Deciduous dental pulp stem cells are involved in osteoclastogenesis during physiologic root resorption†
All authors declare that they have no conflicting interests related to the publication of this article.
Abstract
Multipotent mesenchymal stem cells are derived from the dental pulps of permanent teeth and exfoliated deciduous teeth, and are known to induce bone and dentin generation. However, the role of deciduous dental pulp stem cells (DDPSCs) in physiologic root resorption remains unclear. In this study, dental pulp stem cells (DPSCs) in permanent teeth (P) were retrieved and compared to DDPSCs from deciduous incisors at different root resorption stages: stable (S), middle (M), and final (F). Decalcified teeth sections showed that osteoclasts and resorption lacunae were most prevalent in the M resorption stage. DDPSC proliferation rate was also highest in the M stage. DDPSCs in the F stage produced more calcified nodules than those in the S or M stages. Alkaline phosphatase (ALP) expression was highest in the F stage, indicating that DDPSCs promote mineralization. In addition, the ratio of receptor activator of nuclear factor kappa B ligand (RANKL) and osteoprotegerin (OPG) expression was significantly higher in the M stage, indicating that DDPSCs promote resorption. Dickkopf 1 (Dkk1) expression was remarkably higher in the F and P groups, suggesting that the Wnt pathway is inhibited during the resorption process. Interestingly, despite the fact that Wnt3a down-regulated OPG in osteogenic induction medium and up-regulated RANKL in medium with 1,25-dihydroxy vitamin D3 (VD3), the RANKL/OPG ratio was reduced only with VD3. Collectively, our data indicate that DDPSCs influence osteoclastogenesis during the physiologic root resorption process, and that the canonical Wnt pathway can change the RANKL/OPG expression ratio in DDPSCs. J. Cell. Physiol. 228: 207–215, 2013. © 2012 Wiley Periodicals, Inc.
Abbreviations:
ALP, alkaline phosphatase; BW, osteogenic induction medium and Wnt3a treatment group; CON, control group; CW, VD3 and Wnt 3a treatment group; DDPSCs, deciduous dental pulp stem cells; Dkk1, Dickkopf -related protein-1; DPSCs, dental pulp stem cells; F, final stage; FCS, fetal calf serum; Lef1, lymphoid enhancer factor1; M, middle stage; MEM, modified Eagle's medium; NW, Wnt 3a treatment group; OB, osteogenic induction treatment group; OC, VD3 treatment group; OCN, osteocalcin; OPG, osteoprotegerin; PBS, phosphate-buffered saline; RANKL, receptor activator of nuclear factor kappa B ligand; S, stable stage; SHEDs, stem cells from human exfoliated deciduous teeth; Tcf4, T-cell factor 4; VD3, 1,25-dihydroxy vitamin D3.
The roots of deciduous (primary) teeth are resorbed to allow space for the development of permanent teeth. The physiological process of root resorption is usually initiated on the side of the root of deciduous teeth nearest the permanent successor (Avery, 2002). The majority of data on resorption of hard tissues has been generated by studies on bone osteoclastic resorption (Hammarstrom and Lindskog, 1992). However, the molecular mechanisms underlying root resorption are likely different from bone osteoclastic resorption, since bone undergoes constant remodeling and root resorption occurs only once in primary dentition (Harokopakis-Hajishengallis, 2007).
Odontoclasts are thought to be responsible for the resorption of dental hard tissue. Two key osteoclast-associated genes, the receptor activator of nuclear factor kappa B ligand (RANKL) and the cytokine osteoprotegerin (OPG), have been shown to be related to odontoclast activity (Bhaskar, 1962; Davidovitch et al., 1988; Sasaki et al., 1989; Sahara et al., 1992). Immunohistochemical studies have detected RANKL and OPG expression in odontoblasts, as well as pulp and periodontal ligament fibroblasts and cementoblasts (Hasegawa et al., 2002; Lossdorfer et al., 2002). Interestingly, under non-resorption conditions, the periodontal ligament cells express OPG exclusively (and RANKL expression is undetectable); under resorption conditions, the levels of RANKL expression become up-regulated and those of OPG become down-regulated (Shimizu et al., 1996; Fukushima et al., 2003).
Since the normal pulp structure is usually preserved until the tooth is exfoliated, researchers have tended to assume that dental pulp is not involved in the process of resorption (Kronfeld, 1932; Sahara et al., 1993; Sari et al., 1999). In recent years, investigators have sought a more detailed assessment of pulp status during the resorptive process. Related studies have revealed that RANKL expression was significantly higher in exfoliated deciduous dental pulps than in permanent teeth pulpal tissues (Sasaki et al., 1990; Lossdorfer et al., 2002; Yildirim et al., 2008). Moreover, accumulating evidence has indicated that the postnatal pulp contains several niches of potential stem cells, which may represent a manipulable tool of tissue repair (Gronthos et al., 2000; Shi and Gronthos, 2003; Tecles et al., 2005; d'Aquino et al., 2009; Paino et al., 2010). Miura et al. reported evidence of a multipotent stem cell population in the dental pulps of exfoliated human deciduous teeth (SHED). These SHEDs were characterized as highly proliferative and capable of differentiating into a variety of cell types following exposure to various external stimuli (Miura et al., 2003; Yamaza et al., 2010). Although dental pulp stem cells (DPSCs) and SHEDs have been identified, the role of deciduous dental pulp stem cells (DDPSCs) in the physiologic root resorption process remains unclear. Therefore, we designed the current study to investigate the regulatory mechanism via spatial and temporal tracing of the involved molecules by using DPSCs and DDPSCs in different stages of root resorption.
To date, the odontoclastic genesis-associated molecules RANKL and OPG have only been characterized by expression studies, and their regulatory roles in physiologic root resorption are unknown. In a study of osteogenesis, Spencer et al. identified five functional T-cell factor (Tcf)/lymphoid enhancer factor (Lef) binding sites in the human RANKL promoter, this implicated RANKL as a potential transcriptional target of Wnt signaling in bone (Gary et al., 2006). Wnts, and their downstream signaling pathways, are known to play critical roles in the self-renewal and differentiation of human mesenchymal stem cells, thereby mediating skeletal maturation and tooth formation. Expression of the Wnt antagonist, Dickkopf 1 (Dkk1), was shown to be markedly increased during osteogenic differentiation, which suggested that Dkk1 may promote osteogenesis by down-regulating endogenous Wnt signaling (Liu et al., 2009; Liu and Millar, 2010). In addition, Wnts have been found to down-regulate osteoclastogenesis by repressing RANKL transcription in osteoblasts (Gary et al., 2006). Thus, the current study of root resorption regulatory mechanisms focused on RANKL, OPG, and the canonical Wnt/β-catenin signaling pathway.
Materials and Methods
Tooth samples
Normal deciduous incisors were obtained from children (7–8 years old) undergoing routine dental extractions under general anesthesia. Sound premolar teeth were obtained from patients (13–16 years old) who underwent required orthodontic extractions under general anesthesia. Teeth were subdivided into four groups: incisor teeth with lingual root resorption <1/3 of the root length (stable stage, S group), resorption between 1/3 and 2/3 (middle stage, M group), resorption >2/3 (final stage, F group), and premolar teeth (P group). All subjects were free of any clinical evidence of recent infection. All samples were collected at the Department of Pediatric Dentistry, School of Stomatology, Fourth Military Medical University (Xi'an, China). Written informed consent was provided by all participants or legal guardians, and the study was approved by the hospital's ethics committee.
Histological examinations
Four teeth from each group were immersed in 4% buffered paraformaldehyde for 48 h and decalcified in HAS solution (a complex-acid solution of hydrochloric acid, salicylic acid, and ethylic acid) for 2 weeks. Samples were then sectioned in the axial plane and examined by hematoxylin–eosin staining.
Cell culture
The surface of each intact tooth sample was washed in sterile phosphate-buffered solution (PBS). Teeth were cut around the cementum–enamel junction using sterilized dental fissure burs to reveal the pulp chamber. The pulp tissue was gently separated from the crown and root, and then cut into 1 mm3 pieces. Pulp tissues were separately digested by incubation with type I collagenase (0.66 mg/ml; Sigma–Aldrich, St. Louis, MO) for 40 min at 37°C. Single cell suspensions were generated by filtration through a 70 µm strainer, washing with PBS, and re-suspending in α-minimum essential medium (α-MEM; Gibco BRL, Gaithersburg, MD) supplemented with 10% fetal bovine serum (FBS), 0.292 mg/ml glutamine (Invitrogen, Carlsbad, CA), 100 U/ml penicillin, and 100 mg/ml streptomycin (Gibco BRL). The suspension was then incubated at 37°C in a humidified atmosphere of 5% CO2 and 95% air, with culture medium changes every 3 days. The explants were maintained in 6-well culture dishes (Costar, Cambridge, MA) for 2 weeks until proliferating fibroblasts became sub-confluent.
Cell phenotype analysis by flow cytometry
Cell-surface antigen expression was detected by flow cytometry. Approximately 5 × 105 DDPSCs were washed in PBS and then incubated with mouse anti-human antibodies against CD29, CD34, CD90, CD105 (eBioscience, San Diego, CA), CD45 and CD146 (R&D Systems, Minneapolis, MN) for 30 min at 4°C. Cells were washed twice with cold PBS (2% FBS) and incubated with 1 µg of phycoerythrin (PE)- or fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgM antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) for 30 min at 4°C. Mouse isotype-matched antibodies (BD Bioscience, San Jose, CA) served as controls. Labeled cells were applied to a flow cytometer (Beckman Coulter, Fullerton, CA) to determine phentoypes.
Cell cycle analysis by flow cytometry
After 10 days of culture in α-MEM (10% FBS) in 75 cm2 flasks, DDPSCs and DPSCs (4th passage) were collected by 5 min of trypsinization. Cell precipitates were washed twice with 0.01 M PBS and resuspended in 1 ml physiologic saline with repeated vibration to ensure a single cell suspension. Then, cells were fixed by rapid introduction of 2 ml cold dehydrated alcohol and incubation at −4°C for 24–48 h. Finally, the cells were washed twice with PBS and stained with propidium iodide (100 mg/ml; Sigma–Aldrich) at 4°C for 30 min. Stained cells (5 × 105/sample) were analyzed by flow cytometry to determine the fractions of cells in the G0/G1, S, and G2/M phases of the cell cycle. Intergroup differences in cell cycle distribution and proliferation index (PI) were analyzed by SPSS statistical software (v 18.0; SPSS, Inc., Chicago, IL).
MTT viability assays
DDPSCs and DPSCs (4th passage) were plated in 96-well plates (2 × 103 cells/well) and cultured in α-MEM (5% FBS). The MTT cell proliferation assay was carried out for 8 days according to the manufacturer's protocol (Sigma–Aldrich). Absorbance was measured at 490 nm with a microplate reader (Bio-TEK Instruments, Winooski, VT). All values are presented as mean of quadruplicate samples from triplicate repeats.
Osteogenic and adipogenic in vitro induction assays
For the differentiation study, DDPSCs and DPSCs (4th passage) were plated in 6-well dishes (1 × 104 cells/well) and cultured in α-MEM supplemented with 5% FBS, 100 U/ml penicillin, 100 mg/ml streptomycin, and 50 mg/ml anti-ascorbic acid for 24 h. Then, the medium was replaced with adipogenic induction medium (0.5 mM methylisobutylxanthine, 0.5 mM hydrocortisone, and 60 mM indomethacin; Sigma–Aldrich) or osteogenic induction medium (100 nM dexamethasone, 50 pg/ml of ascorbic acid, and 5 mM β-glycerophosphate; Sigma–Aldrich) and the cells were incubated for 28 or 21 days, respectively, with medium changes every 3 days. Finally, induced cells were fixed in 75% ethanol and stained with Oil red O solution or 2% alizarin red (Sigma–Aldrich), respectively. To quantify the alizarin red stained nodules, the samples were solubilized with 0.5 ml of 5% sodium dodecyl sulfate (SDS) in 0.5 N HCl for 30 min at room temperature. Solubilized samples (0.15 ml) were transferred to a fresh 96-well plate for absorbance measurement at 405 nm.
Immunocytochemistry
DDPSCs and DPSCs (4th passage) were plated in 24-well plates (5 × 104 cells/well) and cultured in basic medium (α-MEM, 5% FBS) for 1 day. The cells were fixed in 4% paraformaldehyde in PBS for 15 min, and then permeabilized with 0.1% Triton X-100 (Sigma–Aldrich) at room temperature. Following overnight incubation with primary antibodies (Col III, s100, and VEGF, 1:200–1:500; Abcam, Cambridge, MA) at 4°C, the samples were subsequently incubated with horseradish peroxidase (HRP)- or rhodamine-conjugated secondary antibodies (Santa Cruz Biotechnology) for 1 h. Isotype-matched control antibodies were used under the same conditions to serve as controls. For immunofluorescent analysis, nuclei were counterstained with Hoechst 33342 (5 µg/ml; Sigma–Aldrich). Imaging and superimposition were carried out with a DP25 inverted fluorescence microscope (Olympus Optical, Tokyo, Japan) and its accompanying DP2 Manager software. The experiments were performed for each group in triplicate.
Quantitative real-time polymerase chain reaction (PCR)
Total RNA was isolated from DPSCs and DDPSCs using the TRIzol reagent (Invitrogen). Approximately, 2–5 µg of total RNA was converted to cDNA using the SuperScript First-Strand Synthesis kit (Invitrogen). The real-time PCR reactions were carried out with reagents from the QuantiTect SYBR Green PCR kit (Toyobo, Osaka, Japan) on a 7500 real-time PCR detection system (Applied Biosystems, Inc., Foster City, CA). Two independent experiments were performed for each sample, which was run in quadruplicate. The gene-specific primers used are reported in Table 1.
| Gene target | Sequences (5′ → 3′) |
|---|---|
| RANKL | Forward: TGATGTGCTGTGATCCAACGA |
| Reverse: AAGATGGCACTCACTGCATTTATAG | |
| OPG | Forward: GAAGGTGAGGTTAGCATGTCC |
| Reverse: CAAAGTAAACGCAGAGAGTGTAGA | |
| Runx2 | Forward: CACTGGCGCTGCAACAAGA |
| Reverse: CATTCCGGAGCTCAGCAGAATAA | |
| OCN | Forward: CCCAGGCGCTACCTGTATCAA |
| Reverse: GGTCAGCCAACTCGTCACAGTC | |
| ALP | Forward: GGACCATTCCCACGTCTTCAC |
| Reverse: CCTTGTAGCCAGGCCCATTG | |
| Dkk1 | Forward: CACTGCATTTGGATAGCTGGTT |
| Reverse: GAAGGTCTGTCTTGCCGGATAC | |
| Tcf4 | Forward: AAATCCGATGACGAGGGTGA |
| Reverse: TCTGCCTTCTGCTCTGGTGTC | |
| Lef1 | Forward: CACAGCGGAGCGGAGATTACA |
| Reverse: AATGAGCTTCGTTTTCCACCATG | |
| β-actin | Forward: TGGCACCCAGCACAATGAA |
| Reverse: CTAAGTCATAGTCCGCCTAGAAGCA |
Wnt3a treatment
DPPSCs (4th passage) were seeded in six T25 culture flasks (5,000 cells/cm2) with α-MEM (5% FBS) and allowed to adhere overnight. Then, the cells were divided into three major groups (two flasks each) for treatment with osteogenic induction medium (group B), 10−8 M 1,25-dihydroxy vitamin D3 (VD3; group C), or PBS (controls; group N). Human recombinant Wnt3a (R&D Systems) was added to one flask of each treatment group at a concentration of 25 ng/ml. Three days later, the six treatment groups (Table 2) were harvested and total RNA was isolated for use in real-time PCR.
| Medium | Wnt3a | |
|---|---|---|
| (−) | (+) | |
| α-MEM (5% FBS) | CON | NW |
| Osteogenic induction medium | OB | BW |
| α-MEM (5% FBS) + 10−8VD3 | OC | CW |
Western blotting analysis
Total proteins were extracted from the cells by lysis in RIPA buffer (10 mM Tris–HCl, 1 mM EDTA, 1% SDS, 1% Nonidet P-40, 1:100 protease inhibitor cocktail, 50 mM β-glycerophosphate, 50 mM NaF). The protein concentration in the extracted lysates was determined by a protein assay kit (Bio-Rad, Hercules, CA) and measuring the absorbance at 595 nm. Cell lysates (20 µg each) were separated by 10% SDS–polyacrylamide gel electrophoresis and then transferred to a polyvinylidene fluoride membrane (Bio-Rad). Non-specific binding sites were blocked by incubating the membrane with 5% milk for 2 h. Then, the membranes were incubated overnight with primary antibodies, followed by incubation with HRP-conjugated anti-rabbit IgG antibodies (Abcam). Immunoreactive bands were visualized with the Western-Light chemiluminescent detection system (Peiqing, Shanghai, China). Polyclonal antibodies against β-catenin and monoclonal antibodies against β-actin (Abcam) were used as normalizing controls.
All data are expressed as mean ± standard deviation (SD) from at least three independent experiments. Intergroup differences were analyzed by the ANOVA Dunnett's T3 test using SPSS v 18.0 software. A P-value <0.05 was considered significant, and <0.001 was considered very significant.
Results
Osteoclasts and resorption lacunae in the pulp cavity
In stable stage teeth and permanent teeth, the inner dentin surface of the pulp canal was relatively smooth and the odontoblast layer was continuous. Neither odontoclasts nor absorption lacunae were found in the pulp cavity (Fig. 1A–C,J–L). In middle stage teeth, many odontoclasts and absorption lacunae were present on the inner surface, from the horn to the apical root, and on the root dentin. In addition, the odontoblast layer was discontinuous. M stage pulpal tissue showed hyperemia and edema, with inflammatory cell infiltration (Fig. 1D–F). In final stage teeth, edema and inflammatory cell infiltration were present, but no hyperemia was observed. In addition, F stage teeth had less odontoclasts and absorption lacunae than M stage teeth (Fig. 1G–I).
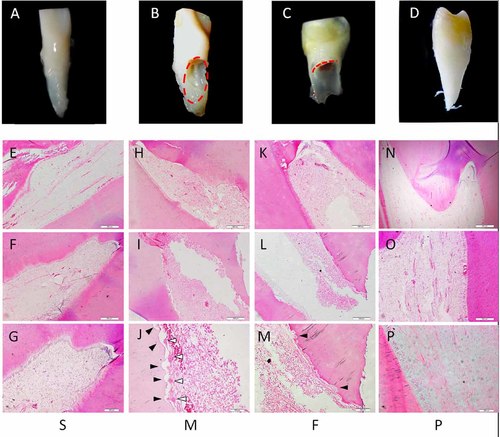
Odontoclastic resorption at the pulpal surface in different stages. Deciduous incisors (A–C) and sound premolars (D) were subdivided into four groups: S, M, F, and P. E–P: Hematoxylin–eosin staining of the dentin and pulp of deciduous teeth and permanent teeth. H–J: Serried odontoclasts (white triangles) and absorption lacunae (black triangles) were observed on the inner dentin surface of the M group. K–M: The F group had relatively less odontoclasts and absorption lacunae. The S group (E–G) and P group (N–P) had smooth and continuous surfaces of pulp canals and odontoblast layers.
DDPSCs and DPSCs possess mesenchymal stem cell properties
The DDPSCs and DPSCs isolated from deciduous incisor teeth and sound premolar teeth (Fig. 1A–D) had typical fibroblast-like morphology (Fig. 2A). As revealed by flow cytometry analysis (Fig. 2B), both cell types expressed mesenchymal stem cell surface markers (CD105, CD146, CD29, and CD90), but were negative for the hematopoietic stem cell marker (CD34) and the leukocyte common antigen (CD45). Immunocytostaining analysis demonstrated that DDPSCs and DPSCs at passage 4 expressed collagen III and nestin, the neural cell marker (Fig. 2C).
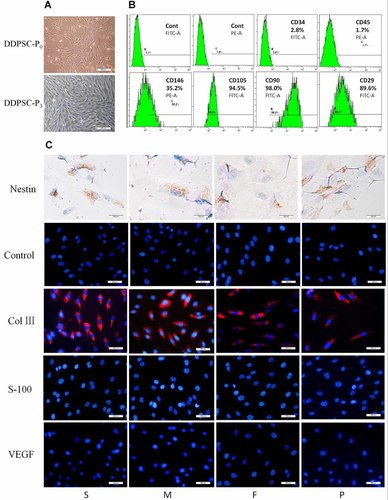
Isolation and identification of DDPSCs. A: The morphology of human-derived DDPSCs was typical of fibroblast-like cells. B: Flow cytometry analysis of cultured DDPSCs at passage 3 (middle stage, equal to SHED cells) indicated positivity for CD146, CD105, CD29, and CD90, and negativity for CD34 and CD45. C: Immunocytochemistry and immunofluorescence showed that DDPSCs expressed nestin and collagen III, but not VEGF or S100. Scale bar: 100 µm.
Characterization of DDPSCs in vitro
DDPSCs in the M group proliferated faster than DDPSCs from any of the other groups, especially from days 5 to 7 of the MTT assay. The proliferation rates in the S and P groups were not significantly different (Fig. 3A). Flow cytometry analyses indicated that the proliferation indexes of DDPSCs in the M and F groups were significantly higher than that in the S group (Table 3).
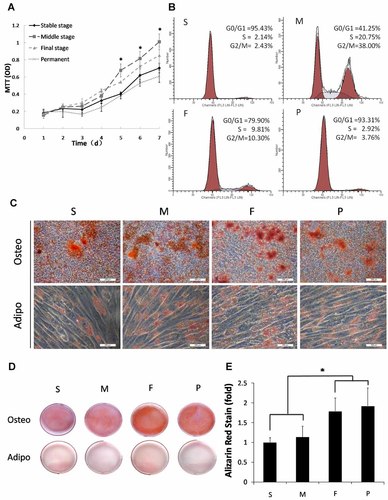
Cell proliferation and differentiation in vitro. DDPSCs and DDPSCs at passage 4 were analyzed by (A) MTT assay (mean ± SD, n = 3) and (B) flow cytometry. C: Cultured DDPSCs and DPSCs formed mineralized nodules (alizarin red staining after 21 days of osteogenic induction) and lipid clusters (Oil Red O staining after 28 days of adipogenic induction). Scale bar: 100 µm. D: Photograph of the alizarin red staning showing Ca2+ deposition after 21 days of osteogenic induction and 28 days of adipogenic induction. E: Graph of the alizarin red-positive area for each group corresponding to total well area (mean ± SD, n = 3). *P < 0.05, ***P < 0.001.
| Group (n) | Cell cycle | Proliferation indexa | ||
|---|---|---|---|---|
| G0/G1 | S | G2/M | ||
| S (3) | 95.62 ± 1.54 | 1.90 ± 0.63 | 2.15 ± 0.51 | 4.05 ± 1.13 |
| M (3) | 41.10 ± 3.53 | 20.58 ± 1.85 | 38.32 ± 1.71 | 58.90 ± 3.54* |
| F (3) | 79.00 ± 2.70 | 9.97 ± 1.69 | 10.80 ± 1.95 | 20.78 ± 3.63* |
| P (3) | 93.86 ± 1.48 | 2.69 ± 0.70 | 3.44 ± 0.78 | 6.13 ± 1.47 |
- All data are presented as % (mean ± SE).
- a PI = ([(S + G2/M) ÷ (G0/G1 + S + G2/M)] × 100).
- * Significantly different from the S group (P < 0.05).
To investigate the potential of DPSCs and DDPSCs to undergo osteogenic/adipogenic differentiation, cells were cultured in osteogenic medium. Both DDPSCs and DPSCs formed mineralized nodules (alizarin red staining, Fig. 3C) and lipid droplets (Oil red O staining, Fig. 3C). Quantitation of alizarin red staining indicated that F stage DPSCs and DDPSCs with higher levels of Ca2+ accumulation had higher potential of osteogenic differentiation (Fig. 3D,E).
Expression of osteoclastogenesis- and osteogenesis-associated genes in DDPSCs
In order to investigate how DDPSCs regulate the root resorption process, the stage-related expression profiles of relevant genes were determined. RANKL expression was found to be markedly up-regulated in the M group. OPG expression was down-regulated in the F group, compared to the S group. The RANKL/OPG ratio was significantly higher in the M group than all other groups (Fig. 4A). Meanwhile, Runx2 expression was also up-regulated in the M group. Osteocalcin (OCN) expression was down-regulated in the F group, compared to the S group. Alkaline phosphatase (ALP) expression was significantly higher in the F group (Fig. 4B), which was consistent with the osteogenic-induction alizarin red staining assay results.
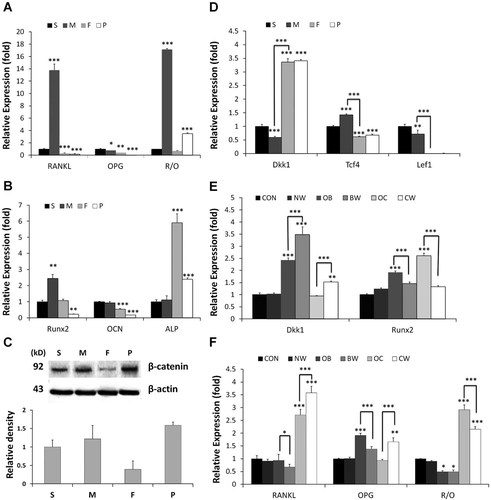
Osteoclastogenesis and osteogenesis relative gene expression. Real-time PCR was carried out to determine the fold-difference in mRNA expression levels for RANKL and OPG (A), Runx2, OCN, and ALP (B), and Dkk1, Tcf4 and Lef1 (D) in DDPSCs and DPSCs. mRNA levels were normalized to β-actin mRNA. Data are presented as mean ± SD (n = 3). C: Semi-quantitative Western blotting analysis was used to determine total β-catenin (relative to β-actin loading control) in DDPSCs and DPSCs. E–F: Real-time PCR was carried out to determine the fold-difference in mRNA expression levels for RANKL and OPG (E), and Runx2 and DKK1 (F) in DDPSCs grown in different induction mediums. mRNA levels were normalized to β-actin mRNA. Data are presented as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001.
To investigate whether DDPSCs were regulated by the Wnt signaling pathway, the levels of total β-catenin protein were detected and compared to the gene expression levels of Dkk1, Tcf4, and Lef1. β-catenin was up-regulated in the M group and down-regulated in the F group (Fig. 4C). Dkk1 expression was remarkably up-regulated in the F and P groups, while Tcf4 and Lef1 expressions were down-regulated (Fig. 4D). These results indicated that Wnt signaling mediated stage-related gene expression changes in DDPSCs.
Effects of Wnt3a supplement on DDPSCs
To investigate whether the canonical Wnt/β-catenin pathway mediated DDPSC function during root resorption by regulating the expression of osteogenic-associated genes, middle stage DDPSCs were induced by osteogenic medium or VD3 and added Wnt3a (Table 2). Cells cultured in osteogenesis medium (OB and BW) showed an obvious up-regulation of Dkk1 expression. Wnt3a treatment (BW and CW) led to significant up-regulation of Dkk1 expression (P < 0.001; Fig. 4E) and down-regulation of Runx2 expression (P < 0.05, Fig. 4E). Osteogenic induction (OB and BW) led to remarkably up-regulated OPG expression. In contrast, VD3 induction (OC and CW) led to up-regulated RANKL expression. Osteogenic induction without Wnt3a stimulation (OB) produced little effect on RANKL expression. Similarly, VD3 induction without Wnt3a stimulation (OC) produced little effect on OPG expression. Wnt3a treatment inhibited both RANKL and OPG expression in osteogenic induction conditions (BW), but promoted both RANKL and OPG expression in VD3 induction conditions (CW). Since the ratio of RANKL/OPG may be a better indicator of osteoclastogenesis, it was calculated for all groups. RANKL/OPG was lower in the osteogenic induction groups (BW and OB), and increased in the VD3 induction groups (CW and OC). Wnt3a stimulation (CW and BW) led to a decrease in the RANKL/OPG ratio (Fig. 4F).
Discussion
Root resorption is a key physiological process of deciduous teeth, and can take place with or without the permanent successor (Obersztyn, 1963; Ten Cate, 1998; Sahara, 2001). The dental follicle of permanent successor teeth and the periodontal ligament both play important roles in root resorption (Marks and Cahill, 1987; Larson et al., 1994; Kanzaki et al., 2002; Fukushima et al., 2003). Meanwhile, odontoclasts, which are mainly aggregated on the pulpal wall, contribute to the resorptive process in the absence of a permanent successor (Lin et al., 2011). This process is further mediated by the pulp itself, which has healing and defense functions to protect against inappropriate or progressive root resorption in deciduous teeth (Simşek and Durutürk, 2005). In the current study, we used human-derived DDPSCs to determine whether and how these cells participate in root resorption.
Some previous studies have investigated the histological differences between resorbing deciduous teeth and non-resorbing deciduous and permanent teeth. The reported resorption activity in resorbing deciduous teeth has been consistently higher than in the other two types of teeth. However, the classification of teeth examined in these studies has been quite broad; therefore, in our current study, we subdivided our resorbing teeth samples into two groups (middle and final stage) and found that the number of odontoclasts and absorption lacunae were much higher in middle stage teeth. These results may reflect the fact that the physiologic resorption of primary teeth is not a continuous process and periods of active resorption are followed by intermittent periods of rest and repair (Fueseth, 1968; Harokopakis-Hajishengallis, 2007). However, no unified standard exists for classifying resorption processes, so the final stage tooth sample and the resorptive activity in our study may be different than that in other previous studies.
Regardless, many previous studies on the root resorption process have discovered stage-related changes in cellular status. Since the particular role of DDPSCs has yet to be determined, we derived pulp stem cells from deciduous teeth at each root resorption stage and used DPSCs from permanent teeth for comparative analysis. In fact, the DDPSCs in our final stage group conceptually equate to SHEDs. Similar to DPSCs, the DDPSCs represent a population of multipotent stem cells that express a distinctive profile of surface molecules. The DDPSCs in our middle stage group exhibited higher proliferation potential than those in the final stage group, and much higher proliferation than those in the stable stage group or the DPSCs. After osteogenic induction, the DDPSCs showed significantly increased mineralization capacity and ALP expression. It is known that stem cells may respond to external stimulation and participate in tissue repair (Stocum, 2001). Moreover, the maintenance and regulation of quiescent stem cell populations are tightly controlled by the local microenvironment (Liu et al., 2011). Therefore, increased proliferation and mineralization of DDPSCs, as observed in our final stage group, may in fact be a response to active resorption, thereby protecting the teeth from excessive resorption.
The RANKL/OPG system is known to regulate osteoclast differentiation and maturation. RANKL and OPG expression have been detected in several kinds of cells derived from the mesenchymal population in deciduous teeth (Shimizu et al., 1996; Oshiro et al., 2002; Miura et al., 2003; Tecles et al., 2005; Yamaza et al., 2010). Our current study demonstrates that the ratio of RANKL and OPG mRNAs expressed in DDPSCs is significantly increased at the middle stage. Runx2, which was also differentially expressed in the various DDPSC stages, is a transcriptional activator of many genes. Overexpression of Runx2 in immature osteoblasts was shown to increase RANKL expression, and subsequently promote osteoblast maturation (Karsenty et al., 1999; Geoffroy et al., 2002). The mechanism underlying the simultaneous increase in Runx2 and RANKL mRNA expression should be investigated in future studies. Many studies have shown that DPSCs and SHEDs are capable of regenerating and repairing dental tissues. However, significantly fewer studies have reported on the interaction between pulp stem cells and root resorption. Our current study provided evidence to support the hypothesis that DDPSCs may promote osteoclastogenesis during the active resorption process, and this function may subside in the final stage.
The role of the canonical Wnt/β-catenin signaling in physiologic root resorption was also evidenced in our current study. Expression of the Wnt antagonist, Dkk1, was remarkably high in our final stage group, while expression of the Wnt-related factors, Tcf4 and Lef1, were decreased. Moreover, the levels of β-catenin were lower in the final stage group. Based on these results, we speculate that the Wnt/β-catenin signaling pathway is dynamically altered in DDPSCs during the physiologic root resorption process. However, the precise role of Wnt signaling in resorption remains unclear.
To gain further insights into how the canonical Wnt signaling pathways may contribute to osteoclastogenesis, we stimulated DDPSCs with exogenous Wnt3a under different conditions and investigated the effects on RANKL and OPG expression. Cells induced by VD3 showed remarkably up-regulated RANKL expression and an increased RANKL/OPG ratio. Cells induced by osteogenic medium had a significantly lower RANKL/OPG ratio. These results are different from those previously reported in a study of osteogenic and VD3 induction of RANKL in human periodontal ligament cells (Fujita and Janz, 2007; Tang and Meng, 2009). Thus, the contribution of Wnt signaling through regulation of RANKL and OPG expression during the root resorption process in various teeth cell types should be further investigated.
Conclusions
Our study demonstrated that the deciduous dental pulp stem cells contribute to osteoclastogenesis, promoting root resorption in the middle stage and suppressing it in the final stage. Moreover, the canonical Wnt signaling pathway is altered during physiologic root resorption, and may act as a dynamic regulator of RANKL and OPG expression under different conditions. Physiologic root resorption is a complicated process regulated directly or indirectly by many cytokines and transcription factors acting through multiple signaling pathways. Elucidating the regulatory mechanism of RANKL and OPG expression in DDPSCs will help our understanding and management of physiologic or pathologic root resorption, and may lead to the identification of molecular targets for effective treatments or preventative therapies.