Transcriptional regulators of the ΔNp63: Their role in limbal epithelial cell proliferation
Abstract
The surface cells of corneal epithelium are regularly shed off and replaced by new cells that are derived from limbal epithelial stem cells (LESC). LESC are believed to reside in the basal layer of the limbal epithelium and are characterized with high expression levels of ΔNp63, a transcription factor (TF) which is believed to play roles in the regulation of LESC proliferation. In this study, we examined the transcriptional regulation of ΔNp63 in limbal epithelial cell. We employed DNA pull down assay followed by LC/MS analysis and cDNA microarray analysis to identify the TFs that were capable of binding to ΔNp63 promoter or were expressed at higher levels in limbus over cornea. The TFs thus selected were further examined for their in vivo ΔNp63 promoter binding by chromatin immunoprecipitation assay. We identified six putative TFs (PAX6, EGR1, CEBPB, JUN, ATF3, and ARID5B) through the aforementioned approaches. Among them, PAX6 and EGR1 were shown to promote the transcription of ΔNp63 and led to increased cell proliferation. In contrast, CEBPB and ATF3 appeared to exert little or no effect on ΔNp63 expression, however, their silencing suppressed cell proliferation. Although JUN exhibited low promoter-binding specificity, however, it affected ΔNp63 expression and limbal epithelial cell proliferation in ways similar to that of PAX6 and EGR1. Intriguingly, ARID5B was highly expressed in the limbal epithelial cell, however, its silencing by siRNA did not obviously affect the expression of ΔNp63, nor did it reduce cell proliferation of the limbal epithelial cell. J. Cell. Physiol. 228: 536–546, 2013. © 2012 Wiley Periodicals, Inc.
Cornea epithelium is composed of terminally differentiated keratinocyte that has a relatively short life span and is replaced through proliferation of a distinct subpopulation of cells, known as the limbal epithelial stem cell (LESC; Ebato et al., 1988; Tseng, 1989; Dua and zuara-Blanco, 2003; Schlötzer and Kruse, 2005). The LESC is believed to reside in the basal layer of the limbal epithelium, which is located at the border of cornea and conjunctiva (Tseng et al., 1990; Yamada and Mashima, 1995; Lavker and Sun, 2000). LESC is characterized by high proliferation potential and unlimited self-renewing ability (Barrandon and Green, 1987; Cotsarelis et al., 1989). It also undergoes asymmetrical division, giving rise to a daughter stem cell and a fast-dividing progenitor cell, termed transit amplifying cell (TAC; Lajtha, 1979; Cotsarelis et al., 1990; Morrison et al., 1997). TAC makes up majority of the proliferative cell population in the corneal/limbal epithelium and after a limited number of cell division, it becomes terminally differentiated.
Under normal physiological conditions, LESC undergoes slow cell cycling and may be activated to become fast cycling cell during corneal wound repair (Lehrer et al., 1998). Numerous mediators have been suggested to involve in the regulation of LESC proliferation and differentiation, including the transcription factor (TF) p63 (Yang et al., 1999; Pellegrini et al., 2001). p63 is a member of the p53 family and is highly homologous to p53. The alternative usage of the first and second promoters of p63 gene gives rise to two groups of isoforms, TAp63 and ΔNp63. Both isoforms can be alternatively spliced at the carboxyl-terminus, resulting in the generation of α, β, and γ isoforms (Yang et al., 1998). p63 is expressed in a confined manner, with the highest expression found in the basal cells of numerous epithelia (Mills et al., 1999; Yang et al., 1999). ΔNp63α was shown to be the major p63 transcript in the limbal epithelium, and was switched to ΔNp63β and ΔNp63γ in activated limbal epithelial cell (Kawasaki et al., 2006). Clonal studies of the cultured limbal epithelial cell showed that ΔNp63α is relatively enriched in the clones with the highest proliferative potential, the holoclone (Pellegrini et al., 2001). p63 was initially shown to play critical roles in embryonic development; p63 knockout mice were shown to exhibit major defects in limb and craniofacial development, and in the maturation of the stratified epithelium resulting in dehydration and death of the animal soon after birth (Yang et al., 1999). Other studies showed that expression of ΔNp63α is required for the proliferation of the zebra fish ectodermal cells (Lee and Kimelman, 2002). Consistently, and upregulation of ΔNp63α was shown to help maintain the proliferation of mammalian epidermis (Bamberger et al., 2002). Our previous study showed that ΔNp63α expression is necessary for the proliferation of cultured rabbit limbal epithelial cell (Wang et al., 2005; Hsueh et al., 2011), strongly suggesting that ΔNp63 plays crucial role in the regulation of LESC proliferation.
Since ΔNp63 appeared to play pivotal roles in regulating LESC proliferation, the regulation governing ΔNp63 expression is therefore, of cell biological significance and deserves to be explored. Recently, Romano et al. (2006) showed that CCAAT-box-binding factor (CBF/NF-Y) and Sp1/Sp3 family proteins are able to bind to the proximal promoter (−164 to 0) of the ΔNp63 gene and activates its transcription in HaCaT cells. Barbaro et al. (2007) further showed the binding of CEBPD (CCAAT enhancer-binding protein delta) to ΔNp63 promoter in limbal epithelial cell. Consistently, in a previous report, we showed that STAT3 binds and activates ΔNp63 promoter in Hep3B cells (Chu et al., 2008). Moreover, we also showed that ΔNp63 promoter activity is similarly regulated by STAT3 in limbal epithelial cell (Hsueh et al., 2011).
To date, studies on the transcriptional regulation of ΔNp63 promoter have focused on the roles of some pre-conceived selected TFs (Teuliere et al., 2005; Romano et al., 2006; Barbaro et al., 2007; Chu et al., 2008). Therefore, a more complete elucidation of the regulation of ΔNp63 expression in normal keratinocytes has remained to be explored. In the present study, we adopted a global approach to screen the putative TFs for ΔNp63 promoter by the employment of genomic (cDNA microarray) and proteomic (liquid chromatography/mass spectrometry, LC/MS) technologies, to screen for the TFs that were differentially expressed in the nuclei of the limbal and cornea keratinocytes. The putative TFs thus screened were then examined for ΔNp63 promoter binding by chromatin immunoprecipitation (ChIP) assay, and the effects of their silencing on ΔNp63 gene expression and limbal epithelial cell proliferation were also examined.
With the above approaches, six putative TFs (PAX6, EGR1, JUN, ATF3, ARID5B, and CEBPB) were selected for further studies. We showed that silencing of PAX6, EGR1, or JUN led to downregulation of ΔNp63 expression and upregulation of cyclin-dependent kinase (CDK) inhibitor p27kip1 leading to downregulation of cell proliferation. Moreover, silencing of CEBPB and ATF3 also suppressed cell proliferation, however, exerted little or no inhibitory effect on ΔNp63 expression. Our work provided a better understanding of the transcriptional regulation of ΔNp63α and its end-point effect on cell proliferation.
Materials and Methods
Materials
Dulbecco's modified Eagle's medium (DMEM), Ham's F-12 nutrition, trypsin–EDTA, fetal bovine serum (FBS), penicillin/ampicillin/streptomycin, and dispase II were purchased from Invitrogen–Gibco (Gaithersburg, MD). Dimethyl sulfoxide (DMSO) and bovine insulin were from Sigma–Aldrich (St. Louis, MO). Mouse receptor grade epidermal growth factor (EGF) was purchased from Upstate Biotech. Inc. (Lake Placid, NY). All plastic cell culture accessories were from Corning/Costar Co. (Oneonta, NY). The primary antibodies used were p63, Pax6, POU5F1, p27kip1, and GAPDH (Chemicon, Temecula, CA); CEBPB, CEBPD, EGR1, and JUN (Santa Cruz, CA); STAT3 (Cell Signaling, Beverly, MA); NANOG (Abcam, Cambridge, MA); ARID5B (Bethyl Laboratories, Montgomery, TX); and ATF3 (AbNova, Taiwan).
Source of tissue donors
The specimens were obtained in accordance with the tenets of the Declaration of Helsinki. Limbo-peripheral cornea tissues were procured within 6 h after cornea transplantation from the Department of Ophthalmology, Chang Gung Memorial Hospital, Taoyuan, Taiwan.
Rabbit limbal biopsy and limbal explant culture
Rabbit biopsy was performed on the healthy eyes from 2-month-old New Zealand white rabbit. The eyelid was sterilized with povidone–iodine, the limbal–corneal tissue that contained epithelial cells and part of the stroma was excised from the superficial limbal–corneal stroma by lamellar keratectomy. The limbal biopsy was inoculated onto the 60 mm plastic dish and cultured in limbal epithelium growth medium (LEGM), containing DMEM/Ham's F12 (1:1, 20 mM Hepes buffered) supplemented with 5% FBS, 0.5% DMSO, 2 ng/ml mEGF and 1 µg/ml bovine insulin. Cultures were incubated in a humidified incubator under 95% air/5% CO2. The culture was maintained for 2 weeks and the medium was replaced with LEGM every 2 days.
Cell culture
Human nasopharyngeal carcinoma cells (NPC 076, ΔNp63 expressing cell line) were cultured in DMEM/Ham's F12 (1:1, 20 mM Hepes buffered) supplemented with 10% FBS. Rabbit limbal epithelial cell (RbLmP1 cells) were harvested from limbal explant culture on day 11 (passage 1) and cultured in LEGM. Cultures were incubated in a humidified incubator under 95% air/5% CO2.
Sequencing of rabbit ΔNp63 upstream DNA
To sequence rabbit ΔNp63 upstream DNA, PCR-based genomic walking assay (TOPO Walker kit; Invitrogen, Carlsbad, CA) was performed according to the manufacturer's instructions. Briefly, rabbit genomic DNA was digested with restriction enzymes (EcoRI, AatII, KpnI, NsiI, PstI, BamHI, and HindIII; New England Biolabs, Hitchin, UK) and dephosphorylated with alkaline phosphatase/CIP. DNA fragments were extended at 5′-end with Taq polymerase using Genome walking primer (GWP1: 5′-TTGTACCTGGAAAACAATGCCCAGACTC-3′), which was designed according to rabbit ΔNp63 proximal promoter sequence, and ligated with an oligonucleotide/TOPO linker. Linker-ligated DNA fragment was amplified with linker primer and GWP2: 5′-GCAAAATCCTGGAACCAGAAGAGAAGA-3′. The linker-ligated DNA PCR products were resolved on 1.5% agarose gel. The protein bands were excised, cloned into pCRII-TOPO sequencing vector (Invitrogen) and sequenced.
Identification of rabbit ΔNp63 transcriptional start site
To identify rabbit ΔNp63 transcriptional start site in the limbal epithelium, 5′ rapid amplification of cDNA ends assay (5′RACE) was used. Total RNA was extracted from limbal epithelium using TRIzol reagent (Invitrogen) and the amount of RNA was quantified. The 5′RACE kit (Clontech, Palo Alto, CA) was used according to the manufacturer's instructions. Briefly, cDNA was synthesized using the gene-specific primer (GSP) GSP2 (5′-ACTACCCCGGCCCGCACAGCTTCGACG-3′) and an oligonucleotide tail was added to it. The oligonucleotide tailed cDNA was then amplified using GSP2 or a nested PCR primer GSP1 (5′-GACCAGCAGATCCAGAACGGCTCCTCG-3′) and the oligonucleotide primer provided in the kit. The GSP primer was designed based on the conserved region of human ΔNp63α cDNA mapping to rabbit genome (http://www.genome.ucsc.edu/). The 5′RACE PCR products were resolved on 2% agarose gel, the bands were excised, cloned into pCRII-TOPO sequencing vector (Invitrogen,) and sequenced.
Analysis of the putative transcription factor-binding sites on ΔNp63 promoter
The human genome sequence was adopted based on the human reference sequence (GRCh37) by the Genome Reference Consortium (February, 2009). The rabbit genome sequence was adopted based on our sequencing result of rabbit ΔNp63 upstream DNA (genomic walking assay) and confirmed with the rabbit genome database [Oryctolagus cuniculus draft assembly; Broad Institute oryCun2 (NCBI project 12,819, AAGW00000000), April 2009]. Alignment of the ΔNp63 upstream region between human (−719 to 0) and rabbit (−740 to 0) were performed using AlignX program (Vector NTI, invitrogen). The matrix sites were identified within the ΔNp63 promoter region (Matinspector; Genomatix, Munich, Germany). Position weight matrices (matrix) were used in motif-finding algorithms to identify cis-regulatory motifs. To predict the putative TF-binding sites, Matrix Family Library Version 8.2 (January 2010) was used, and the group selected were General Core Promoter Elements and Vertebrates (core/matrix sim 0.75/Optimized).
Construction of ΔNp63 promoter-luciferase vectors
The PCR reactions were carried out with the sequence-specific primer pairs: HuDNp63-698 5′-GGACACATTTATCAGGATTCCTA-3′ and 5′-GTACATAATTTAAGGTTTCCTAA-3′; RbDNp63-719 5′-TGGCTTGTTTCCCAGAATTTCAA-3′ and 5′-GTACATAACTTAAGGTTCCCTAA-3′; HuDNp63-308 5′-TCGTGGTGGTGGTGCGGTTTGT-3′ and 5′-GTACATAATTTAAGGTTTCCTAA-3′; and RbDNp63-337 5′-TTCTGAAATGCCTTCTCTAAGTC-3′ and 5′-GTACATAACTTAAGGTTCCCTAA-3′. These primers were all designed to contain a KpnI site and SacI site for the subsequent cloning reactions. Desired DNA fragments were PCR amplified and inserted into luciferase reporter vector pGL3-Basic (Promega, Madison, WI). The inserts were positioned in sense orientation relative to the luciferase coding sequence between KpnI and SacI sites. The nomenclature of ΔNp63 promoter-luciferase vectors were based on the species and the length of the insert upstream to the transcriptional start site of the ΔNp63 promoter.
Analysis of ΔNp63 promoter activity
The human NPC076 and RbLmP1 cells were plated at 1 × 106 cells/60 mm dish. The cultures were incubated with OPTI-MEM for 12 h and were transfected with desired luciferase reporter vectors and β-galactosidase vectors (0.5 µg) for 6 h. After transfection, the cells were further incubated in the growth medium for 48 h. The cells were washed twice with ice-cold PBS and luciferase and β-galactosidase assays were performed using luciferase and β-galactosidase assay reagents, respectively (Promega). Luciferase activity was measured using FB12 luminometer (Berthold Detection Systems, Pforzheim, Germany). The β-galactosidase values used to normalize the luciferase activity were measured by ELISA Reader, SUNRISE (Tecan, Salzburg, Austria).
Identification of TF for ΔNp63 promoter by LC/MS
The limbal tissue was washed with cold PBS twice, incubated in 10× volume of a cytosolic extraction buffer (10 mM HEPES, pH 7.9, 10 mM KCl, 10 mM EDTA, 1 mM dithiothreitol, 0.4% octylphenoxy polyethoxyethanol, protease inhibitors) on ice for 10 min. The epithelial nuclei of the limbal tissue were scraped, and the nuclear clump was disrupted by repetitive pipetting. The suspension was then centrifuged at 14,000g for 3 min at 4°C. The pellet was resuspended in 150 µl of a nuclear extraction buffer containing 20 mM HEPES, pH 7.9, 200 mM NaCl, 1 mM EDTA, 10% glycerol, 1 mM dithiothreitol, protease inhibitors, and was vortexed at full speed for 10 sec. The sample was set on ice for 2 h, shaken once every 20 min, and eventually centrifuged at 14,000g for 5 min at 4°C. The supernatant was collected and designated as the nuclear protein fraction. The nuclear protein extract (200 µg) was pre-cleared with streptavidin-conjugated magnetic beads (Promega) in 0.5 ml of ABCD-binding buffer (20 mM Hepes, pH 8.0, 1 mM EDTA, 100 mM NaCl, 0.5% Nonidet P-40, 1 mM dithiothreitol, 10 mM NaF, 10 mM sodium orthovanadate, 1 mM PMSF, and 1× cocktail protease inhibitor; Sigma-Aldrich) for 30 min at 4°C. The magnetic beads were pelleted by magnet for 1 min at 4°C. The supernatant was further incubated with 10 µg of poly(dI-dC) (Pharmacia Biotech, Piscataway, NJ) and 50 pmol of biotinylated annealed probes at room temperature for 20 min. The annealed probes used were the PCR products amplified from biotinylated rabbit ΔNp63-specific primer (5′-TGGCTTGTTTCCCAGAATTTCAA-3′) and GL2 primer (5′-CTTTATGTTTTTGGCGTCTTCC-3′) from RbΔNp63-719 vector. The DNA–protein complex was precipitated with magnetic beads for 30 min, washed three times in ABCD-binding buffer, eluted by boiling in 2× SDS loading buffer (50 mM Tris–Cl pH 7.8, 0.1% SDS, and 10 mM DTT). Two micrograms of nuclear protein from each group was electrophoresis and 2.6 µg of nuclear protein from each group was digested with trypsin and subjected to LC/MS analysis. Sample was loaded on a C18 reverse column (Thermo Fisher Scientific, Bremen, Germany, 150 mm × 1 mm) coupled with tandame LTQ Orbitrap Velos spectrometer (Thermo). The peptide mass fingerprinting (PMF) and individual MS/MS ion data were then used for TF species identification using Mascot MS/MS Ions search program (Matrix Science, London, UK; version 2.3.2). Carbamidomethylation was set as fixed modification. Variable modifications were deamidated (NQ) and oxidized (M). Taxonomy was set to Chordata (vertebrates and relatives). Peptide mass tolerance and fragment mass were both set at ±0.3 Da.
Analysis of TF expression by c-DNA microarray
Total RNA from the epithelia of peripheral cornea and limbus was isolated by Trizol (Invitrogen), treated with RNase-free DNase and further purified on RNeasy column (Qiagen, Valencia, CA). For array hybridization, 1 µg of total RNA was reverse-transcribed using oligodT as primer. The cDNA samples were labeled with either Cy3 (limbus) or Cy5 (cornea) and were pooled. The RNA in the polled samples (30 µl) was degraded by heating at 65°C for 10 min with the addition of 10 µl of 0.5 M NaOH and 10 µl of 10 mM EDTA. The cDNA was purified by the cDNA cleanup spin column (Affymetrix, Santa Clara, CA), and redissolved in 50 µl of standard saline citrate (15 mM sodium citrate in 150 mM NaCI). The cDNA was hybridized to Human Whole Genome Oligo array chip (contains 41K human unique genes; Agilent Technologies, Palo Alto, CA), washed and scanned. Quantitation procedures and pseudocolor representation of fluorescent image were done in accordance with Genearray Scanner (Agilent Technologies). The scanned image was analyzed using Microarray Suite Version 5 software (Affymetrix) and converted to gene expression level (EScores) by Probe Profiler software (Corimbia, Berkeley, CA).
In vivo binding of TF to ΔNp63 promoter
Chromatin immunoprecipitation assay was performed to confirm the binding of TFs to rabbit ΔNp63 promoter (EZ-ChIP assay kit; Upstate Biotechnology, Waltham, MA) according to the manufacturer's instructions. The limbal and corneal tissues were washed twice with ice-cold PBS and were fixed with 1% formaldehyde (Sigma–Aldrich). The epithelial sheets were scraped in 200 µl of SDS lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris, pH 8.1) and sonicated (10 sec pulses × 5 at 50% output power, Microson XL) to generate chromatin fragments between 200 and 1,000 bp. The TF-DNA complexes were immunoprecipitated using antibodies against either PAX6, CEBPD, CEBPB, STAT3, EGR1, JUN, ATF3, or ARID5B. Normal mouse and rabbit IgG (mIgG and rIgG) antibodies (Millipore, Billerica, MA) were added in parallel tubes and served as negative control. The precipitated immune complexes were heated at 65°C for 4 h to dissociate TF–DNA complexes and the DNA fragments were purified by DNA extraction kit (Protech Technology, Taipei, Taiwan, TW). After purification, PCR was used to analyze the immunoprecipitated DNA (Go Taq Green Master Mix; Promega). The primers used were 5′-AAAACCCTAAAACTAGATTG-3′ and 5′-GGTGATAAGGAATTCTAACT-3′, which recognize proximal rabbit ΔNp63 promoter from −433 to −142 (relative to the transcriptional start site). The primers recognize rabbit ΔNp63 exon 14 from +104,582 to +104,752 (5′-CCCAGTGGTGCCTCTACGGT-3′ and 5′-TTGATGCGCTGCTGCTTGTT-3′) were also used as a negative control. The products of PCR reaction were separated by 1.5% agarose gel electrophoresis and visualized by ethidium bromide staining.
Silencing of the selected TF
siRNA sequences for rabbit TFs were modified from respective human shRNA sequences referenced from the National RNAi Core Facility shRNA database (Academia Sinica, Taipei, Taiwan). The siRNAs were purchased from MWG-Biotech AG (Ebersberg, Germany), including ΔNp63 siRNA, 5′-UCAAUGCCCAGACUCAAUU-3′, CEBPD siRNA, 5′-CGAGAGAAGCUAUACGUGUUU-3′, STAT3 siRNA, 5′-AGUCAGGUUGCUGGUCAAA-3′, PAX6 siRNA, 5′-CGUCCAUCUUUGCUUGGGAAA-3′, EGR1 siRNA, 5′-CCACGCCCAACGCUGACAUUU-3′, JUN siRNA, 5′-ACUCAUGCUAACGCAGCAGUU-3′, ATF3 siRNA, 5′-AGAUGAGAGAAACCUCUUUAU-3′, CEBPB siRNA, 5′-AAGAGGUCGGAGAGGAAGUCG-3′, ARID5B siRNA, 5′-UAAAUGGUUCUCUUUGAAGGC-3′, and non-silencing siRNA, 5′-UUCUCCGAACGUGUCACGU-3′. Epithelial outgrowth of the 5-day-old limbal explant cultures were pre-incubated with OPTI-MEM. The medium was replaced with the same medium on the next day and further incubated overnight. The cells were then transfected with desired siRNA (50 nM) using Lipofectamine 2000 (Invitrogen) for 6 h. After transfection, the cells were further cultured in the LEGM.
Transduction of ΔNp63α
The high expression Adenovirus-ΔNp63α vector was constructed as reported previously (Cheng et al., 2009). A recombinant adenovirus carrying GFP alone was used as a negative control. The vectors, designated Ad-ΔNp63α and Ad-GFP, were propagated in AD-293 cells, collected until the appearance of cytopathic effect, and purified by four rounds of freezing and thawing. Adenoviral particles were titered using the QuickTiter Adenovirus Titer ELISA Kit (Cell Biolabs, Tokyo, Japan) and Ad-GFP was used to detect transductive efficiency of adenovirus to limbal epithelial cell. The epithelial outgrowth of the limbal explant cultures were transduced with Ad-ΔNp63α or Ad-GFP. Briefly, the viral stock (50 µl, 8 × 107 ifu/ml) was diluted into 0.5 ml OPTI-MEM, and added to the 5 ml of OPTI-MEM pre-incubated explant culture on day 7 for 6 h. The transduction was repeated once on day 10, and the transduction efficiency was estimated to be higher than 90% (data not shown). The explant was further cultured in the LEGM.
Extraction of total protein from rabbit corneal and limbal epithelia
Rabbit corneal tissue was punched out by 6.0 mm Barron vacuum cornea punch (Katena, Denville, NJ), limbal tissue was then dissected and removed. The corneal and limbal tissues were washed once with ice-cold PBS and the epithelial layers were scrapped separately into 0.5 ml of Tissue Protein Extraction Reagent (T-PER; Pierce, Rockford, IL) by a surgical blade. The T-PER contains 25 mM bicine and 150 mM sodium chloride (pH 7.6) supplemented with 10 mM sodium fluoride, 10 mM sodium orthovanadate, and 1× protease inhibitor cocktail (Sigma–Aldrich). The suspension was transferred to an eppendoff vial on ice, sonicated to break the cells, and centrifuged for 15 min at 4°C at full speed. The supernatant was pooled and designated as the total protein extract. Protein concentration was determined using a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA).
Extraction of total protein from rabbit limbal explant culture
The epithelial outgrowth of the limbal explant cultures was treated with desired siRNA and Ad-ΔNp63 or Ad-GFP. Forty-eight hours after treatment, two dishes from each group were randomly selected for further processing. The limbal epithelial outgrowth was washed once with ice-cold PBS, and scrapped into 0.1–0.5 ml (depending on outgrowth area) T-PER (contains protease inhibitors and phosphatase inhibitors as described above) by plastic cell scrappers. The suspension was transferred to an eppendoff vial on ice, sonicated to break the cells, and centrifuged at full speed for 15 min at 4°C. The supernatant was pooled and designated as the total protein extract. Protein concentration was determined using a Bio-Rad protein assay kit (Bio-Rad).
Analysis of TF expression by Western blotting
For immunoblotting, 10 µg of cellular protein from each samples were electrophoresed on 7.5% SDS–PAGE and transferred onto Immobilon™-P membrane (Millipore). The membranes were immunoblotted with respective antibodies and incubated with appropriate horseradish peroxidase-conjugated secondary antibodies. The immunoreactive bands were detected by chemiluminescence with ECL (Millipore).
Analysis of limbal epithelial cell proliferation
The limbal epithelial cell proliferation was estimated by area of the epithelial outgrowth in limbal explant cultures. The limbal tissue (1 mm × 2 mm) was implanted with the epithelial side up onto plastic dish and maintained in LEGM. Seven days after the inoculation, dishes with roughly equal size of epithelial outgrowth were chosen for the subsequent experiment. The selected cultures (three in a group) were treated with a desired siRNA together with Ad-ΔNp63 or Ad-GFP vector. Sham control was proceeded with the same treatment except that no siRNA nor adenovector was added. On day 14, the explant cultures were stained with trypan blue, photographed with a Nikon Coolpix 950 digital camera (Nikon Corp., Tokyo, Japan), and the area of epithelial outgrowth compared.
In vitro cell migration assay
Cell migration was measured by the scratch wound method. Briefly, RbLmP1 cells were plated, and grown in 60 mm dishes in LEGM until 80% confluent. The cultures were transfected with desired siRNA (50 nM) as above described, and the cells were further incubated in the LEGM for 48 h. Cultures were then wounded by gently pressing a sterilized razor through the epithelial sheet into the plastic dish to mark the origin and drawing the razor on one side through the monolayer to remove the cells. After the cells were wounded, the LEGM was replaced to remove the floating cells. The keratinocyte migration into the wounded area was permitted for up to 6 h and stopped by fixation with 4% paraformaldehyde. The number of cells migrated into the wounded area was counted under a phase contrast microscope, photographed by Nikon 950 and quantitated by ImageQuant version 5.2 software (Molecular Dynamics, Amersham, UK).
RESULTS
Analysis of human ΔNp63 promoter region
To examine the putative TF-binding site in ΔNp63 promoter region, we first examined the promoter activity by reporter assay. Promoter fragment −698 to 0 or −308 to 0 was inserted into pGL3 luciferase vector (designated HuΔNp63-698 and HuΔNp63-308), transfected into p63 positive human NPC 076 cells separately, and the promoter activity was measured 48 h after transfection. As shown in Figure 1A, the reporter activity is much higher with HuΔNp63-698 than that with HuΔNp63-308, suggesting the presence of active regulators in the region of −698 to −308.
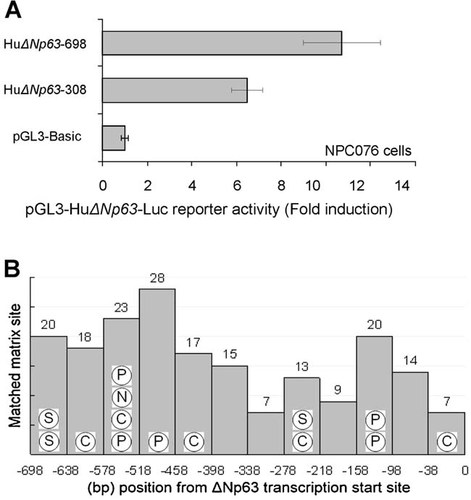
Analysis of the promoter region of human ΔNp63 gene. A: Localization of HuΔNp63 promoter region. Two luciferase reporter vectors containing different length of ΔNp63 promoter region (−698 to 0 and −308 to 0) were established. Either of the two vectors was cotransfected with pCMVLacZ vector into NPC076 cells. Cell lysates were prepared 48 h after transfection, and the luciferase and β-galactosidase activities was quantified. β-Galactosidase was used to normalize the transfection efficiency. Luciferase activity was expressed as fold induction against the baseline activity (pGL3-Basic transfected). Data are mean ± SD from three indepent experiments each with duplicate incubation. B: Distribution of the putative TF-binding sites in ΔNp63 promoter. The putative TF-binding sites in the human ΔNp63 promoter region (−698 to 0) were deduced using MatInspector software (Genomatix). Position weight matrices (matrix sites) were matched to identify cis-regulatory motifs. The human genomic sequences were obtained from UCSC Genome Browser. As shown in the histogram, there were 191 possible matched matrix sites. The matrices appeared to be distributed in two major clusters; with 121 matrices in the region of −698 to −338, and 70 matrices in the region of −338 to 0 (numbers shown on top of each bars). After subtracting the redundant entries, 132 matrix sites and 449 putative TFs were suggested. Among the putative TFs, numerous stem cell-specific regulators were found to be present. The circled alphabets in the bars indicate the presence of various stem cell related TF-binding sites. C = CEBPD; S = STAT3; P = POU5F1 (Oct3/4); and N = NANOG.
We then proceeded to examine the putative TF-binding sites in human ΔNp63 promoter (−698 to 0) using Matinspector software as describe. As shown in Figure 1B, 191 possible matched matrix sites were identified. After subtracting the redundant entries, we suggested the presence of 132 matrix sites and 449 putative TFs. The matrices appeared to be distributed in two major clusters; with 121 matrices in the region of −698 to −338, and 70 matrices in the region of −338 to 0. Among the putative TFs, stem cell specific regulators, such as CEBPD, STAT3, POU5F1/Oct4, and NANOG were found to be present and their putative-binding sites were clustered in the region of −698 to −338. The result suggested that it is preferable to use a longer promoter region (−698 to 0) for screening the possible regulatory factors.
Analysis of rabbit ΔNp63 promoter
To identify the putative TFs that exhibit actual binding to ΔNp63 promoter, we performed DNA pull down assay. Due to the scarce availability of human limbal tissue, we therefore, adopted to use rabbit limbal epithelial cell nuclear protein for the assay. The genomic sequence upstream to the exon 3′ of the RbΔNp63 is shown in Figure 2A, there is a region of 16,332 bp unknown sequence upstream to 369 bp. Since previous studies suggested that some important TFs (STAT3 and CEBPD) bind to regions upstream to the 369 bp region (Barbaro et al., 2007; Chu et al., 2008; Hsueh et al., 2011), it is therefore necessary to sequence this unknown region for rabbit nuclear protein pull down experiment.
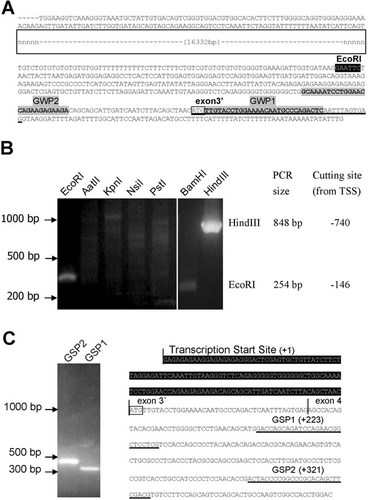
Sequencing and analysis of rabbit ΔNp63 promoter. A: The sequence of rabbit ΔNp63 promoter. Rabbit genomic sequence around ΔNp63 promoter region was obtained from UCSC Genome Browser [Oryctolagus cuniculus draft assembly; Broad Institute oryCun2 (NCBI project 12819, AAGW00000000), Apr. 2009]. The sequence underlined is the first exon (exon 3′) of ΔNp63, and the sequence boxed is the translational start site. The highlighted sequence is the cutting site for EcoRI. GWP1 and GWP2 (highlighted and bold) are primers for Genowalking assay. B: Genome walking assay for sequencing the unknown region of rabbit ΔNp63 promoter. The rabbit DNA was cut with a series of restriction enzymes, including EcoRI, AatII, KpnI, NsiI, PstI, BamHI, and HindIII. The 5′ end of DNA fragments were then ligated with TOPO linker (Invitrogen), and the sequences were amplified by the nester PCR with GWP1 and GWP2 as primers. The right panel shows the size of the PCR products of EcoRI or HindIII treated sample. C: 5′RACE assay for determining the transcriptional start site of ΔNp63 gene promoter. As described in Materials and Methods Section, total RNA was extracted from limbal epithelium, and then converted to cDNA. A known sequence (BD SMART II A oligo, Clonetech) was attached to the 5′ end. The desired cDNA sequence was PCR amplified using gene-specific primers, GSP1 or GSP2. The highlighted sequence is the 5′ untranslated region.
Based on the reporter assay of human promoter construct, rabbit genomic DNA was treated with HindIII and a 848 bp PCR fragment was generated (Fig. 2B). By sequencing this PCR product, we obtained the sequence of a 862 bp fragment upstream to rabbit ΔNp63 exon 3′. To localize the 3′-termius of ΔNp63 promoter, we analyzed rabbit ΔNp63 transcription start site by 5′RACE. We used ΔNp63 GSP to generate a specific 5′-end cDNA product. To avoid the amplification of the genome DNA sequence, the GSP1 and GSP2 designed were located in the second exon (exon 4), and two PCR products with a 98 bp difference were obtained. The PCR product was sequenced to obtain the 5′ untranslated region. The result of the 5′RACE assay suggested that the transcriptional start site of the rabbit ΔNp63 is located at 143 bp upstream to the exon 3′ (Fig. 2C).
Comparison of the rabbit ΔNp63 promoter sequence (−740 to 0) with the corresponding human sequence revealed that there is a 82% similarity, suggesting that the regulation machinery of ΔNp63 expression maybe highly conserved between the two species. Examination of this DNA segment by MatInspector software found that there are 141 possible matrix sites and 514 putative TFs (Fig. 3A). To confirm the rabbit promoter activity, two luciferase reporter vectors containing different length of ΔNp63 promoter regions (−719 to 0 and −337 to 0) were constructed and transfected into RbLmP1 cells. The promoter activities were measured 48 h after transfection. As shown in Figure 3B, RbΔNp63-719 exhibited a higher promoter activity than that of RbΔNp63-337, suggesting a positive regulatory function of the promoter region −719 to −337.
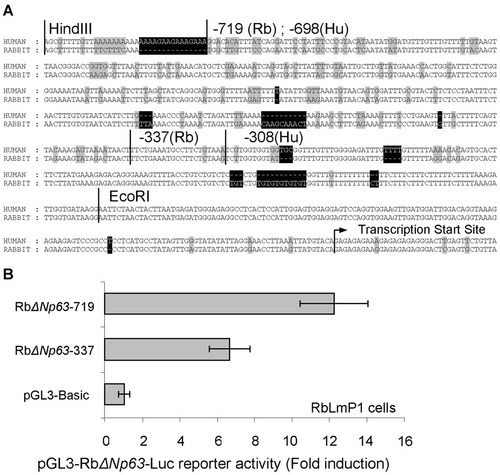
Human and rabbit ΔNp63 promoters are highly conserved. A: Comparison of human and rabbit ΔNp63 promoter sequences. The rabbit sequence (−740 to 0) was obtained from the results of genome walking assay (Fig. 2B), which was aligned to human genome sequence using the Align X program (Vector NTI). The arrow indicates the transcriptional start sit according to the results of 5′RACE (Fig. 2C). The restriction enzyme cutting sites and the segment that was inserted in the reporter construct are also marked. The highlighted nucleotides are the unconserved regions. B: Presence of positive regulatory sites from −719 to −337 in rabbit ΔNp63 promoter. To examine the possible presence of positive regulatory sites from −719 to −337 of the rabbit ΔNp63 promoter sequence, two ΔNp63 promoter luciferase reporter vectors RbΔNp63-719 (−719 to 0) and RbΔNp63-337 (−337 to 0) were constructed. Either of the two vectors was co-transfected with pCMVLacZ report vector into rabbit limbal epithelial cell (RbLmP1 cells), and cell lysates were prepared 48 h after transfection. The reporter activity was evaluated as described in Figure 1A legend.
Analysis of proteins pulled down by rabbit ΔNp63 promoter
Avidin Biotin Conjugated-ΔNp63 promoter DNA pull down assay was performed to examine the TFs that bind to ΔNp63 promoter. Since the transcriptional regulation and the nuclear translocation of TFs are presumed to be different in culture conditions than that in physiological conditions, we collected nucleoprotein from fresh limbal epithelium for the experiment. Limbal tissue is rich in blood vessel and heavily contaminated with albumin that may interfere the LC/MS analysis, therefore, special care was taken to avoid the possible complication. As shown in Figure 4A, the epithelial nuclear extracts prepared were essentially devoided most of the contaminated albumin (about 60 kDa) that was present in the total protein.

PAX6 was identified as a ΔNp63 promoter-binding protein in the ΔNp63 promoter (−719 to 0) DNA pull down assay. A: SDS–PAGE profile of ΔNp63 promoter-binding proteins. Rabbit limbal and corneal tissues were dissected, separated, and nuclear extracts prepared as described. Nuclear extracts (200 µg protein) from limbal or cornea epithelium was incubated with 50 pmoles of biotinylated ΔNp63 promoter DNA (−719 to 0) at room temperature for 20 min. The protein-biotinylated DNA complex was precipitated by streptavidin-conjugated magnetic beads and eluted by boiling in 2× SDS loading buffer. Two micrograms of the eluted protein was fractionated by 7.5% SDS–PAGE and silver-stained. B: Identification of protein species by LC/MS. Limbal and corneal protein eluates (2.6 µg each) were trypsin-digested in separate and subjected to LC/MS analysis. Among the TF candidates, only PAX6 was identified to be limbal specific. Four peptide fragments were obtained from MS analysis (boxed). Comparison of human and rabbit PAX6 protein sequence showed that PAX6 is highly conserved between the two species.
Fifty pmol of biotinlyed-DNA probe (contain rabbit ΔNp63 promoter region −719 to 0) was incubated with 200 µg of limbus or cornea epithelial nuclear protein, and the DNA/protein complex was precipitated using streptavidin-conjugated magnetic beads. Two micrograms of the promoter DNA bound protein was subjected to electrophoretic analysis (Fig. 4A) and the remaining portion was subjected to LC/MS analysis as described. The LC/MS analysis identified 95 bounded protein species in the limbal nuclear extracts, however, only paired box protein 6 (PAX6) was found to possess a putative-binding site in the ΔNp63 promoter construct. This result was consistent with reports shown that PAX6 is implicated in the regulation of LESC proliferation (Funderburgh et al., 2005; Li and Lu, 2005; Li et al., 2008). Comparison of the peptide sequences of human and rabbit PAX6 and Mascot Search matched peptide were shown in Figure 4B.
Transcriptional and translational expressions of the putative transcription factors in human limbal and corneal epithelia
STAT3 and CEBPD have previously been reported to bind to ΔNp63 promoter (Barbaro et al., 2007; Hsueh et al., 2011), however, analysis of the ΔNp63 promoter pulled-down proteins by LC/MS did not show the presence of these two proteins. The inconsistent result indicated the use of promoter pull down assay alone is not sufficient for this study. We therefore, proceeded to use cDNA-microarray combined with ChIP to further screen for TFs that might be involved in the regulation of ΔNp63 expression. Total RNA was extracted from human limbal and corneal epithelia, transcribed to cDNA and analyzed with human whole genome oligo arraychip (Agilent Technologies). The most abundant 2% (8/449) TF genes expressed in the limbal epithelium and their respective expression ratios over that of corneal epithelium were presented in Table 1A. The expression ratio of two known ΔNp63 transcriptional regulators (STAT3 and CEBPD) were also listed (Table 1B). These TFs, except FOXO1 and ATF4 (which exhibited an expression ratio of <2), were selected for further studies to confirm or refute their roles as the regulator of ΔNp63 expression.
| Gene symbol | Entrez gene | Gene title | Log2 | Fold-change | |
|---|---|---|---|---|---|
| Lm | Co | ||||
| (A) Top 2% (8/449) preferentially over-expression in limbus | |||||
| EGR1 | 1958 | Early growth response 1 | 9.4 | 5.1 | 19.54 |
| JUN | 3725 | Jun oncogene | 9.0 | 5.2 | 13.66 |
| ATF3 | 467 | Activating transcription factor 3 | 10.1 | 6.9 | 8.80 |
| ARID5B | 84159 | AT rich interactive domain 5B (MRF1-like) | 8.2 | 5.1 | 8.71 |
| CEBPB | 1051 | CCAAT/enhancer-binding protein (C/EBP), beta | 9.5 | 8.3 | 2.35 |
| FOXO1 | 2308 | Forkhead box O1 | 8.1 | 4.9 | 1.46 |
| ATF4 | 468 | Activating transcription factor 4 | 9.7 | 9.4 | 1.23 |
| PAX6 | 5080 | Paired box protein 6 | 10.3 | 10.1 | 1.12 |
| (B) Stem cell-related regulators | |||||
| CEBPD | 1052 | CCAAT/enhancer-binding protein (C/EBP), delta | 4.3 | 2.4 | 3.90 |
| STAT3 | 6774 | Signal transducer and activator of transcription 3 | 7.8 | 6.5 | 2.44 |
We next compared protein expression of these selected TFs in limbal and corneal epithelia by Western blot analysis. As shown in Figure 5, the expression levels of p63, CEBPD, STAT3, EGR1, JUN, and ARID5B were prominently higher in limbal than in corneal epithelium. In contrast, the protein levels of PAX6, ATF3, and CEBPB were about the same in both tissues, while POU5F1 and NANOG were not detectable.
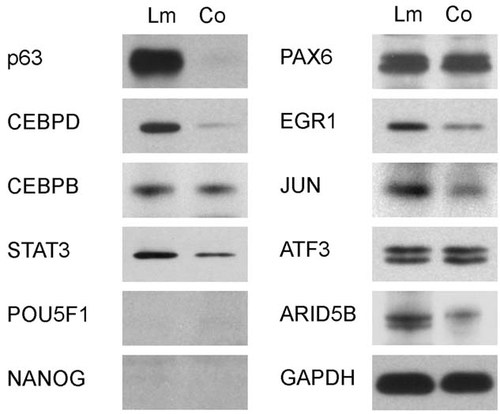
Expression levels of the selected transcription factor proteins in rabbit limbus (Lm) and cornea (Co). Cell lysates prepared from rabbit limbal and corneal epithelia were fractional on 7.5% SDS–PAGE (10 µg/lane). After transfer, the membranes were blotted with antibodies to CEBPB, CEBPD, STAT3, NANOG, POU5F1, EGR1, JUN, ATF3, ARID5B, or PAX6. The expression levels of p63 in limbus and cornea was also evaluated to show its limbal specificity. GAPDH was blotted and served as a loading control.
In vivo binding of selected TFs to ΔNp63 promoter
Chromatin immunoprecipitation assay was performed to examine the in vivo binding of the selected TFs to ΔNp63 promoter. In the nuclei of limbal epithelium, PAX6, EGR1, JUN, STAT3, CEBPB, and CEBPD were found to bind to the proximal region of ΔNp63 promoter (−433 to −142), while in corneal epithelium, CEBPB and JUN were also found to bind to the same region (Fig. 6). The binding of JUN appeared to be less specific, for it also bound to the negative control sequence (+104582 to +104751). The binding of CEBPD and STAT3 to ΔNp63 promoter found here was consistent with previous studies (Barbaro et al., 2007; Chu et al., 2008; Hsueh et al., 2011).
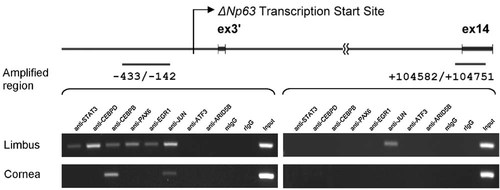
ΔNp63 promoter ChIP assay for selected transcription factors. The in vivo binding of selected TFs on ΔNp63 promoter was investigated in rabbit limbal and corneal epithelia by ChIP assay. Limbal and corneal epithelia were treated with 1% formaldehyde to fix the nucleoprotein–chromatin complexes. The DNA was extracted, fragmented by sonification, and the DNA–protein complexes were immunoprecipitated using mouse or rabbit antibodies against PAX6, CEBPB, CEBPD, STAT3, EGR1, JUN, ATF3, or ARID5B. Non-immunized mouse and rabbit IgG were used as negative control. DNA fragments in the immunoprecipitates were extracted using phenol/chloroform. Primers were designed to PCR amplify the proximal promoter region (−433 to −142) of the ΔNp63 promoter. The primer set spanning over the exon 14 (+104582 to +104751) was also used for PCR reaction to proof the specificity of TF binding.
Regulation of ΔNp63 expression by selected transcription factors
To ascertain the regulatory role of the selected TFs on ΔNp63 expression, TF expression was silenced in limbal epithelial cells by respective siRNA and its effect on ΔNp63 expression was examined. Figure 7A showed that the expression of selected TFs was effectively reduced by respective siRNA. Importantly, silence of STAT3, CEBPD, JUN, PAX6, or EGR1 expression all led to reduced ΔNp63 expression at the protein level (Fig. 7B), suggesting their positive regulation of ΔNp63 expression in the limbus. Moreover, silencing of the above TFs also led to a concomitant upregulation of p27kip1, a CDK inhibitor (Fig. 7B). Our result suggested a linkage of TFs-ΔNp63-p27kip1 pathway in the regulation of limbal epithelial cell proliferation.
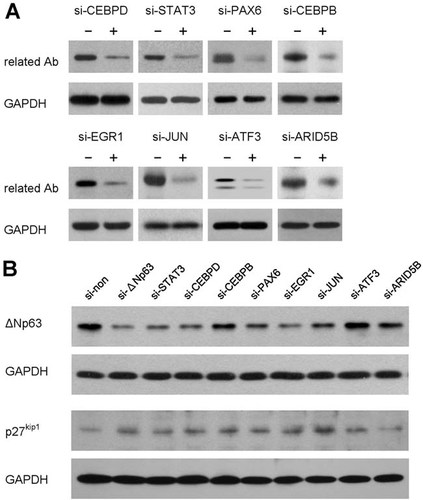
Regulation of p63 and p27kip1 expression by selected transcription factors. A: Silencing of TF expression by siRNA. The silencing effect of the designed siRNA was evaluated by Western blot analysis. Limbal epithelial outgrowth cultures were transfected with desired siRNA or non-silencing siRNA for 6 h and further maintained for 48 h as described in the Material and Methods Section. Total cell proteins were extracted from each experimental group, and fractionated on 7.5% SDS–PAGE (5 µg/lane). First antibodies used included antibodies against CEBPB, CEBPD, STAT3, PAX6, EGR1, JUN, ATF3, and ARID5B. GAPDH was also blotted and served as loading control. B: Effect of TF silencing on ΔNp63 and p27kip1 expression. Silencing of STAT3, CEBPD, PAX6, EGR1, or JUN led to downregulation of ΔNp63 expression with a concomitant upregulation of p27kip1 expression. In contrast, silencing of CEBPB, or ATF3 led to very little or no change of ΔNp63 expression, but upregulated p27kip1 expression. Moreover, silencing of ARID5B exerted very little or not effect on ΔNp63 expression, nor did it change p27kip1 expression. The Western blotting was performed as described in the Material and Methods Section.
Regulation of limbal epithelial cell proliferation
The cell biological effect of TFs-ΔNp63-p27kip1 pathway was examined by the keratinocyte outgrowth of the limbal explants. As shown in Figure 8A, in 14-day explant cultures, the limbal epithelial outgrowth was not suppressed by si-non or Ad-GFP treatment compared to the sham control (Upper panel). The lower two panels showed the inhibition of explant outgrowth by silencing of the individual TF and its rescurability by transduction of ΔNp63α. The data showed that silencing of CEBPD, STAT3, PAX6, or EGR1 effectively suppressed limbal epithelial cell outgrowth and the inhibition was reduced by ΔNp63α. Interestingly, the rescue effect of ΔNp63α in CEBPB, JUN, or ATF3 silenced explant was not as abvious.
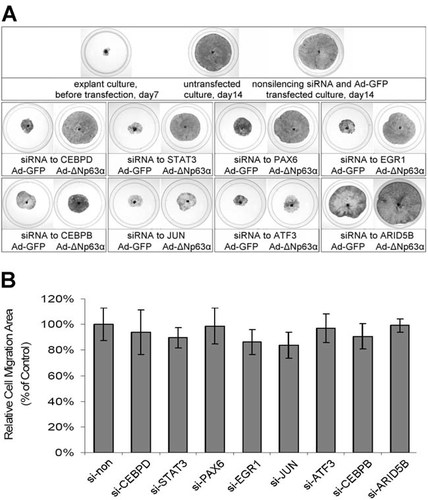
Effect of TF silencing on the proliferation of limbal epithelial cell. A: Evaluation of cell growth by limbal explant culture. Seven days after the initial inoculation, limbal explants cultures with roughly equal size of epithelial outgrowth were chosen for subsequent experiment. The explants were treated with desired siRNA together with Ad-GFP or Ad-ΔNp63α as described in the Materials and Methods Section. The TFs were individually silenced by the respective siRNA. The TFs selectively silenced was PAX6, CEBPB, CEBPD, STAT3, EGR1, JUN, ATF3, or ARID5B. After treatment, the explants were further cultured in the LEGM for 7 days. Cultures were then fixed and stained with 0.25% crystal violet and the area of outgrowth was measures. Six randomly selected dishes were included in each experimental groups. Among them, three were treated with the desired siRNA and Ad-GFP, and the remaining three were treated with same siRNA and Ad-ΔNp63α as indicated. B: Effect of siRNA transfection on the migration of limbal epithelial cell. Limbal epithelial cell was cultured on plastic dishes as described. Upon 80% confluence, the cells were incubated with selected siRNA for 6 h and further cultured for 36 h. The cultures were then wounded by a sterile razor, and cell migration evaluated as described in the Materials and Methods Section. Data are mean ± SD from three independent experiments each with triplicate measures.
To see whether the inhibition of limbal epithelial outgrowth was related to inhibition of cell migration, we evaluated cell migration by the razor wound method in limbal epithelial cell monolayer culture with and without individual TF silencing. Figure 8B showed that the migratory activity of the limbal epithelial cell was not affected by any of the siRNA transfection.
Discussion
It has been reported that p63 is necessary for the development and maturation of many stratified epithelia in mice (Yang et al., 1999). In addition, the expression of ΔNp63 in limbal epithelium is believed to play roles in the homeostasis of limbocorneal epithelium (Pellegrini et al., 2001; Wang et al., 2005; Hsueh et al., 2011). However, the transcriptional regulation of ΔNp63 expression in limbal epithelium has not been fully elucidated and deserves further exploration. Previous studies regarding the transcriptional regulation of ΔNp63 were focused mostly on one or a few pre-conceived TF candidates by examining their direct promoter binding. To gain a better understanding of its transcriptional regulation, we adopted a strategy of functional proteomics coupled with LC/MS and cDNA-microarray combined with ChIP assay to screen for possible ΔNp63 transcriptional regulators. Based on sequencing data of the promoter region (−698 to 0), there were 449 candidate TFs, including some stem cell-related regulators (Fig. 1A). Barbaro et al. (2007) have suggested the presence of TF-binding sites in the further upstream region of ΔNp63 promoter in LESC (Barbaro et al., 2007), however, through our search strategy, we obtained several new TFs that appeared to involve in the regulation of ΔNp63 expression.
We first sequenced the unknown upstream promoter region of rabbit ΔNp63 to validify our subsequent work. The ΔNp63 promoter sequence in the rabbit genome database (Broad/oryCun2) has been updated and supported our sequencing result. In addition, we observed that the transcriptional start sites of the rabbit and human ΔNp63 genes were similar (Human GeneBank; ESTs, DC347501.1, DC347709.1, and DC416276.1). Our study showed that rabbit and human ΔNp63 genes were highly homologous with regard to their promoter regions (82% homology in region 719 to 0), transcriptional start sites, and the coding sequences (95% homology), indicating that ΔNp63 gene is highly conserved in human and rabbit.
Analysis of the promoter pull-down proteins by LC/MS did not identify TFs that were previously reported to bind to ΔNp63 promoter, such as CEBPD and STAT3. It was probably due to the incompletion of rabbit peptide database or the presence of post-translational modifications that made their identification more difficult. Moreover, since the abundance of TFs are usually much lower than that of structural proteins, the presence of TF could easily be obscured by ion suppression and/or mass discrimination effects. This might be the reason why only the highest expressed PAX6 among the 449 candidate TFs was identified by this approach.
Combining the DNA pull down/LC-MS approach with cDNA array/Chip approach, six TFs (including PAX6, EGR1, CEBPB, ARID5B, ATF3, and JUN) were selected for further studies to confirm their roles in the regulation of ΔNp63 expression. Two previously reported transcriptional regulators (STAT3 and CEBPD) of ΔNp63 were also examined. Of the selected TFs, STAT3, CEBPD, PAX6, EGR1, and JUN were found to positively regulate ΔNp63 expression and silence of either of them resulted in a suppression of limbal explant outgrowth, and was rescurable by transduction of ΔNp63. Silencing of ATF3 or CEBPB also suppressed explant outgrowth, however, neither one downregulated ΔNp63 expression. Previous study by others showed that ATF3 can upregulate CDKs (included cyclin D1 and cyclin E; Tamura et al., 2005) and CEBPB was shown to activate cyclin D1 leading to promoted cell proliferation (Eaton et al., 2001). These observation suggested that ATF3 or CEBPB may be involved in a ΔNp63-independent regulation of limbal epithelial cell proliferation.
We previously showed that STAT3 is positively involved in the regulation of ΔNp63 expression in Hep3B cells (Chu et al., 2008) and limbal epithelial cell (Hsueh et al., 2011). Its expression was coincided with promotion of limbal epithelial cell proliferation. In accordance with our result, CEBPD has also been reported to positively regulate ΔNp63 expression and LESC self-renewing (Barbaro et al., 2007). Taken together, our present study not only reconfirmed the earlier reports, but also revealed more regulators for ΔNp63 transcription in limbal epithelial cell.
PAX6 has been shown to be necessary for corneal development and LESC function (Funderburgh et al., 2005; Li and Lu, 2005; Li et al., 2008). Consistently, we showed that PAX6 specifically bound to ΔNp63 promoter, and silencing of its expression downregulated ΔNp63 expression and suppressed limbal explant outgrowth. Intriguingly, no preferential expression of PAX6 in limbal epithelium versus corneal epithelium was observed. EGR1 has been shown to regulate the proliferation of hematopoietic stem cells and to balance between their dormance and self-renewing (Min et al., 2008). The regulatory role of EGR1 shown here suggested that EGR1 is not a stem cell regulator specific to the hematopoietic system.
The mediator(s) between downregulation of ΔNp63 expression and suppression of limbal explant outgrowth has (have) not been identified. In the present study, we found that downregulation of ΔNp63 and associated suppression of limbal explant outgrowth by silencing of individual TFs listed in Figure 7B (except ARID5B) were coincided with the upregulation of p27kip1, suggesting the existence of TF-ΔNp63-p27kip1-cell proliferation signaling cascade in the limbal epithelial cell.
Acknowledgements
This work was supported by grants NSC-95-2320-B-029-MY3 (National Science Council, Taiwan) and CMRPD190531 (Chang Gung Memorial Hospital) to J.K.C.




