Pharmacological inhibition of HSP90 and ras activity as a new strategy in the treatment of HNSCC
Abstract
Advanced head and neck squamous cell cancer (HNSCC) is currently treated with taxane-based chemotherapy. We have previously shown that docetaxel (DTX) induces a ras-dependent survival signal that can be antagonized by farnesyl transferase inhibitors (FTI) such as tipifarnib (TIP). Here we show that the synergistic TIP/DTX combination determines synergistic apoptotic conditions but, at the same time, it modulates the expression of the components of the multichaperone complex that is, in turn, involved in the regulation of the stability of members of the ras-mediated pathway. Therefore, we have stably transfected HNSCC KB and Hep-2 cells with a plasmid encoding for HSP90. The expression of the protein was increased in both transfected cell lines but its activation status was increased in Hep-2 clones and decreased in KB clones. On the basis of these results, we have treated both parental and HSP90-transfected cells with a HSP90 inhibitor geldanamycin (GA). We have found that the antiproliferative activity of GA is dependent upon the activation status of HSP90 and that it is strongly synergistic when added in combination with TIP but not with DTX in cells overexpressing HSP90 and even more in cells with increased HSP90 activity. These data were paralleled by the decreased expression and activity of the components belonging to the ras→mediated signal transduction pathway. The present results suggest that multichaperone complex activation could be a resistance mechanism to the anti-proliferative and apoptotic effects induced by TIP and that the combination of FTIs such as TIP with GA could be a suitable therapeutic strategy in the treatment of HSP90-overexpressing HNSCC. J. Cell. Physiol. 228: 130–141, 2013. © 2012 Wiley Periodicals, Inc.
Head and neck malignant tumors represent, globally, about 10% of cancers in men and 4% in women. Changes in incidence and mortality are considerable, with higher rates in India and northern France (Franceschi et al., 1997; Parkin et al., 1997). The estimated incidence in 2008 for the United States is of 35,310 new cases, with an expected 7,590 deaths due to these cancers (Jemal et al., 2008). The Surveillance, Epidemiology and End Results (SEER) database showed that younger U.S. populations (ages 20–44 years) have been an increased incidence of tonsillar squamous cell carcinomas from 1973 to 2001, while the incidence of squamous cell carcinomas in all other oral and pharyngeal sites remained constant or decreased (Shiboski et al., 2005). Similar rise in the incidence of tonsillar squamous cell carcinomas from 1970–2002 has been shown in Sweden and has been associated with the occurrence of human papillomavirus (HPV) (Hammarstedt et al., 2006; Dayyani et al., 2010). Traditionally, 80–90% of head and neck squamous cell carcinomas (HNSCs) have been attributed to tobacco use and alcohol consumption; nevertheless emerging evidence indicate just in HPV one of the main responsible for HNSC occurrence (Dasgupta et al., 2012).
The median overall survival for recurrent or metastatic head and neck cancer remains less than 1 year despite modern chemotherapy and targeted agents (Price and Cohen, 2012). The treatment of choice for the majority of inoperable patients consists in a combination of chemotherapy and radiotherapy (CRT). Induction chemotherapy (ICT) before CRT seems to decrease systemic relapse. Moreover, incorporation of taxanes to the cisplatin and 5-FU-based ICT has shown increase in response rates (Somani et al., 2011). Indeed, the beneficial effects of Docetaxel (DTX), a conventional chemotherapeutic agent member of Taxanes, plus cisplatin-based induction chemotherapy for patients with unresectable, advanced head and neck cancer have been documented in Western countries, as well as its efficacy has been recently confirmed also in Asian patients (Huang et al., 2012).
DTX induces apoptosis through the interaction with tubulin dimers (Crown and O'Leary, 2000). Overall this interaction stabilizes and promotes the assembly of microtubules, preventing their physiological depolymerization in the absence of GTP and resulting in a parallel disposal and therefore in an incorrect positioning of the mitotic spindle. In the absence of a mitotic spindle with the right form, the chromosomes cannot separate successfully, leading to a process that causes cell death (Crown and O'Leary, 2000). Furthermore, it was also observed that the lack of organization in the microtubules structure induced by Taxanes determines the induction of a signal transduction pathway that in any case lead to apoptotic death (Orr et al., 2003). In fact, several studies have demonstrated that Taxanes induce the phosphorylation of Bcl-2, preventing its ability to form heterodimers with Bax and thus to mediate its anti-apoptotic function (Haldar et al., 1997). Simultaneously, Taxanes induce increased expression of p21—a potent inhibitor of cyclin-dependent kinases cdk2 and cdk4—and of p53—a strong tumor suppressor as well apoptosis induction is realized through the activation/inactivation of different protein kinase (p34cdc2, PKA and PKC). Conversely, experimental evidence has shown that Taxanes are also able to promote a protective effect from apoptosis through the hyper-activation of a ras→ Erk-dependent survival mechanism (Wang et al., 1999).
Consequently, in order to increase DTX anti-tumor potential, we used in combination with DTX a non-peptidomimetic drug, Tipifarnib (TIP, Zarnestra), that inhibits the farnesylation and, in turn, the activation of the oncoprotein ras. The molecular mechanism underlying the antiproliferative activity of the farnesyl transferase inhibitors (FTI) is mainly due to the inhibition of Erk and Akt in several cancer cell models (Sebti and Hamilton, 2000; Selleri et al., 2003). TIP has been tested on a wide range of solid tumors and hematological malignancies, showing antitumor activity in several tumor types, including Myelodysplastic Syndromes and Acute Myeloid Leukemia (AML). TIP was also the first FTI that has prompted, in the first phase of clinical trials, the complete remission of AML; phase II clinical trials confirmed the early report, showing furthermore an acceptable safety profile in patients with this condition (Harousseau et al., 2009). On the other hand, it is emerging that signal transduction pathways and particularly the intracellular stability and the consequent regulation of the activity of their components are regulated by the multi-chaperone complex and by the derived proteasome degradation pathway. The multichaperone complex is, in turn, formed by several heat shock proteins (HSPs) functionally complexed and interacting in order to preserve the correct folding of intracellular proteins. The HSP90 protein is a ubiquitously expressed, highly conserved 90 kDa molecular chaperone that regulates the cellular stress response by maintaining the conformation, stability and function of crucial client proteins (Richter and Buchner, 2001; Blagg and Kerr, 2006). It is well established that HSP90 plays a central role in the conformational maturation of HSP90-associated client proteins (Chen et al., 1998). Over 100 individual HSP90 client proteins have been identified (see the updated list on http://picard.ch), including oncogenic/growth-stimulating proteins such as mutated p53 and BRAF, BCR-ABL, HER2, AKT, C-RAF, CDK4, and others that are frequently found in signal transduction, cell cycle, growth control, and apoptosis pathways. Multiple rounds of chaperone–client protein association/dissociation are driven by cyclical ATP/ADP hydrolysis to maintain the stability and function of client proteins. These functions are as diverse as maintaining steroid receptors competent for ligand binding, or stimulating signal transduction pathways involving kinases such as MAPK, AKT, and IKK. Therefore, multiple components of the ras-dependent pathway are under HSP90 control. In this light, geldanamycin (GA) dissociates mature multi-chaperone complexes by inhibiting HSP90 ATPase activity and the released client proteins are subsequently degraded by the ubiquitin–proteasome pathway (Meyer et al., 2004; Fukuyo et al., 2010). Therefore, GA could sensitize cancer cells to ras inhibitors and/or disrupt escape pathways that occur in response to the anti-proliferative action of DTX.
On the basis of these considerations, we have assessed in the present manuscript the pharmacological interaction between TIP and DTX on HNSC cells. We have indeed found that the two drugs have a strong synergistic interaction on cell growth inhibition of HNSC cells likely based on the disruption by TIP of a ras→Erk and Akt-dependent escape mechanism induced by DTX in cancer cells. Thereafter, we have obtained new experimental cell models from transfection of KB and Hep-2 cells with a plasmid encoding for HSP90. Unexpectedly, the HSP90 overexpressing cells had either increased or reduced HSP90 activity. In these experimental models, we have found that the treatment of these clones with GA strongly synergizes with TIP but not with DTX and the positive pharmacological interaction is dependent from the HSP90 activation.
Materials and Methods
Materials
DMEM, BSA, and FBS were purchased from Flow Laboratories (Milan, Italy). Tissue culture plasticware was from Becton Dickinson (Lincoln Park, NJ). DTX was a gift of Aventis-Pharma. TIP was a gift of Orthobiotech (Orthobiotech, Janssen Research Center, NJ). GA was a gift of InvivoGen (InvivoGen, CA). Protein Sepharose A was purchased from Sigma (St. Louis, MO). Rabbit antisera raised against Erk-1/2, p38 MAPK, pAkt (Ser 473) and pErk MAb were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit antisera raised against HA-Tag, pGSK3 α/β, Akt, and the relative activity evaluation kit were purchased by Cell Signalling (Cell Signaling Technology, Beverly, MA). Anti-pro-caspase3, -Hsp90, and -HSP70 MAbs were purchased from Alexis (Lausen, Switzerland). Anti-pan-ras clone 10 MAb was purchased from Calbiochem (Darmstadt, Germany). The minimum domain of Raf-1, active ras-binding, agarose miscrosfere conjugated, was provided dall'Upstate Biotechnology Inc. (Lake Placid, NY). Anti-α-tubulin MAb was purchased from Oncogene (Cambridge, MA).
Cell culture
The human oropharyngeal epidermoid carcinoma KB, Hep-2, Cal-33, and Cal-27 cells, obtained from the American Type Tissue Culture Collection, Rockville, MD, were grown in DMEM supplemented with heat inactivated 10% FBS, 20 mM HEPES, 100 U/ml penicillin, 100 µg/ml streptomycin, 1% L-glutamine, and 1% sodium pyruvate. The cells were grown in a humidified atmosphere of 95% air/5% CO2 at 37°C.
Cell viability assay
Cell viability was analyzed by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay. Cells were seeded in 96-well plates at the density of 1.5 × 103 cells/well for KB parental and clones, at the density of 1 × 103cells/well for Hep-2 and the corresponding clones, and at the density of 2.5 × 103 cells/well for Cal-33 and Cal-27, in a final volume of 100 µl. Cells were then incubated at 37°C in a humidified atmosphere to allow exponential growth. The following day, cells were treated with DTX at a range of concentrations between 0.09 and 6.2 ng/ml. KB cells and the corresponding clones, Cal-33, Cal-27, and Hep-2 were also treated with TIP, at a range of concentrations between 1.25 and 80 mM, single and in combination with DTX or GA. GA was used to the range of concentrations between 10 and 640 nM, single and in combination with DTX or TIP. TIP and GA were added 24 h after DTX for KB parental and clones, and in concomitant administration for the other cell lines. At 48 h and 72 h from the treatments, cells were exposed to a solution of 10% MTT for 4 h at 37°C to form formazan crystals by reacting with metabolically active cells. The formazan crystals were solubilized in a 1 N isopropanol/HCl 10% solution at 37°C, on a shaking table for 20 min. The absorbance values of the solution in each well were measured at 570 nm using a microplate reader. Cell viability was determined by the formula: Cell viability (%) = (absorbance of the treated wells−absorbance of the blank control wells) / (absorbance of the negative control wells−absorbance of the blank control wells) × 100%. All MTT experiments were performed in triplicate and repeated at least three times.
Drug combination studies
This study allowed the evaluation of the synergistic inhibition of cell growth produced by DTX/TIP combination in KB, Cal-33, Cal-27, and Hep-2 cells, and by DTX/TIP, DTX/GA, and TIP/GA combinations in KB parental and clones. Cells were seeded in 96-well plates at the previously indicated density. After 24 h of incubation at 37°C, cells were treated at the drugs range of concentrations described above. Drug combination studies were based on concentration–effect curves generated as a plot of the fraction of unaffected (surviving) cells versus drug concentration after 48 and 72 h of treatment. To explore the relative contribution of each agent to the synergism, three combinations with different DTX/TIP, TIP/GA, and DTX/GA molar ratios were tested for each schedule: Equiactive doses of the two agents (IC50), higher relative doses of DTX (IC75 of DTX/IC25 of TIP or/IC25 of GA), higher relative doses of TIP (IC75 of TIP/IC25 of DTX or/IC25 of GA), and higher relative doses of GA (IC75 of GA/IC25 of DTX or/IC25 of TIP). Assessment of synergy was performed quantitating drug interaction by Calcusyn computer program (Biosoft, Ferguson, MO). Combination index (CI) values <1, 1, and >1 indicate synergy, additivity, and antagonism, respectively.
Cell cycle analysis
Cells were seeded in 100 mm plates at the density of 6 × 105 for KB and of 1 × 106 for Cal-33, and then incubated 24 h in humidified atmosphere at 37°C. The following day cells were treated with DTX concentrations of 0.047 ng/ml for KB, and 0.006 ng/ml for Cal-33, on concurrent administration with TIP 0.17 µM for Cal-33, and sequential TIP 0.6 µM administration (24 h after DTX) for KB cells. After incubation with DTX and TIP alone or in combination, cells were washed in PBS, pelleted, and directly stained in a Propidium Iodide (PI) solution (50 mg PI in 0.1% sodium citrate, 0.1% NP40, pH 7.4) for 30 min at 4°C in the dark. Flow cytometry analysis was performed using a FACScan flow cytometer (Becton Dickinson, San Jose, CA). To evaluate cell cycle PI fluorescence was collected as FL2 (linear scale) by the ModFIT software (Becton Dickinson). For the evaluation of intracellular DNA content, at least 20,000 events for each point were analyzed in at least three different experiments giving a S.D. less than 5%.
Evaluation of apoptosis by DNA-flow cytometry
Apoptotic cell death was analysed by Annexin-V–FITC staining. Annexin-V–FITC binds to phosphatidylserine residues, which are translocated from the inner to the outer leaflet of the plasma membrane during the early stages of apoptosis. Labeling of apoptotic cells was performed using an Annexin-V kit (MedSystems Diagnostics, Vienna, Austria). Cells were seeded in 100 mm plates at the density of 1 × 106 for Cal-27 and of 4 × 105 for Hep-2, and then incubated 24 h in humidified atmosphere at 37°C. The next day, Cal-27 and Hep-2 cells were treated with 0.004 ng/ml and 0.009 ng/ml DTX concentrations, and with 0.42 µM and 0.47 µM TIP concentrations, respectively, on concurrent administration. Cells were incubated at 37°C for 72 h after the first treatment and then collected and centrifuged for 5 min at 1,300 rpm. Pellet was washed in PBS, incubated with Annexin-V–FITC in a binding buffer (provided by the manufacturer) for 10 min at room temperature, washed, and resuspended in the same buffer as described by the manufacturer. Analysis of apoptotic cells was performed by flow cytometry (FACScan, Becton Dickinson). For each sample, 2 × 104 events were acquired. Analysis was carried out by triplicate determination on at least three separate experiments.
Western blot analysis
KB and Hep-2 parental and clones, and Cal-33 and Cal-27 cells were grown for 48 h with or without DTX and/or TIP in the previously described experimental conditions. For cell extract preparation, cells were washed twice with ice-cold PBS/BSA, scraped, and centrifuged for 30 min at 4°C in 1 ml of lysis buffer (1% Triton, 0.5% sodium deoxycholate, 0.1 M NaCl, 1 mM EDTA, pH 7.5, 10 mM Na2HPO4, pH 7.4, 10 mM PMSF, 25 mM benzamidin, 1 mM leupeptin, 0.025 U/ml aprotinin). Equal amounts of cell proteins were separated by SDS–PAGE. The proteins on the gels were electro-transferred to nitrocellulose and reacted with the different MAbs.
Affinity precipitation of ras
Cells were cultured and treated with DTX and/or TIP and lysed as previously described. Then, 10 µl ras Binding Domain (RBD) conjugated to agarose (Cell Signaling Technology) were added to 1 mg of cell lysate and the resulting mixture was incubated o/n with gentle rocking at 4°C. The agarose beads were collected by microcentrifugation at 14,000g for 5 s and washed thrice with Mg2+ buffer. The agarose beads were boiled for 5 min in 2X Laemmli sample buffer and collected by a microcentrifuge pulse. The supernatants were run on 12% SDS–PAGE, then the proteins were electrotransferred on a nitrocellulose film. The nitrocellulose was incubated overnight with 1 µg/ml of anti- ras Mab, clone RAS10 and with a secondary Mab, a goat amouse HRP conjugated IgG, for 1 h. The film was washed with TBS/0.05% Tween 20 and detected by ECL, chemiluminescence's technique (Amersham). The detection of the expression of active ras was performed as previously described.
AKT kinase assay
Cells were cultured and treated with DTX and/or TIP and lysed as previously described. Then, 20 µl of IgG1 anti-Akt monoclonal antibody agarose beads immobilized (Cell Signaling Technology) was added to 1 mg of cell lysate and the mixture was incubated at 4°C for the night in gentle agitation. The resulting immunoprecipitates were then incubated for 30 min at 30°C with 1 µg GSK-3 fusion protein (Cell Signaling Technology) in the presence of 200 µM ATP and Kinase Buffer (25 mM Tris, pH 7.5, 5 mM β-glycerophosphate, 2 mM dithiotreitol, 0.1 mM sodium orthovanadate, 10 mM MgCl2). The reaction was terminated with the addition of 20 µl 2× SDS sample buffer. The supernatants were boiled for 5 min and electrophoresed by 12% SDS–PAGE and the protein electro-transferred on a nitrocellulose film. Phosphorylation of GSK-3 was detected using as probe an anti-Phospho-GSK-3α/β (Ser21/9) rabbit polyclonal antibody (diluted 1:1,000) and then with a secondary anti-rabbit HRPconjugated monoclonal antibody, (diluted 1:2,000). The film was washed with TBS 13-0.05% Tween 20 buffer and the specific reactivity was detected by chemiluminescence technique (Amersham) according to the manufacturer's instructions (Cell Signaling Technology).
Electroporation
KB and Hep-2 cells were detached from confluent 100 mm-dishes. Cells (100 × 106) were incubated in appropriate electroporation vials with 800 µl of electroporation buffer (20 mM HEPES, 137 mM NaCl, 5 mM KCl, 0.7 mM Na2HPO4 and 6 mM glucose) and 15 mg of the pcDNA3-HSP90-HA-tag plasmid in 20 mM HEPES. Then cells were electroporated at 250 V and at 975 mF for 6 s. The cells were incubated at 37°C. To isolate neomycin-resistant colonies, 5 lg/µl Geneticin (Invitrogen) selection was applied.
Hsp90 ATPase activity assay
In order to evaluate HSP90 ATPase activity in KB and Hep-2 parental and transfected cells, we used Novagen ProteoEnrich ATP-Binders Kit (Novagen, Madison, WI). This Kit contains an affinity resin in which ATP is linked via the γ-phosphate, so it is designed for the purification of proteins that bind nucleotides, included HSP90. Cell extracts were lysed as described before. The protein extracts, endogenous ATP depleted by dialysis, were added to the resin, previously purified and balanced according to the manufacturer's instructions. The resin was then washed with an appropriate buffer (150 mM NaCl, 20 mM MgCl2, 25 mM HEPES, NP-40 0.05% pH 7.2, 1 mM ATP, 1 mM AMP, 1 mM NADH pH 7, 1 mM DTT, 10 mM Na ortovanadato, 25 mM NaF, aprotinin 10 g/ml, PMSF 0.5 mM, leupeptin 10 g/ml), and samples were eluted with an elution buffer (25 mM HEPES, 20 mM ATP, 0.05% NP-40 pH 7.2, 1 mM ATP, 1 mM AMP, NADH pH 7, 1 mM DTT, 10 mM Na ortovanadato, 25 mM NaF, Aprotinin 10 g/ml, 0.5 mM PMSF, leupeptin 10 g/ml). Samples were concentrated, suspended in 20 µl 2X SDS sample buffer, boiled for 5 min, and electrophoresed by 12% SDS–PAGE. The protein were then electro-transferred on a nitrocellulose film. The active fraction of Hsp90 was determined using a monoclonal antibody anti-Hsp90 (diluted 1:1,000) and then a secondary polyclonal antibody (diluted 1:2,000) conjugated with horseradish peroxidase (HRP). The film was washed with TBS 13-0.05% Tween 20 buffer and the specific reactivity was detected by chemiluminescence technique (Amersham) according to the manufacturer's instructions (Cell Signaling Technology).
Statistical analysis
All data are expressed as mean ± SD. Statistical analysis was performed by ANOVA with Neumann–Keul's multiple comparison test or Kolmogorov–Smirnov where appropriate.
Results
Tipifarnib synergizes with Docetaxel in inducing growth inhibition on human epidermoid cancer cells
It has been reported that DTX induces a ras→Erk-dependent escape mechanism from apoptosis (Franceschi et al., 1997). Therefore, we have speculated that the inhibition of this pathway through the use of FTI TIP (Zarnestra®) could prevent this cytoprotective mechanism. As experimental in vitro model we have used the human head and neck squamous cancer cells (HNSC) KB, Cal-33, Cal-27, and Hep-2. We therefore sought, using the MTT cell viability assay, the drug concentrations able to induce the 50% growth inhibition (IC50) of the different HNSC cell lines at 72 h from the beginning of the treatment. The IC50 values found for DTX were 0.78 ng/ml for KB, 0.04 ng/ml for Cal-33 and Hep-2, and 0.02 ng/ml for Cal-27; the corresponding values found for TIP were 8 µM for KB, 2.5 µM for Cal-33 and Cal-27, and 5 µM for Hep-2. Therefore, we have evaluated the growth inhibition induced by different concentrations of DTX in combination with TIP at 72 h on HNSC cells. We have performed these experiments with MTT assay and the resulting data were elaborated with the dedicated software Calcusyn (by Chou and Talalay, see also Materials and Methods section). With this mathematical model synergistic conditions occur when the combination index (CI) is below 1.0. When CI is less than 0.5 the combination is highly synergistic. The experimental CI50s (combination index calculated for 50% cell survival by isobologram analysis) values reveal that the DTX/TIP combination resulted highly synergistic when both agents were used at equitoxic (50:50) concentrations for all cell lines and also in sequential administration for KB (DTX 24 h before TIP) and in concurrent administration for all the remaining cell lines. In fact, the CI50s were 0.25 for KB, 0.26 for Hep-2, 0.52 for Cal-33, and 0.69 for Cal-27 (Table 1). Therefore, the combined use of the two agents resulted highly synergistic on growth inhibition for both KB and Hep-2 and synergistic for the other cell lines. The synergism between the two agents in these experimental conditions is also demonstrated by the representation of the growth inhibition values for the different combinations used for the calculation of CIs (Fig. 1). In fact, in the different combinations the sum of the growth inhibition induced by the single agents was always less than the growth inhibition caused by the combination. Therefore, the combined use of the two agents was highly synergistic on the growth inhibition of both cell lines. Dose reduction index50 (DRI50) represents the magnitude of dose reduction obtained for the 50% growth inhibitory effect in combination setting as compared to each drug alone. In our experimental conditions, the DRI50s for DTX and TIP were 29 and 57 for KB, 10 and 6 for Hep-2, 6.4 and 1.4 for Cal-33, and 4 and 2 for Cal-27 cells, respectively (Table 1). These values were significant for all cell lines, since DRI > 1 values allow a decrease in the dosage, which leads to a considerable reduction of toxicity induced by the drugs.
| Cell lines | Combination ratio | CI50a | DRI50b | Interpetration |
|---|---|---|---|---|
| KB | 0.25 | DTX: 29 | Very strong | |
| DTX/TIP | TIP: 57 | Synergism | ||
| Hep-2 | 1:1 | 0.26 | DTX: 10 | Strong synergism |
| DTX/TIP | TIP: 6.0 | |||
| Cal-33 | 0.52 | DTX: 6.4 | Synergism | |
| DTX/TIP | TIP: 1.4 | |||
| Cal-27 | 0.69 | DTX: 4.0 | Synergism | |
| DTX/TIP | TIP: 2.0 |
- a CI50 were calculated for 50% cell survival (ED50) by isobologram analyses performed with CalcuSyn software.
- b DRI50 represents the order of magnitude (fold) of dose reduction obtained for ED50 effect in combination setting as compared to each drug alone.
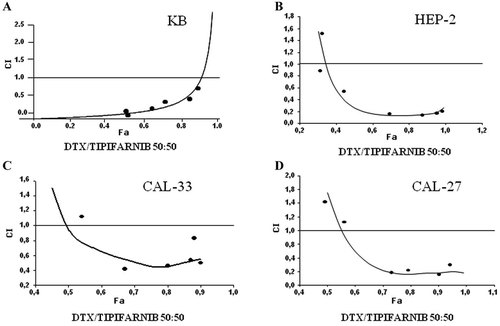
DTX and the TIP have a synergistic effect on KB, Hep-2, Cal-33, and Cal-27 cell growth inhibition. We have evaluated the growth inhibition induced by different concentrations of DTX and TIP at 72 h on KB, Hep-2, Cal-33, and Cal-27 cells. We have performed these experiments with MTT assay and the resulting data were elaborated with the dedicated software Calcusyn (by Chou and Talalay) as described in Materials and Methods section. In the figure, it is shown the isobologram analysis of the effects on growth inhibition of DTX and TIP combinations, used at equitoxic (50:50) concentrations for all cell lines and also in sequential administration (DTX 24 h before TIP) for KB (A) and in concurrent administration for the Hep-2 (B), Cal-33 (C), and Cal-27 (D) cells. CI, combination index. Each point is the mean of at least four different replicate experiments.
The synergistic effect of the DTX/TIP combination on growth inhibition is mediated by the induction of apoptosis
We have selected, for KB, Cal-33, Cal-27, and Hep-2 cell lines, two combinations of DTX and TIP that were highly synergistic at Calcusyn elaboration (0.047 ng/ml and 0.6 µM for KB, 0.006 ng/ml and 0.17 µM for Cal-33, 0.004 ng/ml and 0.42 µM for Cal-27, and 0.009 ng/ml and 0.47 µM for Hep-2, respectively) and we have then evaluated, after 48 h of sequential for KB or concomitant treatment for the remaining cell lines, the induction of apoptosis by FACS analysis after staining with Annexin V-FITC. As shown in Figure 2, we found a significant increase of mean fluorescence intensity (MFI) in cells treated with the drug combination, compared to untreated or treated with single drugs. In details, it was recorded about a 100% MFI increase for KB (A) and a 300% increase for the other cell lines (Cal-33, B; Cal-27, C; Hep-2, D) treated with combination while the single treatment with TIP induced from 20 to 35% MFI increase and with DTX alone no significant changes. In the same experimental conditions, Figure 3 shows cell cycle FACS analysis after propidium iodide (PI) incorporation in KB (A) and Cal-33 cells (B). We observed that low concentrations used for the single treatments with DTX and TIP did not induce significant changes in cell cycle distribution if compared to untreated cells. Conversely, the DTX/TIP combination caused about a 20% increase for KB and 30% for Cal-33 of the sub-G0/G1 peak, presumably due to apoptosis onset, and a parallel decrease of cells in G0/G1 phase. Moreover, we have found about a 60 and 40% decrease of pro-caspase 3 expression in Cal-27 (C) and Hep-2 (D) cells, respectively, with western blot assay after treatment with the combination (Fig. 3C,D). DTX alone was again not effective while TIP alone induced about a 15% decrease of pro-caspase 3 expression on both cell lines. The decrease of pro-caspase 3 expression suggested that caspase-3 was cleaved and, therefore, activated to trigger the apoptotic program.
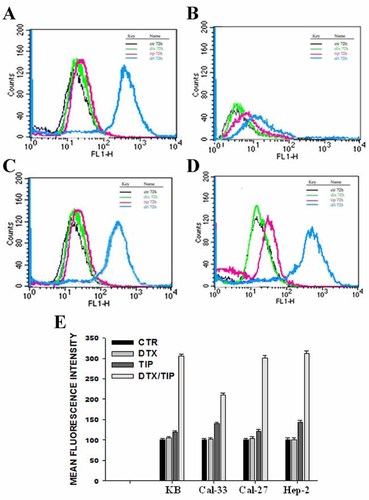
Effect of the DTX/TIP combination on the induction of apoptosis in KB, Cal-33, Cal-27, and Hep-2 cells. We have selected for KB (A), Cal-33 (B), Cal-27 (C), and Hep-2 (D) cells a combination of DTX and TIP that was highly synergistic by Calcusyn elaboration at 72 h. We have evaluated the apoptotic effects of these combinations by FACS analysis, after labeling with Annexin V-FITC. The employed DTX/TIP combinations were 0.047 ng/ml and 0.6 µM for KB, 0.006 ng/ml and 0.17 µM for Cal-33, 0.004 ng/ml and 0.42 µM for Cal-27, 0.009 ng/ml, and 0.47 µM for Hep-2, respectively. E: Mean Fluorescence Intensity (MFI) expressed as a percentage of untreated cells (CTR). The experiments were performed at least three different times and the results were always similar. Bars, s.e.'s
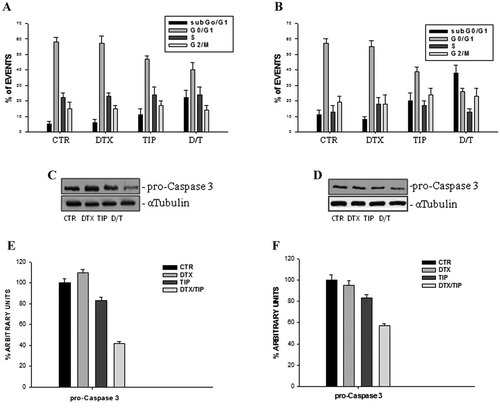
Effect of the DTX/TIP combination on the induction of apoptosis in KB, Cal-33, Cal-27, and Hep-2 cells. We have evaluated the distribution of KB (A) and Cal-33 (B) cells in the different phases of the cell cycle, after the treatments with DTX and/or TIP as previously described. This experiment was performed by FACS analysis after PI staining as described in Materials and Methods section. Cal-27 (C) and Hep-2 (D) cells have been cultured as described before. Cells were then processed for the determination of the expression of pro-caspase3, evaluated after blotting with an anti-pro-caspase3-specific Mab as described in Materials and Methods section. The house-keeping protein β-tubulin was used as loading control. CTR, untreated; DTX, DTX treated cells as described before; TIP, TIP treated cells as described before; D/T, DTX + TIP treated cells as described before. Each point is the mean of three different evaluations performed in at least three different experiments. Scan of the bands associated with pro-caspase-3 expression in Cal-27 (E) and Hep-2 (F) cells, normalized with the house-keeping protein, was performed with a dedicated software and the intensities of the bands were expressed as arbitrary units (%, mean of three different experiments). Bars, s.e.'s.
These data indicate that the synergistic combination DTX/TIP is able to induce apoptosis in the HNSCs.
Effects of DTX/TIP combination on ras-dependent survival signal transduction and on stress related proteins
On the basis of the synergistic interaction between DTX and TIP on both growth inhibition and apoptosis of HNSC cells, we have evaluated the effect of the single agents and the combination on the modulation of both expression and activity of ras, Erk-1/2, and Akt [KB (A, E), Cal-33 (B, F), Cal-27 (C, G), and Hep-2 (D, H) in Fig. 4]. In details, the combination induced a 200–300% decrease of ras activity in all cell lines without changes in ras protein expression (Fig. 4 A–D). The single agents induced no or significantly less effects on ras activity. In details, TIP alone induced about a 75 and 25% decrease of ras activity in Cal-33 and Hep-2, respectively, and no effects on the other cell lines. DTX induced a 20 and 35% decrease of ras activity on Cal-33 and Hep-2 cells, respectively, and no effects on the other cell lines. The combination induced similar effects also on the activity of the downstream Erk-1/2 without affecting their expression. The effects were again maximal on KB and Cal-27 cells (about 300% decrease) and DTX alone did not induce significant effects while TIP alone induced about a 45% decrease in Cal-33 cells. Similar effects were also recorded on the activity of the survival enzyme Akt. In details, the combination caused about a 200% decrease of Akt activity in KB and 400% in the other cell lines without affecting the total protein levels of the enzyme. Again DTX alone did not induce any significant changes in Akt activity while TIP alone caused about a 55% decrease in all cell lines (Fig. 4 A–D).
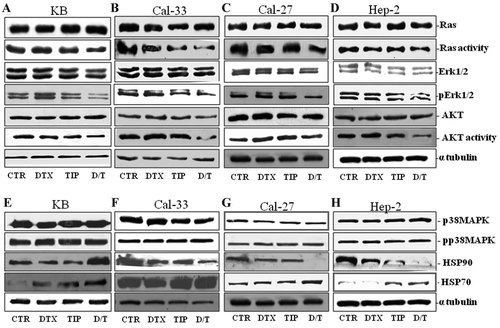
The DTX/TIP combination affects both the ras-dependent survival pathway and the expression of stress-related proteins. KB (A), Cal-33 (B), Cal-27 (C), and Hep-2 (D) cells were treated for 72 h with the following DTX/TIP combinations: 0.047 ng/ml and 0.6 µM for KB, 0.006 ng/ml and 0.17µM for Cal-33, 004 ng/ml and 0.42 µM for Cal-27, 0.009 ng/ml, and 0.47µM for Hep-2 cells. Thereafter, both the expression and activity of ras, Erk, and Akt were evaluated after blotting with specific antibodies, as described in Material and Methods section. Also the expression of the stress-related proteins HSP90, HSP70, and p38MAPK, was determinated with western blot assay. The house-keeping protein β-tubulin was used as loading control. CTR, untreated; DTX, DTX treated cells as described before; TIP, TIP treated cells as described before; D/T, DTX + TIP treated cells as described before. Each point is the mean of three different evaluations performed in at least three different experiments.
Thereafter, we have analyzed the modulation of a number of proteins involved in mechanisms of cellular response to stress. In particular, p38MAPK and its phosphorylated isoform did not undergo to substantial changes after treatment with the single agents or the combination in all cell lines (Fig. 4 E–H). On the other hand, the different treatments induced significant changes in the expression of the heat shock proteins HSP70 and HSP90, whose modulation deserves a special attention since they are involved in the regulation of the expression of proteins that control cell proliferation and survival. The single treatment with TIP induced a significant increase of HSP70 expression in all cell lines (250% in KB, 30% in Cal-33, 30% in Cal-27, and 200% in Hep-2 cells). DTX alone caused no significant effects on Cal-33, Cal-27, and Hep-2 cells while it induced about a 180% increase of HSP70 in KB cells. Conversely, the combination induced about a 100% increase of HSP90 expression in KB cells and about a 200% decrease of the same protein on all the remaining cell lines. The single agents had opposite effects on KB cells reducing HSP90 expression of about 50% if compared to untreated cells while on the other cell lines they caused similar but less pronounced effect than the combination. Increased levels of HSP70 made us to assume that the pharmacological combination played a role in inhibiting HSP90, since it is known that a correlation between HSP70 levels and the activation status of HSP90 exists (Shiboski et al., 2005). In particular, it was reported that HSP90 inhibition determines increased levels of HSP70. However, the biological meaning of the different modulation of HSP90 and the state of activation of the latter in KB and the other three cell lines remained to be clarified.
The HSP90 inhibitor, Geldanamycin, has a dose-dependent inhibitory effect on HNSC growth
The HSP90 inhibitor GA can induce anti-tumor effects competing with ATP for binding to HSP90 and inhibiting its activation. The final effect should be the reduction of the proteasome-dependent degradation of its targets involved in the control of cell proliferation and survival. Therefore, we have evaluated the effect induced by GA on HNSC growth inhibition (Fig. 5A). GA induced a time and dose-dependent growth inhibition without causing toxic effects. The IC:50 at 48 h was reached with 160 nM GA for KB, 640 nM for both Cal-33 and Cal-27, and 320 nM for Hep-2.
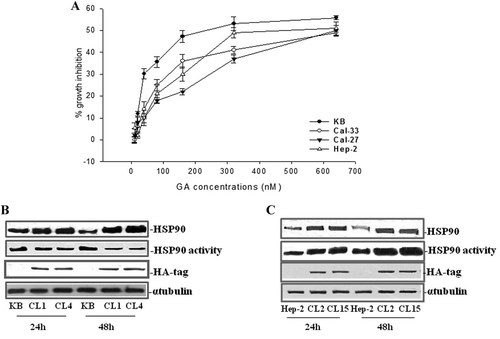
Dose-dependent inhibitory effect of GA on human epidermoid cancer cells and effects of HSP90 overexpression on HSP90 activation status in KB and Hep-2 cells. KB, Cal-33, Cal-27, and Hep-2 cells were seeded and treated with GA (A) at different concentrations as described in Materials and Methods section. Percentage of growth inhibition induced by different concentrations of GA after 48 h of treatment in human KB (●), Cal-33 (○), Cal-27 (▾), and Hep-2 (Δ) cells was evaluated with MTT assay. KB (B) and Hep-2 (C) parental and clones were seeded and then harvested after both 24 h and 48 h. Thereafter, cells were processed for western blot determination of HA-tag and of both the expression and activity of HSP90, after blotting with specific antibodies, as described in Material and Methods section. The house-keeping protein α-tubulin was used as loading control. Each point is the mean of three different evaluations performed in at least three different experiments.
These results suggested a different sensitivity of the different cell lines to GA that could be based upon a different basal activation status of HSP90. In fact, it was reported that cell susceptibility to GA is a function of HSP90 activity (Hammarstedt et al., 2006).
To define the functional role of HSP90 in HNSC and the biological significance of its different modulation in response to combined treatment, we overexpressed this protein in KB and Hep-2 cells. In particular, the sequence encoding HSP90 was cloned in the expression vector pcDNA3-HA tagged. Cells were transfected by electroporation and stable clones were selected as described in Materials and Methods section. The HSP90 expression was subsequently verified by SDS–PAGE by comparing total protein extracts prepared from parental or transfected clones. Among the obtained clones, around fifteen for each cell line (data not shown), we selected those that, through western blotting analysis, showed a more marked induction of HSP90, i.e., clones 1 and 4 for KB, and clones 2 and 15 for Hep-2 cells (Fig. 5 B,C). As additional evidence of the efficiency of transfection, we evaluated, even by western blotting, the expression of HA in the transfected cells (Fig. 5 B,C). Thereafter, we have correlated HSP90 levels with the activation status in the different clones. For this purpose we used an HSP90 ATPase activity assay. We surprisingly observed that whereas in the Hep-2 cells the increased expression of HSP90 was paralleled by an enhancement of its activity, opposite effects occurred in KB, where we observed a marked reduction of the activity of this protein when HSP90 was over-expressed (Fig. 5 B,C).
These results led us to make an important consideration about the correlation between the different modulation of HSP90 in KB and Hep-2, subsequently to treatment with the drug combination.
Study of the pharmacological interaction between DTX, TIP, and GA on parental and HSP90-overexpressing KB and Hep-2 cells
We evaluated the susceptibility of KB (A–C) and Hep-2 (D–F) cells over-expressing or not HSP90 to DTX (A,D), TIP (B,E) and GA (C,F) (Fig. 6). The dose-response curves obtained after 48 h by MTT assay showed for DTX the following IC50 values: 3.1 ng/ml for parental KB and 1.5 ng/ml for both KB clones, and 6.2 ng/ml for both parental and Hep-2 clones (Table 2). The IC50 values found for TIP were 20 µM for KB clones and 10 µM for parental KB and both parental and transfected Hep-2 cells (Table 2). Interestingly, the sensitivity of clones and parental cells to GA was different. In particular, IC50 values found for this drug were the following: 160 nM for parental KB and Hep-2 clones, and 320 nM for parental Hep-2 and KB clones (Table 2).
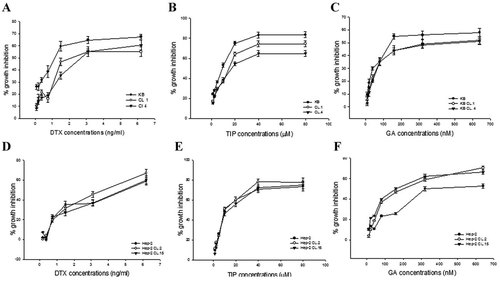
Dose-dependent inhibitory effect of DTX, TIP, and GA on human epidermoid cancer cells. KB (●), KB-clone1 (○), and KB-clone4 (▾) were seeded and treated with DTX (A), TIP (B), and GA (C) at different concentrations as described in Materials and Methods section. Percentage of growth inhibition induced by different concentrations of drugs after 48 h of treatment in human KB, clone1, and clone4 was evaluated with MTT assay. Hep-2 (●), Hep-2-clone2 (○), and Hep-2-clone15 (▾) were seeded and treated with DTX (D), TIP (E), and GA (F) at different concentrations as described in Materials and Methods section. Percentage growth inhibition induced by different concentrations of drugs after 48 h of treatment in human Hep-2, clone2, and clone15 was evaluated with MTT assay. Each point is the mean of at least four different replicate experiments. Bars, s.e.'s.
| Docetaxel (ng/ml) | Tipifarnib (µM) | Geldanamycin (nM) | ||
|---|---|---|---|---|
| KB | 3.1 | 10 | 160 | |
| KB-HSP90 CL.1 | 1.5 | 20 | 320 | |
| KB-HSP90 CL.4 | IC50a | 1.5 | 20 | 320 |
| Hep-2 | 6.2 | 10 | 320 | |
| Hep-2-HSP90 CL.2 | 6.2 | 10 | 160 | |
| Hep-2-HSP90 CL.15 | 6.2 | 10 | 160 |
- a IC50 represent the drug concentration able to induce the 50% growth inhibition.
These data were in complete agreement with previous findings on HSP90 expression; it is well known that GA is more effective in cells with increased HSP90 and, conversely, its inhibitory effect is less effective in cells with reduced HSP90.
Based on previous data showing that KB clones and Hep-2 clones, had revealed a different response than the corresponding parental cells to the drug treatments, we evaluated for these cells, by MTT assay, the effects of DTX/TIP, DTX/GA, and TIP/GA combinations on cell proliferation. The obtained data were then processed with the dedicated software Calcusyn, as previously described. In details, we found that the DTX/TIP combination produced synergistic effect in both parental and KB clones, when the two drugs were used in sequential treatment (DTX 24 h before TIP) at equitoxic concentrations. The corresponding CI50s were 0.65, 0.70, and 0.62, respectively, for parental KB, clone 1 and clone 4 (Table 3). Interestingly, the synergism was higher in Hep-2 clone 2 and 15 (CI50 = 0.31 and 0.44, respectively) than in parental counterpart (CI50 = 0.63) (Table 4). In addition, the DRI50s calculated for DTX and TIP were 3.8 and 3.4 for KB, 3.7 and 5.9 for clone 1, and 4.6 and 5.0 for clone 4, 3.6 and 3.0 for Hep-2, 4.8 and 4.4 for clone 2 and 6.8 and 5.4 for clone 15, respectively (Tables 3 and 4). In contrast, the DTX/GA combination was additive for all parental or transfected cells, when the two drugs were used in the sequential treatment (DTX 24 h before TIP) and at equitoxic concentrations (Tables 3 and 4). We also demonstrated that the TIP/GA combination was synergistic only for clones. In fact, when the two drugs were used in concomitant administration and at equitoxic concentrations, CI50 values were 0.67 for KB clone 1 and 0.70 for KB clone 4, 0.41 for Hep-2 clone 2 and 0.34 for Hep-2 clone 15. On the other hand, the CI50 values for KB and Hep-2 untransfected cells were 1.0 and 0.93, respectively (Tables 3 and 4).
| Combination ratio | CI50a | DRI50b | Interpetration | ||
|---|---|---|---|---|---|
| DTX/TIP | 0.65 | DTX 3.8 | Synergism | ||
| TIP 3.4 | |||||
| KB | DTX/GA | 1:1 | 1.0 | DTX 1.2 | Additivity |
| GA 2.2 | |||||
| TIP/GA | 1.0 | TIP 1.3 | Additivity | ||
| GA 4.0 | |||||
| DTX/TIP | 0.70 | DTX 3.7 | Synergism | ||
| TIP 5.9 | |||||
| KB-HSP90 CL.1 | DTX/GA | 1:1 | 0.80 | DTX 2.2 | Additivity |
| GA 2.8 | |||||
| TIP/GA | 0.67 | TIP 3.9 | Synergism | ||
| GA 7.8 | |||||
| DTX/TIP | 0.62 | DTX 4.6 | Synergism | ||
| TIP 5.0 | |||||
| KB-HSP90 CL.4 | DTX/GA | 1:1 | 0.90 | DTX 2.0 | Additivity |
| GA 2.4 | |||||
| TIP/GA | 0.70 | TIP 3.6 | Synergism | ||
| GA 7.0 |
- a CI50 were calculated for 50% cell survival (ED50) by isobologram analyses performed with CalcuSyn software.
- b DRI represents the order of magnitude (fold) of dose reduction obtained for ED50 effect in combination setting as compared to each drug alone.
| Combination ratio | CI50a | DRI50b | Interpetration | ||
|---|---|---|---|---|---|
| DTX/TIP | 0.63 | DTX 3.6 | Synergism | ||
| TIP 3.0 | |||||
| Hep-2 | DTX/GA | 1:1 | 0.83 | DTX 2.6 | Additivity |
| GA 2.2 | |||||
| TIP/GA | 0.93 | TIP 2.5 | Additivity | ||
| GA 2.1 | |||||
| DTX/TIP | 0.31 | DTX 4.8 | Strong Synergism | ||
| TIP 4.4 | |||||
| Hep-2-HSP90 CL.2 | DTX/GA | 1:1 | 0.91 | DTX 2.1 | Additivity |
| GA 2.2 | |||||
| TIP/GA | 0.41 | TIP 5.2 | Strong Synergism | ||
| GA 5.5 | |||||
| DTX/TIP | 0.44 | DTX 6.8 | Strong Synergism | ||
| TIP 5.4 | |||||
| Hep-2-HSP90 CL.15 | DTX/GA | 1:1 | 0.94 | DTX 2.8 | Additivity |
| GA 2.4 | |||||
| TIP/GA | 0.34 | TIP 6.6 | Strong Synergism | ||
| GA 6.4 |
- a CI50 were calculated for 50% cell survival (ED50) by isobologram analyses performed with CalcuSyn software.
- b DRI50 represents the order of magnitude (fold) of dose reduction obtained for ED50 effect in combination setting as compared to each drug alone.
These results suggested that the activity of a combination based on the use of a ras activity inhibitor (TIP) with HSP90 inhibitor (GA) could be useful in cells over-expressing HSP90 especially when the chaperone activity is also increased. On the other hand, it appears that GA does not potentiate the anti-cancer activity of DTX nor is synergistic with TIP when HSP90 expression/activity is not increased.
Effect of HSP90 overexpression on ras signalling in HNSC
We also investigated the effect of HSP90 overexpression on the modulation of proteins involved in the ras-dependent signal transduction pathway. Western blot analysis of KB (Fig. 7A) and Hep-2 (Fig. 7B), parental and transfected cells, cultured for 24 and 48 h, showed that the expression of total ras, Erk, and Akt proteins (and their derived activities) paralleled the activity of HSP90. In fact, reduced expression and activity of ras, Erk-1/2, and Akt were recorded in KB clones if compared to parental counterparts as in the clones the HSP90 activity was reduced. On the other hand, increased expression and activity of ras, Erk-1/2, and Akt were recorded in Hep-2 clones if compared to parental counterparts as in the clones the HSP90 activity was increased.
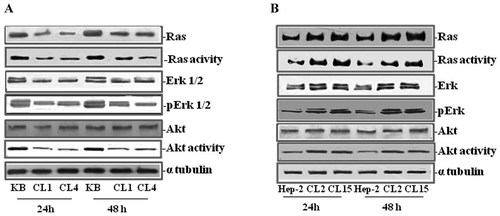
Effect of HSP90 overexpression on ras-dependent pathways. KB (A) and Hep-2 (B) parental and clones were seeded and then harvested after both 24 h and 48 h. Thereafter, cells were processed for western blot determination of both the expression and activity of ras, Erk, and Akt, as described in Material and Methods section. The house-keeping protein α-tubulin was used as loading control.
Taken together these results clearly demonstrate that HSP90 activity is essential for the stabilization of the active counterpart of the key regulators of proliferative and survival pathways in HNSC. Moreover, the strong synergism between GA and TIP recorded in Hep-2 clones could be explained on the basis of the upregulation of ras-dependent signal transduction pathways and on the consequent signaling addiction of the clones as compared to parental cells. On the other hand, less synergistic conditions were recorded on KB clones where the basal activity of the HSP90-ras-dependent pathways was lower.
Discussion
DTX, a member of taxane class, is one of the most potent antineoplastic agents commonly used in cancer therapies. Several studies on the anti-mitotic mechanism of taxanes have shown how the interaction of DTX with the tubulin dimers determines the triggering of a set of events that finally leads to apoptotic cell death (Wang et al., 1999). However, as we previously proved (Caraglia et al., 2005) and as reported in the literature (Wang et al., 1999), DTX is, at the same time, capable of inducing the activation of the ras→ Erk-mediated cell proliferation pathway. Therefore, we sought to enhance its antineoplastic action by adding, in combination, another drug that selectively inhibited this anti-apoptotic pathway.
TIP is one of the most potent and selective non-peptidomimetic competitive FTI (End, 1999), which in various studies both in vivo and in vitro have shown to have antitumor activity. Although TIP was initially considered a ras inhibitor, it is now clear that its antitumor activity is expressed on a variety of proteins that require post-translational modifications through the prenylation (Kelland et al., 2001). Ras stimulation induces the triggering of different proliferative and anti-apoptotic pathways and causes drastic changes in cell morphology. One of the most significant ras-mediated pathways is the MAPK (mitogen activated kinase) signaling, an intracellular cascade of three kinases (MAPKKK, MAPKK, and MAPK), activated in succession by phosphorylation events (McCubrey et al., 2011; Whelan et al., 2012). Another important ras-mediated pathway is the anti-apoptotic Akt/PKB signaling (Zhou et al., 2000; von Gise et al., 2001). The Akt-mediated protection from apoptosis may be mediated at mitochondrial level, as Akt is involved in regulating Bcl-like proteins (Kuo et al., 2001; Liu et al., 2001; Mitsui et al., 2001).
Given the importance in inhibiting ras farnesylation in order to enhance the antitumor activity of Taxanes, we evaluated the effects produced by TIP/DTX combination on growth inhibition and apoptosis in HNSC.
In this light, we demonstrated that the two agents, used in combination at equitoxic concentrations, were strongly synergistic to the Calcusyn elaboration. This provided an important input about the opportunity to employ DTX/TIP combination in the treatment of advanced HNSC. Moreover, we demonstrated that the highly synergistic combinations produced also an enhancement of the pro-apoptotic effects in HNSC when compared with the single agents. According to our previous results (Caraglia et al., 2005) and to literature (Wang et al., 1999), we have also shown that the DTX-induced Erk phosphorylation was significantly inhibited by the combined treatment in KB, as well as in the remaining cell lines. The DTX/TIP combination was also able to reduce ras and Akt activation showing, therefore, its effectiveness in hindering the two important ras-mediated escape machanims from apoptosis. In addition, the analysis of stress mediator levels revealed that the expression of HSP70, except for Cal-33 cells in which it remained unchanged, was substantially increased in HNSC cells treated with the combination, compared to untreated cells. On the other hand, the HSP90 levels were strongly increased in KB and, on the contrary, reduced in the other cell lines. It is known that, predominantly in tumor cells, HSP90 is only slightly expressed as free form, whereas largely participates in the formation of a multi-molecular complex named multi-cheperone (Xu et al., 2003). When HSP90 is in the ADP-linked inactive form, it interacts with HSP70 and HSP60Hop, and oversees the ubiquitination and subsequent proteasome-dependent degradation of a series of “client” proteins involved in survival, differentiation, and cell cycle regulation. Conversely, the ATP linked multi-chaperone activated form, characterized by the assembly of HSP90 with p50CDC37 and p23, favors the maintenance of “client” proteins in their native structure preventing ubiquitination and subsequent degradation (Powers and Workman, 2006). Therefore, we hypothesized that DTX/TIP combination played a role in the maintenance of multi-chaperone in the inactive form, directing cells towards the degradation of the proteins involved in the proliferative processes. It is, indeed, described that the increase of HSP70 levels represents a biomarker of HSP90 inhibition (Eloa and Kaarnirantab, 2005; Shu et al., 2005). It remained to be understood the biological significance of the HSP90 modulation in response to treatment with the combination in HNSC, and especially if it was possible to relate the latter with the state of activation of that protein. For this purpose, we stably transfected KB and Hep-2 cells, with the pcDNA3-HSP90-HA-tag plasmid. However, the overexpression of HSP90 was differently correlated with the activity of the multichaperone complex. In fact, we selected two clones from KB and other two from Hep-2 but the activity of HSP90 was increased in Hep-2 and decreased in KB cells if compared to parental cell lines. This result should be interpreted on the basis of the concomitant profile of expression of the other HSPs whose binding to HSP90 influences its activation status such as HSP70 (Eloa and Kaarnirantab, 2005; Shu et al., 2005). In this light, HSP90-transfected KB clones were more resistant to GA treatment if compared to parental cells while HSP90-transfected Hep-2 clones were, on the contrary, more sensitive to the growth inhibition induced by GA than the non transfected counterpart. These results can be explained by the evidence that HSP90 inhibition is more effective where the protein is more active (Budillon et al., 2005). Subsequently, we have evaluated the effects of GA in combination with either DTX or TIP in the treatment of Hep-2 and KB clones and parental cells. We have found that the effect of GA in combination with DTX on cell growth inhibition was only additive on both KB and Hep-2 clones and parental cells. On the other hand, the combination of GA with TIP induced only an additive effect on both parental KB and Hep-2 cell lines, but it became synergistic in HSP90 overexpressing KB clones and highly synergistic in HSP90 overexpressing Hep-2 clones where HSP90 activity was increased. The synergistic activity of TIP and DTX was also confirmed in our experimental conditions. However, the combination was strongly synergistic in Hep-2 clones where HSP90 activity was increased. These data are not surprising in our opinion because the increase in HSP90 activity induced an increase of the ras→dependent survival signaling that, in turn, caused a pharmacological addiction of cancer cells to the ras→mediated survival pathway. Therefore, the inhibition of this pathway with a ras inhibitor such as TIP caused a strong impairment of cancer cell survival functions determining a highly synergistic effect on cell growth inhibition.
These results suggested that the activity of a combination based on the use of a ras activity inhibitor (TIP) with HSP90 inhibitor (GA) could be useful in cells over-expressing HSP90 especially when also the chaperone activity is also increased. On the other hand, it appears that GA does not potentiate the anti-cancer activity of DTX nor is synergistic with TIP when HSP90 expression/activity is not increased. These results are in agreement with our previous findings suggesting that an increase of the ras→dependent pathways makes the cells more sensitive to the inhibition of this pathway with FTI such as TIP (Caraglia et al., 2005; Caraglia et al., 2007). Finally, the study of the modulation of both main signal transduction ras-dependent pathways, allowed us to demonstrate how, in agreement with previous data (Zhang and Burrows, 2004; Hashiramoto et al., 2012), even for KB and Hep-2, HSP90 plays a pivotal role in the stabilization of the activated status of the proteins involved in proliferative and survival pathways.
In conclusion, our experimental evidence about the correlation between the HSP90 levels and its activation status, confirmed the hypothesis that the modulation of HSP90 induced by the DTX/TIP combination was, for both KB and Hep-2 cells, in any case, an indicator of decreased activity of the multichaperone complex. The combination of GA with DTX was not able to induce any synergistic effects on cell growth inhibition of HNSC cells independently from HSP90 expression and activation status. On the other hand, the combination of GA with TIP is potentially able to give synergistic effects on the growth arrest of HNSCC cells above all when HSP90 is overexpressed and even more when it is over-activated. The basal activation status of the multichaperone complex could, therefore, represent a resistance mechanism to the antiproliferative activity of chemotherapy agents such as DTX commonly used in the treatment of HNSCC. Moreover, these data, in our opinion, open a new scenario in the treatment of advanced HNSCC based upon the combination of HSP90 inhibitors with isoprenylation inhibitors such as TIP depending upon the evaluation of HSP90 expression in the primary tumour samples.
Since the inactivation of HSP90 implies the simultaneous abrogation of multiple signaling pathways regardless of the mutational status of the substrates, it represents an undeniable advantage for the treatment of tumoral diseases, such as head and neck tumors, characterized by multiple mutational events.
Finally, the HSP90 levels, increased in KB and decreased in Hep-2, could represent an indicator of cell response to treatment with the DTX/TIP combination.
Acknowledgements
This work was dedicated to the beloved memory of Prof. Alberto Abbruzzese who was our mentor and high scientific contributor. This work was supported by the Italian Ministry of Education and Research (MIUR) (2009EHW394 and RBAP11884M_004) and by Laboratori Pubblici “Hauteville” from Regione Campania.