiments exposed key cell cycle regulatory molecules affected by manipulating RKIP levels (Al-Mulla et al., 2011b). RKIP silencing induced the expression of molecules that drive DNA synthesis, G1/S and G2/M transitions (such as Cyclin D1, Cyclin E2, CDK6, SKP2, micro-chromosome maintenance family, and NEK6), and attenuated the expression of molecules involved in guarding the cell cycle checkpoint, such as p21Cip1, Aurora B, Cyclin G1, Sertuin, and Anaphase promoting complexes 11 and 7 (Al-Mulla et al., 2011b). Cyclin D1 and SKP2 are considered to be directly relevant to RKIP because they are regulated by GSK3β. As previously mentioned, GSK3β is inactivated by RKIP downregulation or loss; therefore, cyclin D1 and SKP2 are stabilized at the protein level. Overall, RKIP depletion significantly influences processes that accelerate cellular proliferation and growth by directly or indirectly modulating the expression of genes involved in DNA replication, transition through G1/S phase, G2/M checkpoints, and genomic stability (Al-Mulla et al., 2011b). However, it is still unclear how RKIP can modulate the expression of these fundamental cell-cycle associated molecules.
RKIP in Cell Motility and Invasion
Cell migration is one of the major cellular processes regulated by Raf-MEK-ERK signaling cascade (Matallanas et al., 2011). Wound healing and embryonic development are physiologically regulated by the process of cell migration. The deregulation of this process may cause the cells to acquire an abnormal or pathological status. Studies correlating RKIP levels and cell migration have been controversial. The inhibition of the ERK signaling pathway in malignant cells has been shown to inhibit cell motility and invasion in vivo. Conversely, elevated ERK levels in non-invasive cancerous cells increase cell migration (Krueger et al., 2001). By default, these observations implicate RKIP as a key player in cellular migration because it is the only physiological negative regulator of the MAPK pathway. Lee et al. (2006) observed that the restoration of RKIP expression repressed cell migration and motility in human hepatoma cells (HHC). This report is consistent with other observations indicating that RKIP overexpression in melanoma cells clearly reduced their invasive potential (Schuierer et al., 2004; Das et al., 2012). Contrary to other studies, RKIP silencing slowed down the migration of MDCK epithelia cells compared to control cells expressing RKIP, while RKIP overexpression significantly affected the behavior of MDCK cells by favoring migration (Zhu et al., 2005). Similarly, Ma et al. (2009) demonstrated that RKIP overexpression promoted cell migration in rat hepatic stellate cells (HSC), while RKIP inhibition decreased HSC migration. We have shown that RKIP depletion in HEK-293 cells favored migration by inducing the p38-mediated phosphorylation of GSK3β. This process induces GSK3β degradation, subsequently stabilizing cell migration regulatory molecules such as β-catenin (Al-Mulla et al., 2011b). Microarray expression profiling confirmed this observation because β-catenin expression was threefold higher in RKIP-silenced HEK-293 cells compared to control. Interestingly, wide-screen expression profiling also revealed additional cell migration and motility regulatory proteins abrogated after RKIP depletion. C-MET, a transcriptional factor of β-catenin and a growth factor that promotes cell cycle and cell migration (Gherardi et al., 2012), was significantly upregulated twofold (Al-Mulla et al., 2011b). In addition, PAK1, a protein kinase involved in cell proliferation and motility (Sells et al., 1999), was also overexpressed in RKIP-silenced HEK-293 cells. These observations strongly support the hypothesis that RKIP loss may directly or indirectly influence the cell proteome and transcriptome to favor migration. Moreover, Beshir et al. (2010) postulated that the suppressive function of RKIP in metastasis and cell migration may be attributed to its ability to downregulate the expression of certain matrix metalloprtoeinases (MMPs), namely MMP-1 and MMP-2. The authors reported that RKIP silencing rendered the cells highly invasive and associated significantly with higher expression levels of MMP-1 and MMP-2. This study concluded that RKIP controls the invasion of cancerous cells by negatively regulating NF-κB, which controls the expression of MMPs and hence, cellular invasion (Beshir et al., 2010). Similarly, RKIP was shown to downregulate MMP-2 and MMP-9, cathepsin B and urinary plasminogen activator and E-cadherin (Xinzhou et al., 2011). Ciarmela et al. (2012) reported that in normal trophoblastic tissues, RKIP is expressed in the cytotrophoblasts but not in the syncytiotrophoblasts, and its inhibition by Locostatin reduced cytotrophoblastic invasion and motility. However, Locostatin may influence cellular motility independent of its interaction with RKIP (Shemon et al., 2009; Al-Mulla, 2012b).
The inconsistencies between these observations with respect to RKIP expression and cell migration may be attributed to the use of different cell lines and may therefore be cell-type dependent. For example, and as stated above, RKIP stabilizes GSK3β in its active form (Al-Mulla et al., 2011a). Moreover, active GSK may inhibit growth cone extension (Eickholt et al., 2002; Etienne-Manneville and Hall, 2003). Because MDCK cells rely on activated GSK3β for their motility (Farooqui et al., 2006), RKIP modulation may have specifically influenced the motility of these cells solely by modifying GSK3β activation.
RKIP Tissues and Subcellular Localization
RKIP is expressed in almost all normal tissues in humans and mammals (Fig. 3). However, RKIP is highly expressed in the nervous system, adrenals, and thyroid. RKIP is moderately expressed in the liver, testes, and prostate, and it is weakly expressed in white blood cells, the heart and other tissues (Ponten et al., 2008; http://www.proteinatlas.org/ENSG00000089220/normal). Interestingly, even within neural tissues, RKIP expression levels vary. For example, RKIP mRNA is intensely expressed in prefrontal cortex and amygdala, but it is expressed to a lesser degree in the pons (Fig. 3). Moreover, embryological evidence suggest that RKIP expression may be time-dependent because its expression was reduced after 1 month of skeletal muscle development (Sun et al., 2009). The presence of RKIP in various tissues, suggests that RKIP possesses more roles than the mere inhibition of the Raf/MEK/ERK pathway. At the cellular level, RKIP is localized toward the negatively charged inner leaf of the plasma membrane (Banfield et al., 1998). However, immunohistochemical analysis using polyclonal antibodies have demonstrated RKIP localization in the cytoplasm and plasma membranes in several tissues (Seddiqi et al., 1994; Al-Mulla et al., 2006c). Recently, RKIP was found to be secreted by senescing MCF7 cells after they were treated with ionizing radiation (Han et al., 2012); however, this observation requires more elaborate confirmation. Occasionally, immunohistochemistry experiments have demonstrated intense nuclear expression of RKIP, as p-RKIP (Hagan et al., 2005). This is not surprising because RKIP can associate with the centrosomes and kinetochore of nuclear chromosomes (Eves et al., 2006). Using a normal tissue microarray and a polyclonal antibody generated in our laboratory, we have documented nuclear RKIP expression in the bladder, thyroid, endocrine pancreatic cells more frequently than in other tissues (Al-Mulla et al., 2006c). Moreover, we found that the monoclonal RKIP antibody that was generated by Epitomics, yields more intense nuclear localization than polyclonal antibodies (unpublished data). However, data from the Protein Atlas database using three polyclonal antibodies indicate that nuclear RKIP expression is a rare event in normal as well as cancerous tissues (http://www.proteinatlas.org/ENSG00000089220/normal). It has been well documented that different methods of fixation and antigen retrieval can influence tissue staining (Leong and Leong, 2011). It is also worth noting that various commercial polyclonal and monoclonal RKIP antibodies are now available and these antibodies should be objectively evaluated and compared to establish their clinical efficacy.
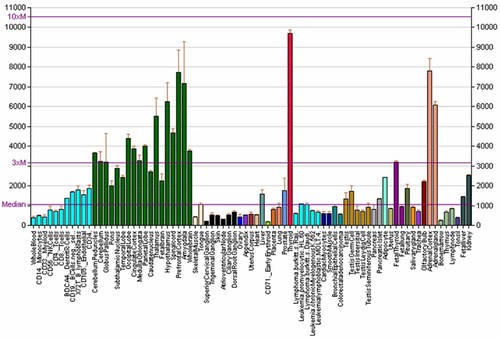
Expression of RKIP in human and mouse tissues using the Affymetrix 210825_s_at probe modified from Su et al. (2004) (http://en.wikipedia.org/wiki/File:PBB_GE_PEBP1_210825_s_at_tn.png).
Clinical Significance of RKIP and pRKIP Perturbation
Numerous reports have implicated RKIP in various health- and disease-associated conditions. RKIP clearly regulates several key signaling pathways in cells and tissues. Several studies have implicated RKIP as a member of novel class of proteins that behave as metastasis suppressors. The loss of such proteins may trigger cancer progression and the development of malignancy. Experimental results have demonstrated that RKIP loss is highly associated with several aggressive cancers. To date, RKIP loss or diminution has been reported in almost all cancers and it is associated with poor overall survival and reduced disease-free survival periods in almost all cancer types, with the exception of acute myloid leukemia (AML). In AML, the reduction or loss of RKIP expression was associated with longer relapse-free survival and overall survival in uni- and multi-variate analyses (Zebisch et al., 2012). Keller et al. (2004) reported that RKIP gene expression was significantly reduced in metastatic compared to non-metastatic prostate cells; this observation initially linked RKIP loss with the development of metastatic behavior in cancerous cells. Clinically, RKIP expression is higher in benign tumors than in cancerous tissues while its expression is completely absent in metastases. Keller et al. concluded that the loss of RKIP leads to the progression of prostate cancer. Fu et al. (2006) reported that prostate cancers with reduced RKIP expression had a significantly higher chance of disease recurrence, signifying RKIP as a potential positive predictive marker of prostate cancer progression and metastasis. This result may occur by partially enhancing angiogenesis in prostate cancer (Fu et al., 2003). Consistently, RKIP was also identified as a useful prognostic marker for identifying early colorectal cancer with potential metastatic recurrence. Al-Mulla et al. delineated a strong relationship between the reduced RKIP expression, metastatic recurrence and the overall survival independent of the stage of colorectal cancer (Al-Mulla et al., 2006c). These results have also been confirmed by independent studies (Minoo et al., 2007; Zlobec et al., 2008a, b). The role of RKIP has been assessed in hepatocellular carcinoma (HCC), in which its expression was significantly reduced, likely accounting for the hyper-activation of ERK cascade in human HCC (Schuierer et al., 2006; Xu et al., 2010; Notarbartolo et al., 2011; Walker et al., 2011; Wu et al., 2011). RKIP expression was also diminished in pancreatic cancer (Kim et al., 2010; Song et al., 2012), cancer of the ampulla of Vater (Kim et al., 2012a), non-melanocytic skin cancers (Libra and Torrisi, 2009; Zaravinos et al., 2009), esophageal cancer (Birner et al., 2012; Gao et al., 2012; Guo et al., 2012a; Kim et al., 2012b), gastrointestinal stromal tumors (Martinho et al., 2009) and its response to Imatinib (Valadao et al., 2012), gastric cancers (Fujimori et al., 2012; Jia et al., 2012; Guo et al., 2012a; Zhang et al., 2013), renal cell carcinoma (Moon et al., 2012), endometrial cancer (Martinho et al., 2012a), and brain tumors (Gimenez et al., 2010; Maresch et al., 2011; Martinho et al., 2012b). In breast cancer, lymph node metastases displayed significantly reduced levels of RKIP (Hagan et al., 2005; Minn et al., 2012). Moreover, an inverse correlation between RKIP and Her2/neu signaling was recently documented in breast cancer (Zhang et al., 2008). A proteome analysis revealed differentially expressed proteins in nasopharyngeal carcinoma (NPC) in which RKIP was significantly downregulated compared to normal pharyngeal epithelial tissue, and reduced RKIP has been associated with cancer progression and metastasis (Chen et al., 2009). Furthermore, Li et al. reported that RKIP expression was clearly reduced in ovarian cancer (OVCA). RKIP expression was inversely related to the invasive potential of OVCA. Similarly, RKIP overexpression reduced cellular proliferation and adhesion, implicating RKIP as a metastasis suppressor gene in human OVCA (Li et al., 2008). Similarly, the role of RKIP was also investigated by Wang et al. (2009) who stated that RKIP-inhibited OVCA metastasis and OVCA cell growth. In melanoma, the role of RKIP has been more controversial. Although RKIP manipulation in melanoma cell lines is generally consistent with our understanding of RKIP's metastatic suppressive capabilities (Park et al., 2005; Das et al., 2012), few studies have explored the relationship between RKIP and melanoma. In a study by Houben et al. (2008) neither RKIP nor p-ERK expression showed a significant correlation with the clinical outcomes of patients. Therefore, the role of RKIP, B-RAF and the Raf-MEK-ERK signaling cascades warrant further study in melanoma to elucidate this niche. Taken together, these data clearly indicate a pivotal role for RKIP in tumor growth and cancer metastasis. Nevertheless, the precise mechanisms that promote metastasis after RKIP loss or reduction remain elusive. Is it the ability of RKIP to modulate cellular motility, growth, and genomic stability responsible for its metastasis suppression functions? This question has been difficult to answer due to the multi-modular functions of this protein. Metastasis itself is a complicated process that sometimes appears gradual; however, in some cancers it emerges abruptly and is tissue specific. For example, some breast cancers have stronger tendencies to metastasize to bone (Kang et al., 2003), colorectal cancer often metastasizes to the liver, bypassing lymph nodes metastasis completely (Al-Mulla et al., 1999). Years of daunting research established that metastasis may be controlled and the metastatic potential of a primary tumor is predetermined by its genomic and transcriptomic/proteomic signatures (Poste and Fidler, 1980; Liotta and Kohn, 2001; Al-Mulla et al., 2006a, b; Nguyen et al., 2009; Sethi and Kang, 2011; Shoushtari et al., 2011). RKIP's ability to control and modulate many pivotal signaling pathways is consistent with the complicated metastasis process. Recently, a mechanism by which RKIP suppresses the invasion and metastasis of breast cancer cells to bone was proposed by Dangi-Garimella and colleagues. The authors revealed that the suppression of breast cancer cell metastasis was mediated by RKIP through the activation of mir let-7 (Dangi-Garimella et al., 2009). This process, which has been described above, offers a unique mechanistic example by which RKIP loss may specifically activate bone marrow homing proteins in breast cancer cells. Therefore, other tissue-specific signaling pathways that are modulated by RKIP may also exist in different cancer types in addition to the general ability of RKIP to modulate growth, motility, apoptosis, cell cycle, and genomic stability. In support of this hypothesis, RKIP was recently shown to inhibit MDA-9, which is required for melanoma progression and metastasis (Das et al., 2012).
Overall, most of the research regarding the role of RKIP as a metastasis suppressor has focused on RKIP but not its phosphorylated form (p-RKIP). Huerta-Yepez et al. recently reported the effect of p-RKIP expression perturbation on the prognosis of lung cancer. Their study revealed that the total RKIP level was less valuable than p-RKIP in predicting the outcome of lung cancer. The authors demonstrated that patients whose cancers expressed high p-RKIP levels survived longer than patients with relatively low p-RKIP expression levels. These data indicate the importance of assessing p-RKIP and RKIP expression levels to delineate the role of RKIP in different cancers (Huerta-Yepez et al., 2011). However, although extensive clinical research experiments have indicated that RKIP is an important prognostic and therapeutic target in cancer patients, well-conducted, randomized clinical trials tailored specifically to early-stage cancer patients must be employed to determine whether the level of RKIP and/or p-RKIP in tumor cells may influence prognostication and/or therapy.
What is the role of RKIP in other diseases? RKIP is highly expressed in neurons and the nervous system. The hippocampal cholinergic neurostimulating peptide (HCNP) is released by hippocampal neurons and is involved in the development of the hippocampus, thus supervising the process of learning and memory. RKIP was reported to be the precursor of HCNP (Fig. 2), implicating RKIP in neural development (Seddiqi et al., 1994; Mitake et al., 1995, 1996a, b). HCNP also reportedly accumulates in Hirano bodies, which dominate in CA1 region of the hippocampus and are strong indicators of Alzheimer's disease. However, the roles of RKIP and HCNP in the pathophysiology of other neurological disorders are still unclear (Mitake et al., 1995). Furthermore, RKIP and its product, HCNP, have been shown to play a role in cardiac physiology because they were both secreted into the circulation with catecholamines (Goumon et al., 2004). Similarly, complexes between dimerized RKIP and GRK-2 were localized in murine heart tissues, confirming the pathophysiological role of RKIP in the heart (Lorenz et al., 2003; Deiss et al., 2012). Because the deregulation of GRK-2 expression has a notable negative impact on cardiomyocytes function and heart hypertrophy (Koch et al., 1995; Raake et al., 2008) and RKIP is an inhibitor of GRK-2, the involvement of RKIP perturbation in cardiomyopathy may be worth exploring.
RKIP is involved in spermatogenesis and RKIP has been identified in the testis and epididymis of adult rat. Perry (1994) reported that RKIP was localized to elongated spermatids and residual bodies and Leydig cells; thus, RKIP is strongly associated with the organization of sperm membranes and proteins during spermatogensis. Interestingly, it has been shown that mice lacking RKIP diplay reduced reproduction rates due to altered sperm capacitation (Moffit et al., 2007). In relation to diabetes, PKC signaling was found to be implicated in diabetic neuropathy (Yasuda et al., 2003). Because RKIP is one of the molecules that acts downstream of PKC, RKIP may play a role in diabetic neuropathy. Theoretically, such association was partially elucidated when bioinformatics analysis identified an unknown upregulated protein (1.54-fold) in the mouse diabetic type 1 kidney as RKIP (Thongboonkerd et al., 2004). It will be interesting to apply further comprehensive techniques to clearly elucidate the role of RKIP in diabetes. To that end, GSK3 kinases have been well-established to play important roles in glycogen metabolism, insulin signaling, cellular proliferation, neural functions, embryo development, and oncogenesis (Rayasam et al., 2009). Therefore, the mere ability of RKIP to protect GSK3β from degradation indicates a more direct role for RKIP in diabetes than was previously thought. A similar argument can be proposed to connect RKIP and diseases associated with the perturbation of oxidative stress responses, especially type 2 diabetes (Bitar et al., 2005; Bitar and Al-Mulla, 2012).
The association between ethanol (EtOH) consumption and pancreatitis has been well-established. In an elegant study, EtOH has been shown to induce the sensitization of secretagogue Ca2+ signaling in pancreatic aciner cells and to induce subsequent adverse reactions, resulting in pancreatitis (Kim et al., 2012c). Kim et al. showed that EtOH induced the PKC-dependent phosphorylation of RKIP. The inhibition of RKIP expression in rodent aciner cells averted the condition by inhibiting the activating effect of ethanol on the Colecystokinin-induced chymotrypsin, a digestive enzyme whose aberrant activation in the aciner cells is indicative of pancreatitis. These data highlight RKIP as a pivotal mediator of EtOH cytotoxic effect on pancreatic aciner cells. Therefore, the inhibition of RKIP expression may be utilized as a therapeutic treatment strategy to prevent alcohol-related pancreatitis, which, if left untreated may eventually develop into cancer (Kim et al., 2012c). This study also suggests an important role for p-RKIP in Ca2+ homeostasis, which may be significantly relevant to other diseases associated with Ca2+ perturbations. The ability of EtOH to activate both the classical isozyme PKCα, and the novel isozyme PKCε and to induce the phosphorylation of RKIP at S153 is intriguing because this process activates both the RAF-MEK-ERK as well as the GPCR pathways and may offer a preliminary connection between the excessive use of EtOH and cancer. Conversely, EtOH confers the opposite effect on PKC in neural tissue. It has been shown that EtOH impairs brain-derived neurotrophic factor (BDNF) signaling, which is required to activate Raf/MEK/ERK through PKC and p-RKIP. Therefore, excess EtOH consumption has been shown to impair neuronal differentiation and interfere with the development of neuronal network by maintaining RKIP inhibitory binding to Raf-1 (Hellmann et al., 2009, 2010). Similarly, cag Pathogenicity Island of Helicobacter pylori and IL6 have been shown to induce the phosphorylation of RKIP at the S153, partly through PKC-dependent activation (Moen et al., 2012). The presence of Helicobacter pylori and nuclear p-RKIP appear to induce the transcriptional expression of RKIP, which subsequently causes apoptosis in gastric cells. This intriguing connection, highlights a pivotal role of RKIP and p-RKIP in Helicobacter pylori-associated gastritis and cancer (Moen et al., 2012).
RKIP: A Challenging Target for Drug-Induced Apoptosis
Metastasis suppressor genes represent potential diagnostic and therapeutic targets due to their role in regulating the metastatic process. Recent data have underscored the role of RKIP in sensitizing cells to radio and chemotherapy. The RKIP expression level in tumor cells is an inevitable apoptotic determinant, in which RKIP upregulation sensitizes cancerous cells to drug-induced apoptosis, whereas its downregulation reduces apoptosis. Chatterjee et al. confirmed such an observation by demonstrating that RKIP expression sensitizes breast and prostate cancer cells to chemotherapeutic agents (Chatterjee et al., 2004). Similarly, RKIP expression is induced in response to the chemotherapy drug Rituximab, which then inhibits the Raf-MEK-ERK pathway and enhances apoptosis in non-Hodgkin's lymphoma B cells (Jazirehi et al., 2004; Jazirehi and Bonavida, 2005). Another promising observation reported by Baritaki et al. (2007) involved the sensitivity of tumor cells to TRAIL-mediated apoptosis in relation to RKIP expression, NF-κB, and the zinc finger transcriptional regulator YY1, which regulates cell growth and tumor suppression. It is noteworthy that cancerous cells could escape the host immune system by employing several strategies in a process known as immune surveillance. A crucial outcome of immune surveillance is the downregulation of death receptors, which renders the cell resistant to cytotoxic effector cells and hence apoptosis. Baritaki et al. (2007) demonstrated that RKIP overexpression in prostate and melanoma cells inhibits YY1. Under supra-physiological RKIP levels, YY1 and NF-κB may be chemically inhibited, leading to the overexpression of the TNF-related apoptosis inducing ligand (TRAIL) death receptor DR5 and consequently sensitizing the cell to TRIAL-induced apoptosis. These data highlight RKIP as a potential immune surveillance cancer gene, and suggest that cancerous cells may downregulate RKIP to evade cytotoxic effecter cells (Baritaki et al., 2007). Ignatoski and colleagues tested the effect of RKIP expression levels on radiation-induced apoptosis, and they concluded that RKP upregulation may sensitize prostate cancer cells to radiation therapy, consistent with previous chemotherapy-related observations (Woods Ignatoski et al., 2008). The loss of RKIP diminished radiation-induced apoptosis, protecting cells against death.
We recently, demonstrated an alternative mechanism of therapeutic resistance relating RKIP to the KEAP1/NRF2 pathway. Using RKIP-inducible in vitro models, we noted a significant positive correlation between RKIP and KEAP1 and a negative correlation between RKIP and NRF2 levels (Al-Mulla et al., 2012). It is noteworthy that NRF2, a molecule that upregulates intracellular antioxidants and enhances cellular survival (Venugopal and Jaiswal, 1996). NRF2 is overexpressed in head and squamous cancers (Stacy et al., 2006), and it confers chemo-resistance in type II endometrial cancer (Jiang et al., 2010), while KEAP1 is inactivated in lung cancer (Ohta et al., 2008; Solis et al., 2010). RKIP depletion apparently enhances NRF2 stability by accelerating KEAP1 proteosome-based degradation. The apoptosis rate was directly associated with the RKIP/KEAP1 expression levels in colorectal cancer tissues, which delineated for the first time, an additional mechanism by which RKIP abrogation may confer resistance to therapy (Al-Mulla et al., 2012).
Transcriptional and Post-Transcriptional Regulation of RKIP
Transcriptional and post-transcriptional mechanisms are involved in the regulation of RKIP expression. Although transcriptional control appears to be the major mechanism by which RKIP is downregulated, most clinical studies focused on studying the protein (using immunohistochemistry) rather than mRNA expression. Although RKIP protein expression is highly reduced in metastatic cells, the mechanisms underlying RKIP deregulation remain unclear. At the genomic level, RKIP mutations have not been identified or reported. However, we have yet to observe serious RKIP gene sequencing efforts from various cancers and metastases to confirm this observation. Perhaps such data may soon emerge from the cancer genome project using next generation sequencing techniques. Recent studies have shed some light on the transcriptional and post-transcriptional regulation of RKIP. The human RKIP gene is located on chromosome 12q24.23 (Chromosome 12:118573663-118583390 of the forward strand according to hg-19). The gene is 9728 bases long and harbors 4 exons that yield two transcripts. PEBP-001, a 1,697 base pair mRNA that encodes a 187 amino acids protein (including the first methionine) and PEBP-004 is a 708 base pair, non-protein coding transcript. The RKIP promoter region harbors several DNA sequences involved in the transcriptional control of the gene (Fig. 4A). Among the well-characterized sequences are the E1-Box, E2-Box, CpG islands, and androgen response elements (ARE). The p53-binding consensus identified 572 bases downstream of the ATG start site that requires urgent attention (Fig. 4A). DNA methylation and histone modifications are common epigenetic mechanisms by which tumor suppressor genes are deregulated (Baylin and Herman, 2000). Various epigenetic modifications are partially responsible for the initiation and progression of cancer (Kristensen et al., 2009; Kurdistani, 2011). For example, DNA methylation at CpG islands is a well-known epigenetic system involved in gene transcriptional control (Baylin and Herman, 2000). DNA methylation at CpG islands in the RKIP promoter results in its transcriptional silencing and this phenomena has been reported in normal, premalignant and cancerous tissues. Minoo et al., reported that 8 of 11 (73%) serrated adenomas and 5 of 5 associated colorectal cancers harbored a methylated RKIP promoter (Minoo et al., 2006). Interestingly, in the same study, the RKIP promoter was also methylated in 4 of 7 (57%) normal colonic mucosa indicating the potential importance of this aberration in colorectal cancer initiation (Minoo et al., 2006). Similarly, the same group reported a significant association between decreased RKIP expression and a high CpG island methylator phenotype (CIMP-H; Zlobec et al., 2011). Using methyl-specific PCR (MSP), we found that 51 of 82 (62%) of microsatellite stable colorectal cancer cases had methylated RKIP CpG islands, and this silencing was significantly associated with the loss of RKIP protein expression in vivo (Al-Mulla et al., 2008). Similarly, RKIP promoter methylation was reported in esophageal squamous cell carcinomas (Guo et al., 2012b) and aggressive gastric cancer (Guo et al., 2012a). However, other studies have found little evidence of RKIP CpG island methylation in colorectal cancer (Minoo et al., 2007), gastrointestinal stromal tumors (Martinho et al., 2009), and HCCs (Poma et al., 2012). Therefore, this avenue may benefit from studies that employ more sensitive and methylation-specific technologies and involve a larger number of cases.
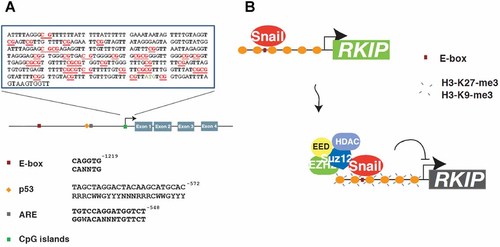
RKIP transcriptional control. A: Shows downstream promoter sequences involved in the control of RKIP transcription. CpG islands are depicted in red fonts. B: Depicts transcriptional control of RKIP by SNAIL and EZH2 histone deacetylase complexes.
RKIP is repressed by Snail, a transcriptional factor that is highly expressed in metastatic breast and prostate cancers (Beach et al., 2008). In the RKIP promoter, Snail binds to E-box 1 (and to a lesser extent the E-box 2) cis-elements (Fig. 4A), and it recruits mSin3A, histone deacetylases and transcriptional repressor complexes (Beach et al., 2008). A recent study by Ren et al. demonstrated that in the presence of Snail, the enhancer of Zeste homolog 2 (EZH2) inhibits RKIP expression at the transcriptional level, thus hastening cellular invasion. The authors also showed that EZH2 directly suppresses RKIP expression in breast and prostate cancers via the recruitment of The polycomb-repressing complex (PRC2) and EZH2-mediated H3-k27-me3 at the RKIP promoter (Fig. 4B; Ren et al., 2012). The PRC2, has been shown to alter the trimethylation of histon 3 at lysine 27 or 9 (H3-K27-me3 or H3-K9-me3), leading to gene silencing (Kondo et al., 2008). This event is catalyzed by EZH and other proteins that form the PRC2 complex (Morey and Helin, 2010; Margueron and Reinberg, 2011). The downregulation of EZH2 decreased the proliferation of prostate cancer cells in vitro (Varambally et al., 2002), while its overexpression induced anchorage-independent growth and invasion of breast epithelial cell line (Kleer et al., 2003). The direct correlation between EZH2 upregulation and tumor aggressiveness may be attributed to the E-cadherin deactivation by EZH2 (Herranz et al., 2008). The expression pattern of E-cadherin is similar to those of other metastasis suppressors genes, such as RKIP (Granovsky and Rosner, 2008). Like E-cadherin, reduced RKIP expression may enhance angiogenesis leading to vascular invasion, drug resistance, and enhanced cellular immortality. Recently, MDA-9 was shown to inhibit the transcription of RKIP in melanoma, although the mechanisms by which MDA-9 accomplished this require further confirmation and study (Das et al., 2012). Nevertheless, this finding raises the possibility that the mechanisms used to suppress RKIP transcription in cancer may be cell type dependent.
In vivo and in vitro results suggest that RKIP is an androgen target gene. 5α-Dihydrotestosterone has also been shown to induce the expression of RKIP in normal prostate epithelial cells via the androgen receptor binding (Zhang et al., 2012). Indeed, the RKIP promoter harbors an androgen receptor binding consensus termed ARE (Fig. 4A). These data illuminate a possible avenue through which RKIP levels can be induced in cancer, although they may confer other hormone-related side effects. It would be interesting to determine whether other hormones, such as estrogen and progesterone may also influence the expression of RKIP. This possibility is under intensely investigation in our laboratories.
Micro-RNA has emerged as an intriguing system that can control gene expression at the transcriptional, translational and post-translational levels (Lujambio and Lowe, 2012). Recent data have demonstrated that RKIP gene expression can be inhibited by microRNAs-224 (miR-224) in breast cancer cell lines. Huang et al., showed that the RKIP 3′-UTR contains a target sequence that is identified directly by miR-224. The authors found that upregulation of miR-224 inhibited RKIP gene expression and that of stroma-associated RKIP target genes, which increased breast cancer cellular invasion (Huang et al., 2012). Similarly, miR-375 had been shown to inhibit the expression of RKIP in gastric cancer cell lines (Tsukamoto et al., 2010). Such a pivotal mechanism may represent a key method of controlling RKIP gene expression in cancer metastases.
RKIP regulation at the post-translational level is less well understood. Using a wide-proteome-based approach, mir-Let-7, mir-1, mir-16 overexpression were shown to mildly enhance RKIP protein translation (Selbach et al., 2008). Alternatively, the overexpression of mir-155 conferred the opposite effect on RKIP protein stability (Selbach et al., 2008; Schwanhausser et al., 2009; http://psilac.mdc-berlin.de). Moreover, in two independent studies utilizing global proteome screens, RKIP was found to be ubiquitylated at K47 and K113 residues in HCT 116 cells (Kim et al., 2011), and it was found to be a substrate for the cullin-RING ubiquitin ligases (Emanuele et al., 2011). In support of these data, the exposure of neuroblastoma cells to nanomolar Doxorubicin treatment induced the ubiquitylation of RKIP (Mandili et al., 2012). Helicobacter pylori, was also shown to induce the proteosome-mediated degradation of RKIP (Moen et al., 2012). The extent to which ubiquitylation of RKIP contributes to its degradation in precancerous and cancer tissues remains to be elucidated. Taken together, these results suggest that several of these mechanisms may act together to reduce RKIP expression in cancer.
Pharmaceutical Manipulation of RKIP and Its Feed-Back Circuits
The major question that remains to be answered is how can the expression of RKIP be induced in cancer (especially in early-staged disease). To address this topic, it is vital to understand the signaling circuits that regulate RKIP (Fig. 5A). As stated above, Snail is a transcriptional inhibitor of RKIP (Beach et al., 2008), while RKIP itself has been shown to inhibit the transcription of Snail at the mRNA level (Dangi-Garimella et al., 2009; Al-Mulla et al., 2011b). Therefore, these two “foes” control an important signaling circuit, which is disrupted by the dominant action of Snail in cancer (Fig. 5B). Consequently, the reduction or inhibition of Snail is an obvious target in cancer therapeutic intervention. Unfortunately, with the exception of shRNAs, no specific agents are currently known to inhibit Snail, which is an area that may be of exceptional benefit for cancer therapy. The Snail protein can be endogenously inhibited by GSK3β (Yook et al., 2005), while its transcription can be inhibited by RKIP and nitric oxide (NO; Baritaki et al., 2010); therefore, the RKIP-GSK3-Snail circuit may represent another avenue for intervention. To that end, Baritaki et al. investigated the effect of NO on EMT phenotype in metastatic cells. The EMT is generally mediated by the constitutive expression of Snail, and it can be reversed by the expression of RKIP and E-cadherin (Cano et al., 2000; Baritaki et al., 2010). Moreover, the NO donor (Z)-1[2-(2-aminoethyl)-N-(2-ammonioethyl) aminodiazen-1-ium-1, 2-diolate (DETANONOate) inhibits NF-κB through the S-nitrosylation of NF-κB (p50; Bonavida et al., 2008; Baritaki et al., 2010; Bonavida and Baritaki, 2011). It has been well-documented that NF-κB induces Snail expression at the transcriptional level (Julien et al., 2007; Wu and Bonavida, 2009). Baritaki et al. (2010) have shown that treatment of human prostate cancer cells with DETANONOate inhibited EMT and reversed their invasive property. The same group reported another promising observation, implicating RKIP-Snail-NF-κB circuitry as a therapeutic target for drug-induced apoptosis in prostate cancer cell lines. Their data illuminated the mechanism by which the proteasome inhibitor NPI-0052 may induce apoptosis and inhibit the EMT phenotype. The authors demonstrated that NPI-0052 induced RKIP expression by inhibiting NF-κB, which is required for Snail activation (Baritaki et al., 2009). Singhal et al. (2012) provided another promising observation regarding the positive regulation of RKIP. Their work implicated a role for Didymin as a potential drug for treating neuroblastoma. Didymin appears to induce RKIP expression, thus inhibiting neuroblastoma growth in vivo and in vitro (Al-Mulla, 2012a; Singhal et al., 2012). However, the precise mechanism by which this Flavin induces RKIP remains unknown. Similarly, using a proteomic approach to investigate possible mechanism behind the ability of ascorbic acid to inhibit xenograft tumor growth in nude mice, Park et al. (2009) discovered that ascorbic acid induced RKIP expression. Moreover, 4-shogaol, a phytoconstituent isolated from dried red ginger, appears to induce RKIP expression, and it has been shown to inhibit MDA-MB-231 cellular invasion and metastasis (Hsu et al., 2012). Similarly, (−)-Epigallocatechin 3-gallate, a constituent of green tea, has also been shown to induce the expression of RKIP by modulating the histone deacetylase activity (Kim and Kim, 2013). Therefore, in addition to NO, Didymin, 5α-dihydrotestosterone, ascorbic acid and 4-shogaol, Epigallocatechin 3-gallate may now be added to the list of natural products and pharmaceutical drugs that can induce RKIP expression (Fig. 5C). However, when considering the inhibition of cancer progression and the reversal of therapeutic resistance, the real benefit to cancer patients awaits well-planned randomized clinical trials.

Major intracellular signaling circuits modulated by RKIP. A: Depicts RKIP inhibition of ERK, NFκB, SNAIL (burgundy lines) and the activations of GSK3β. B: Loss of RKIP abrogates the inhibitory/activation signaling leading to cellular invasion and metastasis. C: Pharmacological and natural compounds found effective in the induction of RKIP and restoring its controlled circuitry acting to inhibit the NFκB and reduce SNAIL (red arrow) or induce RKIP expression level directly (green arrows).
In Conclusion
The name Raf Kinase Inhibitory Protein does not adequately describe the multitude of diverse interactions and functions attributed to RKIP. This small protein plays cardinal roles in modulating fundamental cellular signaling processes and circuits that are perturbed in many diseases. The challenge of modern medicine is to gain more insight into the various functions of RKIP in healthy and disease, understand its roles in theranostics and find better avenues for manipulating its expression in many disease conditions.
Acknowledgements
We are grateful for Kuwait Foundation for The Advancement of Sciences (KFAS) for fully funding our project number 2012-130-201.




