Erythropoietin-activated ERK/MAP kinase enhances GATA-4 acetylation via phosphorylation of serine 261 of GATA-4†
Conflicts of interest: nothing to declare.
Abstract
GATA-4, a zinc finger transcription factor, plays a critical role in heart development. Previous studies have shown that p300-targeted GATA-4 acetylation increases GATA-4 stability and transcriptional activity, which then stimulates hypertrophy of cardiomyocyte. Erythropoietin (EPO), an essential hypoxia-induced hormone for normal erythropoiesis, is known to exert cardioprotective effects against heart disease of either ischemic or non-ischemic origins. Although, various action mechanisms of EPO have been proposed in the diseased heart, its action mechanism in normal condition has not been investigated. In this study, we aimed to investigate the influence of EPO-induced ERK signaling on the regulation of GATA-4 protein action. EPO treatment increased the protein level of endogenous GATA-4 via ERK signaling pathway. Inhibition of ERK activity by U0126, suppressed EPO-induced expression of GATA-4 protein in rat cardiac myocytes. In addition, ERK activation by over-expression of constitutively active MEK1 strongly increased GATA-4 phosphorylation and subsequently enhanced its acetylation in P19 cells. EPO-induced ERK activation further increased the association of GATA-4 with p300. On the other hand, knock-down of p300 using siRNA diminished ERK-induced GATA-4 acetylation. As EPO-induced GATA-4 phosphorylation via ERK signaling pathway directly correlated with GATA-4 acetylation, we investigated to identify the ERK-dependent phosphorylation sites in GATA-4. Site-directed mutagenesis implicated that Ser-261 in GATA-4 played an important role for ERK-mediated GATA-4 acetylation. Taken together, these results indicated that EPO-induced ERK signaling activation increased GATA-4 phosphorylation and acetylation, partly via increase in the association between GATA-4 and p300, and these processes required the phosphorylation of GATA-4 at Ser-261 residue. J. Cell. Physiol. 228: 190–197, 2013. © 2012 Wiley Periodicals, Inc.
Erythropoietin (EPO) is a well-known important mediator in promoting the proliferation, differentiation, and survival of a wide range of cell types including both erythrocytes and non-hematopoietic cells (Smith et al., 2003; Grasso et al., 2006). EPO has been used as a therapeutic protein for reducing organ damage caused by ischemia-reperfusion (I/R) injury besides for the treatment of anemia. EPO attenuates cardiac myocyte apoptosis by regulation of diverse cellular signaling pathways including phosphatidylinositol 3 kinase (PI3K)/AKT and extracellular signal-regulated kinase (ERK)1/2, which lead to reduction of myocardial ischemia injury (Smith et al., 2003). It also improves cardiac function from myocardial I/R injury via reduction of reactive oxygen species (ROS) overexpression (Tada et al., 2006; Asaumi et al., 2007). Furthermore, EPO has been known to play a role in myocardial remodeling after I/R injury.
The GATA family is one of the transcription factor groups which include six members with two highly conserved zinc finger DNA-binding domain (Cys-X2-Cys-X17-Cys-X2-Cys) that directly interacts with the nucleotide sequence elements 5′-(A/T)GATA(A/G)-3′ in promoter regions of tissue-specific genes. The GATA family acts as a critical regulator for the differentiation, growth, and survival of various cell types (Merika and Orkin, 1993). The GATA-1, -2, and -3 subfamily members are essential for hematopoietic cell development (Simon, 1995), whereas the GATA-4, -5, and -6 subfamily members are expressed in various mesoderm- and endoderm-derived tissues, and cells of the cardiovascular system (Molkentin, 2000). In the heart, GATA-4 has been shown to be involved in transducing nuclear events and the early stage of cardiogenesis as well as cardiac myocyte hypertrophy (Akazawa and Komuro, 2003). As a regulator of inducible gene expression in cardiac myocytes in response to hypertrophic stimulation, it mediates expression of anti-apoptotic protein and stimulates cell survival signals and stress-induced gene expression (Kim et al., 2003; Kobayashi et al., 2006). Being one of the hypertrophy-responsive transcription factors, GATA-4 expression is upregulated during myocardial-hypertrophy. In addition to hypertrophic stimuli, diverse intracellular signaling pathways also participate in the activation of GATA-4 DNA binding and transcriptional activities in cardiac myocytes (Molkentin and Olson, 1997). GATA-4 is phosphorylated by the activation of MEK1/ERK1/2 which lead to DNA-binding activation (Liang et al., 2001). In vivo, MEK1-induced GATA-4 phosphorylation resulted in concentric left-ventricular hypertrophy in cardiac myocytes (Bueno et al., 2000).
A potential GATA-4 co-activator, p300 is an adenovirus E1A-associated protein and possesses an intrinsic histone acetyltransferase (HAT) activity that plays a central role in various physiological processes including proliferation, differentiation, and apoptosis (Goodman and Smolik, 2000). p300 also acts on other transcription factors that participate in the interaction of DNA-binding transcription factors and promoting gene expression in the physiological and pathological growth of cardiac myocytes during development (Backs and Olson, 2006). p300-deficient mice demonstrated cardiac structural defects and reduced trabeculation, whereas a knock-in approach elucidated that HAT domain activity of p300 was essential for heart formation (Yao et al., 1998; Shikama et al., 2003).
In addition to transcriptional regulation, GATA-4 can be modified by post-translational modifications such as phosphorylation, acetylation, and ubiquitination. Phosphorylation of GATA-4 by various signaling pathways results in diverse effects. Its effects are on not only GATA-4 function and heart development but also on transcriptional activity of hypertrophy-response genes. Transgenic mice overexpressing a constitutively active MEK1 showed a dramatic increase in cardiac function and concentric hypertrophy without signs of cardiomyopathy or lethality up to 12 months of age (Bueno et al., 2000). Regulation of GATA-4 transcriptional activity and stability is important for hypertrophic responses to heart failure as well as heart development. EPO increased GATA-4 phosphorylation, and enhanced the transcriptional activation of GATA-4 during hypertrophic responses and/or I/R injury (Liang et al., 2001; Shan et al., 2009). p300-mediated GATA-4 acetylation was one of the critical nuclear events in cardiac myocyte during hypertrophic responses (Takaya et al., 2008). However, it is still unclear whether EPO-induced changes in GATA-4 protein level is involved in the modulation of GATA-4 phosphorylation and acetylation, and is associated with p300 in cardiac myocytes under normal condition. In this study, we aimed to investigate the role of EPO-induced ERK activation in GATA-4 protein level and post-translational modifications.
Materials and Methods
Primary culture of rat cardiac myocytes
The animal procedures were performed with approval by the committee for the Care and Use of Laboratory Animals, Yonsei University College of Medicine and conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (Institute of Laboratory Animal Resources et al., 1996). Neonatal cardiac myocytes were isolated as previously described (Kim et al., 2008). In brief, 11- to 3-day-old Sprague–Dawley neonatal rat pups were sacrificed by cervical dislocation. The hearts were dissected, and ventricles washed with phosphate-buffered saline (PBS) under sterile conditions. The hearts were minced, and digested using an enzyme mixture containing 0.1% (w/v) collagenase I, 1% trypsin–EDTA in HEPES. The dissociated cells were mixed with alpha-minimum essential medium (α-MEM) containing 10% fetal bovine serum (FBS), 100 units/ml penicillin, and 100 µg/ml streptomycin and were centrifuged and pooled. These cells were incubated in 100 mm culture plate for 1 h to reduction of fibroblast contamination. Non-adherent cells were collected and seeded at 5 × 105 cells/ml. After 6 h, cells were rinsed twice with cell culture medium and cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS and 100 units/ml penicillin, and 100 µg/ml streptomycin. EPO was purchased from calbiochem (La Jolla, CA). Culture media and supplements were purchased from WelGENE (Seoul, Korea).
Real-time PCR
Expression levels of GATA-4 mRNA in rat cardiac myocytes were examined by real-time PCR. Total RNA isolation and real-time PCR analysis was performed as previously described (Jun et al., 2011). RNeasy-mini kits were purchased from Qiagen (Valencia, CA). Maxime RT PreMix kit and WEST-ZOL (plus) were purchased from iNtRON Biotechnology (Sungnam, Korea). SYBR premixEx Taq™ was purchased from TaKaRa (Otsu, Japan). Each sample was examined in quadruplicate and GATA-4 gene was normalized to the reference housekeeping gene, glyceraldehydes-3-phosphate dehydrogenase (GAPDH). Fold differences were then calculated for each treatment group using normalized CT values for the control. The primer sequences for real-time PCR were as follows: forward 5′-AAG CAG CCT TGG TGA CTA TG-3′ and reverse 5′-GCA AGC AAG CTA GAG TCC TG-3′ for GATA-4; forward 5′-CAA CAG CAA CTC CCA CTC TT-3′ and reverse 5′-TGT TGC TGT AGC CGT ATT CA-3′ for GAPDH.
DNA constructs and site-directed mutagenesis
The full-length GATA-4 coding sequence (NM_008092) was generated by cDNA synthesis and was inserted into the T-Easy vector (Promega, Madison, WI). For determination of orientation, the full-length GATA-4 cDNA including KpnI and XbaI site was amplified using the following specific primers: forward 5′-GCCA GGT ACC ATG TAC CAA AGC CTG GCC-3′ and reverse 5′-GCCA TCT AGA TTA CGC GGT GAT TAT GTC-3′. To generate the His-tagged expression vectors, the PCR products representing GATA-4 was ligated into the pcDNA 3.1/V5-His TOPO TA expression vector (Invitrogen, Carlsbad, CA), and digested with KpnI/XbaI. The gel-purified fragments were end-filled and religated with its vectors. The GATA-4 S105A and S261A mutants were generated by the QuickChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. The primers used were as follows: forward 5′-ACC CCG CCG CCC GTG GCC CCG CGC TTC TCT TTC-3′ and reverse 5′-GAA AGA GAA GCG CGG GGC CAC GGG CGG CGG GGT-3′ for S105A; forward 5′- CCT CAG CGC CGC CTG GCC GCT TCC CGC CGG GTA-3′ and reverse 5′-TAC CCG GCG GGA AGC GGC CAG GCG GCG CTG AGG-3′ for S261A. All constructs were confirmed by sequencing. The HA-p300 expression plasmid was previously reported (Jun et al., 2010). pcDNA and the constitutively active MEK1 plasmid (pFC-MEK1) were purchased from Stratagene.
Cell culture and transient transfection
Since the primary cardiac myocytes have low transfection efficiency, we used P19 embryonal carcinoma cell lines which can be differentiated to beating cardiac myocytes. P19 cells or H9c2 embryonic rat cardiac cells were maintained in α-MEM or DMEM supplemented with 10% FBS and 100 U/ml penicillin, and 100 µg/ml streptomycin at 37°C in 95% humidified air plus 5% CO2. The cells were transfected using Lipofectatmine 2000 reagent as recommended by the manufacturer (Invitrogen Life Technologies, Carlsbad, CA). Cells were cultured in 60 or 100 mm dishes for 16 h, the medium was changed with antibiotic- and serum-free medium, and these cells were transfected with DNA construct or with pcDNA empty vector. After 6 h, the medium was replaced with growth medium. The cells were lysed and subjected to assays after 24–30 h of transfection.
Immunoprecipitation and immunoblot analysis
After appropriate treatments, the cells were washed with ice-cold PBS and lysed in cell lysis buffer supplemented with 20 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, a protease inhibitor mixture and phosphatase inhibitor cocktail-2 and -3 (Sigma, St. Louis, MO). After measuring protein concentrations using BCA reagents, 1 mg of protein from each cell lysate was immunoprecipitated with the appropriate primary antibodies and protein G-agarose beads. After binding reactions for 16 h at 4°C with continuous rotation, the beads were collected and washed, and bead-bound proteins were eluted by boiling in 1× Laemmli sample buffer with 1 M DTT and then subjected to SDS–PAGE and immunoblot analysis. Anti-acetyl lysine, anti-phosphoserine for MAPK/CDK substrates (PXS*P or S*PXR/K motif), anti-phospho ERK, anti-ERK, the MEK1/2 inhibitor U0126, and cell lysis buffer were purchased from Cell Signaling Technology (Beverly, MA). The p38 inhibitor SB203580 and the JNK inhibitor II SP600125 were purchased from Calbiochem. Anti-HA monoclonal antibody was purchased from Upstate Biotechnology (Berkley, CA). Anti-p300 antibody was purchased from Upstate Biotechnology (Lake Placid, NY). Anti-GATA-4, Anti-Actin, and HRP-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phospho GATA-4 at Ser 105 was purchased from Abcam (Cambridge, MA). Each experiment was performed at least three times.
Determination of GATA-4 stability
P19 cells were transiently transfected with His-GATA-4 expression plasmids. Transfected cells were treated with 10 µg/ml cycloheximide (CHX) for protein synthesis inhibition (Sigma). After 1 h, cells were further incubated in the presence or absence of U1026 (10 µM) and/or EPO for the indicated time. The level of GATA-4 was analyzed by immunoblot analysis.
Gene knockdown by small-interfering RNA (siRNA)
On-TARGETplus SMARTpool siRNAs for mouse p300 and a non-targeting siRNA were purchased from Dharmacon (Chicago, IL). Transfection of siRNA into P19 cells was performed according to the manufacturer's instructions. At 6 h post-transfection, cells were transiently transfected with His-GATA-4 and/or caMEK1 expression vectors for 6 h, and incubated for 24 h in culture medium. These cells were washed and harvested, and immunoprecipitation and immunoblot analysis was performed.
Immunocytochemistry and measurement of cell surface area
Cultured H9c2 cells were fixed and stained for β-myosin heavy chain (MHC) antibody (Abcam) using the indirect immunoperoxidase method. We used Dako REAL™ EnVison™ Detection system kit according to the manufacturer's instructions (DAKO, Glostrup, Denmark). Anacardiac acid and CTPB were purchased from Enzo Life Sciences (Farmingdale, NY). Then, the cell surface area was measured semiautomatically using ImageJ software (Wayne Rasband, National Institutes of Health, Bethesda, MD).
Statistical analysis
All results were expressed as mean ± SE. The statistical significance was analyzed by one-way analysis of variance (ANOVA) or Student's t-test followed by Bonferroni correction. A P-value of <0.01 was considered significant.
Results
EPO increased the GATA-4 protein level in rat cardiac myocytes
EPO is known to provide cardioprotective effects by regulating various signaling pathways and transcription factors against I/R injury (Lipsic et al., 2006; Shan et al., 2009). Their molecular mechanisms under normoxic condition, however, have been not fully elucidated. To investigate the role of EPO in rat cardiac myocytes under normoxic condition, firstly, we examined whether EPO treatment affected GATA-4 mRNA level in primary rat cardiac myocytes. Real-time PCR results demonstrated that EPO did not exert a significant effect on GATA-4 mRNA level in a dose dependent manner although it was weakly induced in 5 IU/ml of concentration of EPO in serum starved medium (Fig. 1A). EPO (20 IU/ml) also did not exert a significant effect on GATA-4 mRNA level in a time-dependent manner (Fig. 1B). Then, we measured the change of endogenous GATA-4 protein level by EPO treatment in primary rat cardiac myocytes (Fig. 1C). EPO (20 IU/ml) markedly increased the total GATA-4 protein level. EPO (20 IU/ml) significantly increased phosphorylated GATA-4 level as well as total protein level in primary rat cardiac myocytes (Fig. 1D). At this time point, ERK activation was still observed in the EPO-treated cells. These results suggest that EPO can regulate the post-translational modification of GATA-4 in normoxic condition.

EPO increased the GATA-4 protein level in rat cardiac myocytes. EPO did not affect GATA-4 mRNA expression. Rat cardiac myocytes were serum-starved or not for 16 h and were treated EPO (0–50 IU/ml) for 8 h (A) and these cells were serum-starved for 16 h and treated with or without EPO (20 IU/ml) for 8 and 24 h (B) and then real-time PCR were performed. Data represent the mean ± SE (n = 4). *P < 0.01, compared to baseline values. C: EPO increased endogenous GATA-4 protein level in primary rat cardiac myocytes. These cells were treated with or without EPO (0–50 IU/ml) for 24 h. Endogenous GATA-4 protein level was determined by immunoprecipitation (IP) and immunoblot (IB) analysis. D: EPO increased endogenous GATA-4 phosphorylation. Rat cardiac myocytes were serum-starved for 16 h and treated with or without EPO (20 IU/ml) for 24 h. Endogenous GATA-4 phosphorylation and protein levels were determined by IP and IB analysis. EPO, erythropoietin.
ERK activation is involved in EPO-enhanced GATA-4 phosphorylation and total protein levels
P19 cells were transiently transfected with His-tagged GATA-4 expression plasmids and incubated with or without EPO for 24 h (Fig. 2A). EPO increased exogenously expressed GATA-4 protein level and GATA-4 phosphorylation in P19 cells. As the MAP kinase pathway is one of the major downstream signaling pathways for GATA-4 and is also activated by EPO signaling pathway (Liang et al., 2001; Shan et al., 2009), we examined which MAP kinases were responsible for EPO-dependent increase in GATA-4 protein level. P19 cells transiently overexpressing GATA-4 were pretreated with kinase-specific inhibitors for 1 h before EPO treatment (Fig. 2B). SB203580, SP600125, and U0126 are selective inhibitors of the p38, JNK, and ERK/MAP kinases, respectively. U0126 completely blocked EPO-dependent increases in GATA-4 total protein level, while SB203580 weakly decreased total GATA-4 protein level. There was no effect on JNK signaling pathway. Furthermore, we observed that EPO treatment (0–50 IU/ml) for 24 h stimulated ERK phosphorylation (Fig. 2C). We also checked EPO-induced ERK phosphorylation in primary rat cardiac myocytes (data not shown). After then, we examined whether EPO-enhanced GATA-4 protein level was related with the increase in GATA-4 stability by ERK signaling pathway in P19 cells (Fig. 2D). EPO significantly delayed GATA-4 protein degradation. U0126, however, blocked EPO-induced GATA-4 stabilization and significantly enhanced GATA-4 degradation compared to the control. These results suggest that ERK signaling pathway is critical for the maintenance of GATA-4 protein stability even in normoxic condition.
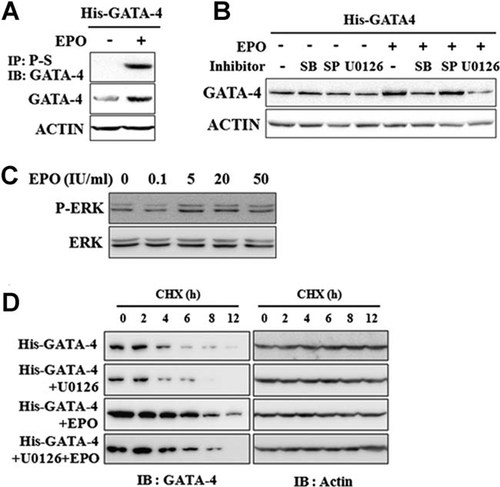
ERK activation is involved in EPO-enhanced GATA-4 phosphorylation and stability. A: EPO increased exogenously expressed His-GATA-4 phosphorylation and protein levels. P19 cells were transiently transfected with His-GATA-4 expression plasmids, incubated for 24 h in the presence or absence of EPO, and subjected to immunoprecipitation (IP) and immunoblot (IB) analysis. B: U0126, ERK inhibitor suppressed GATA-4 protein level. P19 cells were transiently transfected with His-GATA-4 expression vectors and incubated for 16 h. These cells were pretreated with vehicle (DMSO) or inhibitors for 1 h and further incubated in the presence or absence of EPO for an additional 24 h. Exogenous GATA-4 protein level was determined by IB analysis. C: EPO-induced ERK activation. P19 cells were serum-starved for 16 h and treated with EPO (0–50 IU/ml) for 24 h. Whole cell lysates were prepared and subjected to IB analyses. D: EPO treatment stabilized GATA-4 protein, whereas U0126 enhanced degradation of GATA-4. P19 cells were transiently transfected with His-GATA-4 expression vectors. Twenty-four hours after transfection, these cells were treated with cycloheximide (CHX, 10 µg/ml) in the presence or absence of U0126 and EPO for predetermined durations. The levels of GATA-4 and actin were determined by IB analysis. EPO, erythropoietin. P-S, anti-phosphoserine antibody.
EPO-activated ERK increased GATA-4 acetylation
As previously demonstrated, EPO activated the GATA-4 phosphorylation in rat cardiac myocytes. Phosphorylation and acetylation of GATA-4 are the main mechanisms of increasing its DNA-binding activity and transcriptional activity (Takaya et al., 2008). Their relationship in GATA-4 regulation in normoxic condition, however, has not been clearly identified. We evaluated whether EPO could up-regulate GATA-4 acetylation and protein levels via ERK-induced GATA-4 phosphorylation. Inhibition of GATA-4 phosphorylation by U1026 mitigated EPO-induced GATA-4 acetylation and protein stability (Fig. 3A). To clarify the direct effect of ERK activation on GATA-4 protein stability, we elucidated the effect of ERK activation on exogenously expressed His-GATA-4 (Fig. 3B). Overexpression of constitutively active MEK1 as well as EPO significantly increased GATA-4 phosphorylation, acetylation, and total protein level. These results showed that EPO increased GATA-4 phosphorylation which led to stimulation of GATA-4 acetylation and its protein stability mainly via ERK signaling pathway in normoxic condition.
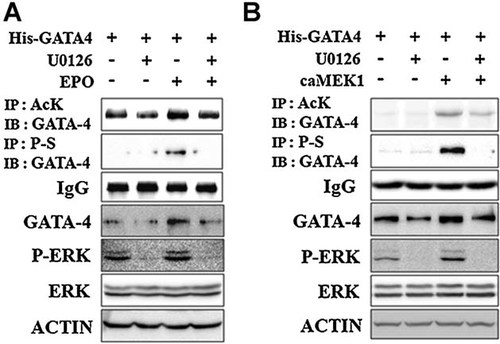
EPO-activated ERK kinase increased GATA-4 acetylation. P19 cells were transiently transfected with His-GATA-4 expression vector and incubated for 16 h. These cells were pretreated with vehicle (DMSO) or U0126 for 1 h and further incubated in the presence or absence of EPO for an additional 24 h (A). P19 cells were transiently transfected with His-GATA-4 and constitutively active MEK1 expression vectors and incubated in the presence or absence of U1026 for 24 h (B). Immunoprecipitation and immunoblot analysis were then performed. P-S, anti-phosphoserine antibody; Ack, anti-acetylated lysine antibody; EPO, erythropoietin.
EPO-induced ERK activation enhanced the association between GATA-4 and p300
Previous studies have demonstrated that the association between GATA-4 and p300 increased GATA-4 acetylation and identified p300-targeted acetylated sites of GATA-4 during hypertrophic responses in cardiac myocytes (Yanazume et al., 2003; Takaya et al., 2008). Therefore, we examined whether the association between GATA-4 and p300 was influenced by EPO-ERK-mediated phosphorylation of GATA-4. P19 cells were transiently transfected with His-GATA-4, HA-p300 expression plasmids and treated with EPO in the presence or absence of U1026 (Fig. 4A). p300-induced GATA-4 acetylation and total protein levels were synergistically increased by EPO-activated ERK phosphorylation. We also observed that the physical interaction between GATA-4 and p300 increased GATA-4 acetylation and its total protein levels (Fig. 4B). Additionally, overexpression of MEK1 increased the interaction between GATA-4 and p300 as well as the total protein levels of GATA-4 and p300. U1026 diminished these ERK activation-induced effects. The necessity of p300-mediated acetylation for ERK-induced GATA-4 protein level was then confirmed through knocked down endogenous p300 by siRNA (Fig. 4C). The efficiency of sip300 was confirmed using immunoblot analysis. Similar to Figure 4B, MEK1 overexpression markedly increased endogenous p300 protein level. siRNA for p300 suppressed ERK-induced p300 protein level as well as the basal level of p300. Furthermore, knock-down of p300 inhibited ERK-induced GATA-4 acetylation and its total protein levels. These results indicated that p300 was involved in part, in ERK-induced GATA-4 acetylation even in normoxic condition.
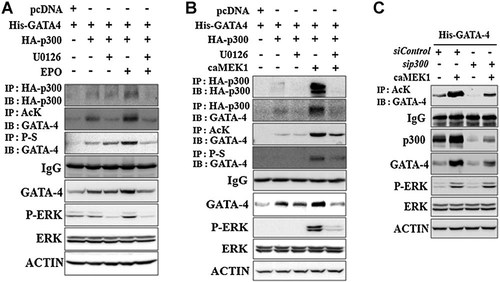
EPO-activated ERK kinase stimulated the association between GATA-4 and p300. A: 19 cells were transiently transfected with pcDNA, His-GATA-4, and HA-p300 expression vectors and incubated for 16 h. These cells were pretreated with vehicle (DMSO) or U0126 for 1 h and further incubated in the presence or absence of EPO for an additional 24 h. B: Nineteen cells were transiently transfected with His-GATA-4, HA-p300, and constitutively active MEK1 expression vectors and incubated in the presence or absence of U1026 for 24 h. Immunoprecipitation (IP) and immunoblot (IB) analysis were then performed. C: Nock-down p300 using siRNA suppressed ERK-mediated GATA-4 acetylation and total protein levels. P19 cells were transfected with siRNAs for p300 (sip300) and a non-targeting control siRNA (siControl). After the first transfection for 6 h, the second transfection with His-GATA-4 and MEK1 expression vectors were performed, and the cells were incubated for an additional 24 h, and then IP and IB analysis were performed. P-S, anti-phosphoserine antibody; Ack, anti-acetylated lysine antibody.
Ser-261 of GATA-4 played an important role in GATA-4 acetylation and stabilization
Considering that EPO-induced GATA-4 acetylation and stabilization were suppressed by an ERK-specific inhibitor, the ERK kinase should be an important signaling molecule for regulating the post-translational modification of GATA-4 and its DNA binding, and transcriptional activity. As a next step, we evaluated which phosphorylation site of GATA-4 by EPO-ERK signaling pathway has an impact on GATA-4 acetylation and stabilization. Previous reports demonstrated that MEK1-ERK signaling pathway activated phosphorylation of serine 105 of GATA-4 (Liang et al., 2001), while cAMP/PKA and ERK-RSK signaling pathway activated phosphorylation of serine 261 of GATA-4 (Tremblay and Viger, 2003; Li et al., 2012). To identify specific phosphorylation site in GATA-4 acetylation activated by EPO-ERK signaling pathway, the effects of S105A and S261A mutants on ERK-induced GATA-4 acetylation and total protein levels were observed. These mutations were confirmed by sequencing (Fig. 5A). S105A mutant was also confirmed by immunoblot analysis using a specific antibody for phosphorylated GATA-4 at serine 105 (Fig. 5B). As expected, EPO-induced GATA-4 phosphorylation was decreased in GATA-4-S105A and GATA-4-S261A mutants. In GATA-4-S105A mutant, however, EPO stimulation still increased acetylation and total protein levels compared to GATA-4-S105A control despite reduced EPO-induced phosphorylation (Fig. 5B). On the contrary, GATA-4-S261A mutant demonstrated almost complete suppression of EPO-induced GATA-4 acetylation and total protein levels as well as its phosphorylation. To confirm this effect on EPO-ERK signaling pathway, we used constitutively active MEK1 instead of EPO (Fig. 5C). GATA-4-S261A mutant abolished ERK-induced GATA-4 acetylation and stabilization. Furthermore, GATA-4-S261A mutant decreased ERK-induced association between GATA-4 and p300, resulting in reduced GATA-4 acetylation and its total protein levels (Fig. 5C). These results suggest that ERK-induced phosphorylation of serine 261 of GATA-4 plays an important role in the association between GATA-4 and p300, which led to stimulation of GATA-4 acetylation and stability under normoxic condition.
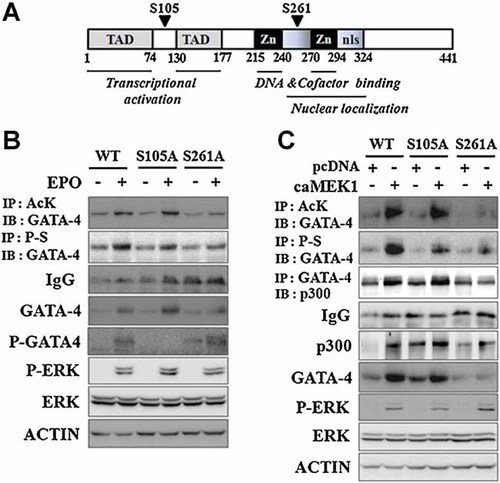
Ser-261 of GATA-4 played an important role in GATA-4 acetylation and stabilization. A: The structure of GATA-4 with candidate phosphorylation sites. P19 cells were transfected with wild-type (WT)-, S105A-, or S261A-GATA-4 expression vectors concomitantly with either pcDNA or MEK1 as indicated and incubated for 24 h (B,C). The levels of acetylated and phosphorylated GATA-4, and the association of GATA-4 with endogenous p300 were detected by immunoprecipitation (IP) and immunoblot (IB) analysis. P-GATA-4, phosphorylation of Ser-105 of GATA-4. P-S, anti-phosphoserine antibody. Ack, anti-acetylated lysine antibody.
EPO-enhanced cell hypertrophy through ERK-signaling pathway in H9c2 cells
To test whether GATA-4 stabilization by EPO-induced ERK activation was involved in the hypertrophy responses in the absence of a hypertrophic stimulation, H9c2 embryonic rat cardiac cells were treated with or without EPO, U0126, CTPB, and anacardic acid (AA), and subjected to immunocytochemical staining with the anti β-MHC antibody. As shown in Figure 6A, H9c2 cells stimulated by EPO and CTPB (p300 activator) demonstrated an increase in cell size compared to control cells, whereas treatment with U1026 and AA (p300 inhibitor) attenuated these responses. Treatment with U0126 in addition to EPO significantly inhibited EPO-induced cell size changes. We could also observe that GATA-4 overexpression increased cell size in H9c2 cells. However, EPO-induced cell size changes could not be observed in GATA-4-S261A mutant (Fig. 6B). These results suggest that EPO-ERK-induced GATA-4 phosphorylation is involved in GATA-4 acetylation and stabilization that lead to hypertrophic responses in the absence of a hypertrophic stimulation in normoxic condition, and that these processes require phosphorylation of GATA-4 at Ser-261 residue.
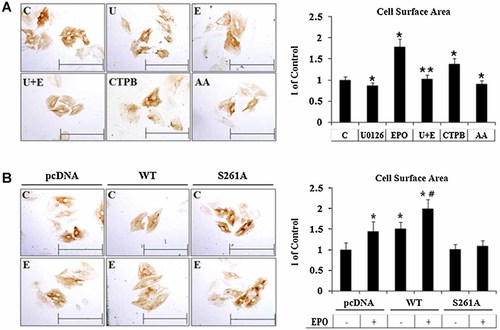
EPO enhanced cell hypertrophy through ERK signaling pathway in H9c2 cells. A: H9c2 embryonic rat cardiac cells were pretreated with or without U0126 (10 µM) for 1 h and further incubated in the presence or absence of EPO (20 IU/ml), CTPB (100 µM), AA (10 µM) as indicated for an additional 24 h. These cells were stained with anti-β-MHC antibody (brown signals). B: H9c2 cells were transfected with pcDNA, wild-type (WT), or S261A-GATA-4 expression vectors and treated with or without EPO for 24 h, and subjected to immunocytochemistry staining using anti-β-MHC antibody (brown signals). Scale bar, 20 µm. A,B (right parts): Cell surface areas were measured as described under Materials and Methods section. Data represent the mean ± SE (n = 50). *P < 0.01, compared with control (A) or pcDNA control (B); **P < 0.01, compared to EPO treatment; #P < 0.01, compared to GATA-4-WT overexpressed group.
Discussion
GATA-4 is a key transcription factor for cardiac morphogenesis and mediates hypertrophy of cardiac myocytes (Molkentin and Olson, 1997; Pikkarainen et al., 2004). It is also known that GATA-4 phosphorylation stimulated by various signaling pathways is subject to regulation of its DNA binding and transcriptional activities (Liang et al., 2001). In the current study, we observed that EPO stimulation could regulate GATA-4 post-translational modification. Novel findings of this study performed under normoxic condition are (i) EPO did not affect GATA-4 mRNA expression, (ii) EPO increased GATA-4 protein level, (iii) EPO stimulated GATA-4 phosphorylation via ERK phosphorylation, (iv) GATA-4 acetylation and stabilization were increased by EPO-induced ERK activation which were suppressed by U1026, (v) constitutively active MEK1-induced GATA-4 phosphorylation also increased GATA-4 acetylation and total protein levels, (vi) ERK activation increased p300 protein level and the association between GATA-4 and p300, (vii) knock-down of p300 expression abolished ERK-mediated GATA-4 acetylation and stabilization, (viii) the phosphorylation of GATA-4 at Ser 261 played an important role in ERK-mediated GATA-4 acetylation and stabilization, (ix) EPO-induced GATA-4 acetylation and stabilization led to cell hypertrophy via ERK-induced GATA-4 phosphorylation at serine 261.
Previous report showed that pressure overload by aortic banding activated GATA-4 post-translational modifications without increasing GATA-4 gene expression in the left ventricle (Hautala et al., 2001). Similarly, we also observed that EPO did not affect GATA-4 mRNA expression (Fig. 1A,B), although EPO stimulation increased endogenous GATA-4 protein level in primary rat cardiac myocytes (Fig. 1C) and exogenous GATA-4 protein level in P19 cells (Fig. 2A) even in the absence of a hypertrophic stimuli. In these processes, ERK activation but not JNK signaling pathway was involved in P19 cells (Fig. 2B). The EPO-induced ERK activation increased GATA-4 phosphorylation for 24 h in P19 cells (Fig. 2).
The GATA-4 activity is regulated through modulating the level of GATA-4 protein expression via transcriptional as well as translational modification. The nuclear localization and transcriptional activation domains of GATA-4 have been identified (Pikkarainen et al., 2004). GATA-4 has a domain of two adjacent zinc fingers (N-terminal and C-terminal) and most of the protein–protein interactions of GATA-4 were mediated by its DNA sequence recognition domain of the C-terminal zinc finger (Arceci et al., 1993; Lee et al., 1991). p300, a transcriptional co-activator of GATA-4, possesses an intrinsic HAT activity. There were direct physical interactions between cysteine/histidine-rich region 3 (C/H3) of p300 and C-terminal zinc finger domains of GATA-4, which were able to acetylate GATA-4 leading to a subsequent increase in its transcriptional activity (Dai and Markham, 2001). GATA-4 acetylation was critical for the differentiation of embryonic stem cells into cardiac myocytes (Kawamura et al., 2005). In accordance with previous reports, we also observed that the physical interaction between GATA-4 and p300 was increased by EPO-ERK activation and these processes required the phosphorylation of GATA-4-S261 in the current study. Recently, there was a report that curcumin, a p300 HAT inhibitor, was able to inhibit GATA-4 acetylation and prevent heart failure (Morimoto et al., 2008). Additionally, we reported that the activation of ERK signaling increased endogenous p300 acetylation and its total protein levels in mouse myoblast cell line (Jun et al., 2010). Similar to those results, EPO-activated ERK phosphorylation synergistically increased p300 protein level in P19 cells (Fig. 4A,B). Furthermore, in the current study, we observed that EPO-induced ERK activation increased not only phosphorylated but also acetylated GATA-4 level and its total protein level partly via the association between GATA-4 and p300 (Fig. 4), a distinct feature of EPO signaling transduction in GATA-4 regulation, which has not been reported hitherto.
It has been shown that protein kinase A (PKA)-induced GATA-4 phosphorylation was associated with recruitment of transcriptional co-activator CREB-binding protein (CBP; Tremblay and Viger, 2003). CBP and p300 are expressed in most tissues as multifunctional molecules that can exert both positive and negative effects on transcription and cell differentiation, and also acetylase activity. Thus, CBP and p300, and their interaction are able to regulate acetylation (Blobel, 2000). Since ERK-mediated GATA-4 acetylation was not completely abolished by knock-down p300, we can suspect that p300 was partly involved in ERK-induced GATA-4 acetylation following EPO stimulation, and an another molecule may be required for this acetylation.
Previous studies demonstrated that GATA-4 in cardiac myocytes was phosphorylated in response to hypertrophic stimuli such as phenylephrine (Morimoto et al., 2000) and endothelin-1 (Kitta et al., 2001). Ser 105 at GATA-4 was also phosphorylated by ERK (Liang et al., 2001), p38 mitogen activated protein kinases (Kerkela et al., 2011), and hepatocyte growth factor (Kitta et al., 2003). We also observed SB203580, a p38 inhibitor, suppressed the EPO-induced increase in GATA-4 total protein level (Fig. 2B). These results implicate that EPO can modulate the post-translational modification of GATA-4 via the p38 signaling pathway.
In the current study, GATA-4-S105A mutant suppressed EPO-ERK-mediated phosphorylation compared to GATA-4 wild type, while acetylation was not changed in this mutant (Fig. 5B). On the contrary, GATA-4-S261A mutant decreased both EPO-ERK-mediated phosphorylation and acetylation, and the association between GATA-4 and p300. Therefore, serine 261 in GATA-4 may play a critical role in EPO-ERK-mediated up-regulation of GATA-4 acetylation, stabilization, and the association of between p300 and GATA-4. It was reported that C-terminal zinc finger domain of GATA-4 was more important than N-terminal zinc finger domain in physical interaction with p300 (Dai and Markham, 2001). Serine 261 in C-terminal zinc finger domain of GATA-4 may be a potent residue for EPO-ERK-mediated enhancement of the association between GATA-4 and p300. GATA-4 stabilization by EPO-induced ERK activation was involved in the hypertrophic responses in H9c2 embryonic rat cardiac cells even in the absence of hypertrophic stimuli or I/R injury in the current study. CTPB treatment also increased cell size compared to control cells. However, in spite of EPO stimulation, these hypertrophic responses could not be observed in GATA-4-S261A mutant as a dominant-negative form (Fig. 6B). EPO-induced phosphorylation of Ser-261 of GATA-4 could enhance GATA-4 acetylation and stabilization resulting in hypertrophic responses under normoxic condition in the absence of hypertrophic stimulation.
Previously, in the field of cardiology, concerns for EPO have been focused on its protective effects against I/R injury and on cardiac remodeling after heart disease while its effects on normal or less injured myocardium was not critically considered. Recently, however, issues dealing with tumor progression associated with EPO receptor stimulating agents have been raised (Blau, 2007). These findings force the researchers to investigate the effect of EPO in different conditions from damaged myocardium. The post-translational modification on GATA-4 is important for its functional performance in the cellular physiological as well as pathological processes and is clearly involved in hypertrophic responses of cardiac myocytes. Therefore, our results demonstrated the possibility of unwanted myocardial remodeling in normal myocardium during systemic use of EPO for various clinical purposes. However, additional in vivo studies whether an acute or chronic use of EPO in normal myocardium would result in clinically remarkable modulations in myocardial differentiation and morphologic changes seems to be required.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0007099).