Involvement of CaMK-IIδ and gelsolin in Cd2+-dependent cytoskeletal effects in mesangial cells
Abstract
Cadmium is a toxic metal with pleiotropic effects on cell death and survival. The mesangial cell is particularly responsive to Cd's effects on kinase signaling pathways and cytoskeletal dynamics. Here we show that CaMK-II is a participant in the cytoskeletal effects of Cd2+. A major mesangial cell isoform, CaMK-IIδ, was identified in pellets of DNase I pull-downs and cytosolic immunoprecipitates of G-actin. CaMK-IIδ was also present in Triton X-100-insoluble cytoskeletal preparations and translocated to the cytoskeleton in a concentration-dependent manner in Cd-treated cells. Translocation was suppressed by KN93, an inhibitor of CaMK-II phosphorylation. In vitro actin polymerization studies indicated that recombinant CaMK-IIδ sequestered actin monomer. Cytoskeletal preparations from Cd-treated cells decrease the rate of polymerization, but KN93 co-treatment prevents this effect. Over-expressed CaMK-IIδ also translocated to the cytoskeleton upon Cd exposure, and this was prevented by KN93. Conversely, siRNA silencing of CaMK-IIδ increases the effect of cytoskeletal extracts on actin polymerization, and abrogates the effect of Cd. The actin capping and severing protein, gelsolin, translocates to the cytoskeleton in the presence of Cd2+, dependent upon the phosphorylation of CaMK-II, and is recovered together with actin and CaMK-IIδ in G-actin pull-downs and F-actin sedimentation. Translocation is accompanied by generation of a 50 kDa gelsolin fragment whose appearance is prevented by KN93 and CaMK-IIδ silencing. We conclude that cytoskeletal effects of Cd in mesangial cells are partially mediated by Cd-dependent activation of CaMK-IIδ, binding of CaMK-IIδ and gelsolin to actin filaments, and cleavage of gelsolin. J. Cell. Physiol. 228: 78–86, 2013. © 2012 Wiley Periodicals, Inc.
Cadmium is a toxic metal of environmental importance that adversely affects not only human health (Fowler, 2009; Joseph, 2009; Nordberg, 2009) but that of wildlife (Larison et al., 2000). While its effects as an environmental pollutant in industrialized areas are well recognized (Hotz et al., 1999), contamination of groundwater from intensive agricultural practices (Bandara et al., 2010) and the growing industry of recycling e-waste in developing countries (Schmidt and Hall, 1998; Zheng et al., 2008) are presenting new concerns. Among the various effects of Cd on cultured cells, we have shown Cd-dependent activation of kinases such as Erk, p38, Jun kinase, and Ca2+/calmodulin-dependent protein kinase II (CaMK-II) in renal mesangial cells (Liu and Templeton, 2008; Xiao et al., 2009). Additionally, Cd causes cytoskeletal disruption in mesangial cells by affecting the balance between actin polymerization and depolymerization (Wang and Templeton, 1996b; Apostolova et al., 2006), and inhibition of CaMK-II is partially protective against this effect (Liu and Templeton, 2010). The present study was undertaken to elucidate the Cd-dependent role of CaMK-II in mesangial cell cytoskeletal dynamics.
The involvement of CaMK-II in regulating actin dynamics is complex and probably dependent on cell type. Certain isoforms of CaMK-II co-localize with the actin cytoskeleton and regulate cytoskeletal and focal adhesion dynamics (Caran et al., 2001; Easley et al., 2008; Lin and Redmond, 2008; Seward et al., 2008; Sanabria et al., 2009). The CaMK-II isoforms α and β are homologous, but an insertion of about 30 amino acids in a variable region of the β isoform creates a domain that targets it for actin binding. Thus, CaMK-IIβ regulates actin assembly by binding to G-actin monomer (preventing polymerization) and bundling F-actin (stabilizing the filament) (Lin and Redmond, 2008; Sanabria et al., 2009). F-actin binding is disrupted when CaMK-IIβ is activated by binding to Ca2+/calmodulin, or by phosphorylation. However, inactivation of the kinase activity of CaMK-IIβ does not affect actin binding (Lin and Redmond, 2008). The major CaMK-II isoforms expressed in vascular smooth muscle cells (Jones et al., 2007) and smooth muscle-like mesangial cells (unpublished observations) are designated γ and δ. The γ isoform is primarily involved in regulating contractility, whereas the δ isoform is implicated in signaling events that control cell migration and proliferation (Jones et al., 2007).
The CaMK-IIδ isoform has also been reported to interact directly with the actin cytoskeleton, potentially regulating actin dynamics. It possesses an F-actin targeting domain between the central variable and association domains (Caran et al., 2001). There are several variants of CaMK-IIδ arising from alternative splicing of common and unique exons in the variable domain; CaMK-IIδC is the simplest, containing only the two common exons (30 residues), while δE has one additional unique exon of 14 residues. CaMK-IIδE co-localizes with F-actin in the perinuclear region and at cellular extensions in 3T3 fibroblasts. Constitutively active CaMK-IIδE has been found to induce F-actin-rich extensions either by inducing polymerization or by stabilizing the resultant actin filament (Caran et al., 2001). Overexpression of CaMK-IIδC results in its distribution throughout the cell, but with perinuclear enrichment and significant concentration at the leading edge of migrating 3T3 cells. Both inhibition and constitutive activation of CaMK-II inhibit cell motility by excessive stabilization or destabilization of focal adhesions (Easley et al., 2008). Perinuclear enrichment of endogenous CaMK-IIδC is also characteristic of vascular smooth muscle cells, and active CaMK-IIδC (phosphorylated at Thr287) is enriched at the leading edge of migrating cells. It does not, however, colocalize with cortical actin filaments or stress fibers (Mercure et al., 2008).
We found that Cd2+ increased both CaMK-II phosphorylation/activation and cytoskeletal disruption in rat mesangial cells (RMC), while inhibition of CaMK-II prevented Cd-induced disruption of the cytoskeleton and protected against disruption of focal adhesions (Liu and Templeton, 2007; Liu and Templeton, 2010; and unpublished data). As CaMK-IIδ is a major isoform in RMC, we investigated the mechanism of CaMK-IIδ involvement in Cd-induced actin disruption.
Methods
Cell culture
Rat mesangial cell cultures were established as previously described (Wang and Templeton, 1996a), grown in RPMI-1640 medium with 10% FBS in a humidified atmosphere of 5% CO2 at 37°C, passaged by trypsinization, and used between passages 5 and 15. For most experiments, cells were allowed to grow overnight before starvation in 0.2% FBS for 48 h to render them quiescent. During CdCl2 treatment, this medium was replaced with serum-free medium, and parallel serum-free controls were included.
Western blotting
Cytosol and cytoskeletal-enriched fractions were prepared as described (Wang and Templeton, 1996b) with minor modifications. Cells were lysed with buffer A (10 mM Tris·HCl, pH 7.4, with 2 mM MgCl2, 138 mM KCl, 25 mM NaF, 1 mM Na3VO4 with proteinase inhibitors and 0.2% Triton X-100). The lysates were centrifuged at 10,000g for 15 min, and supernatants were collected as cytosol. The pellets were resuspended in buffer G (5 mM Tris·HCl, pH 8.0, with 0.2 mM CaCl2 and 200 µM ATP), sonicated, and spun at 10,000g for 5 min. The supernatants were referred to as the cytoskeletal fraction. Equal amounts of protein were mixed with 5 × SDS loading buffer, heated for 5 min at 95°C and resolved on 8 or 10% SDS–PAGE gels. Proteins were transferred to nitrocellulose membranes for western blotting. The antibodies and their dilutions were anti-phospho-Thr287-CaMK-II (1:2000, BioSource, Camarillo, CA), anti-α-smooth muscle actin (1:10,000), and anti-Flag (1:10,000) from Sigma (St. Louis, MO), polyclonal anti-gelsolin (c-20) (1:3,000), and anti-His (1:4,000) from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-CaMK-IIδC (1:2,500) was provided by Harold Singer (Albany Medical College, NY).
DNase I-Sepharose pull-down assay
Ten milligrams of DNase I (Sigma) was coupled to 400 mg CNBr-activated Sepharose 4 fast flow according to manufacturer's instructurions (GE Healthcare, Mississauga, ON) was pre-equilibrated in Buffer A and incubated with 300 µg of cytosolic extracts overnight at 4°C. The beads were then washed with 50 mM Tris·HCl, pH 7.4, containing 100 mM NaCl, 0.3 M MgCl2, and 1 mM EGTA for 15 min at room temperature. This incubation with 0.3 M MgCl2 was included to depolymerize any actin filaments still associated with the beads. The beads were washed twice more with cold PBS. The bound proteins were separated by SDS/PAGE and probed with polyclonal anti-gelsolin and anti-CaMK-IIδC antibodies.
F-actin co-sedimentation assay: F-actin and associated proteins were sedimented by ultracentrifugation according to Chan et al. (2007). Cells were suspended in a detergent-free buffer (2 mM Tris·HCl, pH 7.4, with 1 mM EGTA, 0.2 mM ATP, 0.2 mM MgCl2, 0.2 mM dithiothreitol, and 2 mM phenylmethylsulfonyl fluoride), containing 1 µM phalloidin to stabilize F-actin. Cell lysates were suspended several times with a narrow-gauge pipette tip and equal amounts of total proten were sedimented (100,000g, 1 h at 4°C). The pellet, which contained actin filament complexes, was dissolved in 2 × SDS loading buffer, separated by SDS–PAGE and blotted with anti-gelsolin, anti-CaMK-IIδC, and anti-α-smooth muscle actin antibodies.
CaMK-IIδE-pcDNA3 constructs for RMC transfection
CaMK-IIδE-pcDNA3 constructs (γ/δ chimeric) encoding wild type CaMK-II and a constitutively active CaMK-II (T287D) mutant were obtained from Robert Tombes (Virginia Commonwealth University) (Caran et al., 2001; Seward et al., 2008). These constructs contain Flag and monomerized EGFP tags at their N-termini, which are followed by the 316-residue catalytic, autoinhibitory and calmodulin binding domains of the γ isoform (which are highly conserved among CaMK-II isoforms), and the 44-residue variable domain and 130-residue association domain of CaMK-IIδ. The sub-cellular targeting domain of CaMK-IIγ/δ is between the central variable and association domains (Caran et al., 2001). This γ/δ chimeric construct showed similar localization as reported for full-length CaMK-IIδE expressed in 3T3 fibroblasts (Caran et al., 2001). Here, we refer to these chimeric constructs as CaMK-IIδE. The CaMK-II cDNA insert was removed by digestion with BspE1 and BamHI and blunt-ended with T4 DNA polymerase (New England Biolabs, Pickering, ON) to make the empty vector for transfection control. Localization of the CaMK-IIδE isoform is unaffected by the tags, and has been well documented (Caran et al., 2001).
Transfection experiments
1 × 106 cells were seeded in 10-cm plates overnight and at 60–70% confluence cells were transfected with 8 µg CaMK-IIδE-pcDNA using 16 µl lipofectamine 2000 (Invitrogen, Carlsbad, CA) for 48 h as recommended by the manufacturer. Cells were starved for 24 h and followed by up to 6 h Cd treatment. Transfection efficiency was followed by western blotting with anti-Flag antibody and EGFP fluorescence at λex = 485 nm and λem = 520 nm.
Immunofluorescence
Cells were seeded in 24-well plates on 12 mm coverslips and grown overnight, then starved for 48 h in 0.2% FBS. After treatment with CdCl2, cells were washed with cold PBS twice and fixed in 4% paraformaldehyde for 10 min at room temperature. Cells were permeabilized with 0.5% Triton X-100 in Hepes buffer, pH 7.4, for 5 min and then incubated with a solution of PBS containing 5% BSA for 1 h to block nonspecific binding. Cells were stained with rhodamine-phalloidin (1:100 dilution; Molecular Probes, Eugene, OR) for 2 h. After two washes with PBS containing 0.2% BSA and one wash with BSA, the samples were mounted in mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlington, ON).
Purification of recombinant CaMK-IIδE
The CaMK-IIδE insert was excised from pcDNA3 with BspE1 and BamHI, subcloned into the NdeI and BamHI sites of ProEX (Lu et al., 2003), and verified by sequencing (ACGT Corp., Toronto, ON). The expressed protein carries an N-terminal hexahistidine tag. E. coli bacterial suspension (100 ml) was grown at 37°C to an optical density at 600 nm of 0.8–1.0, induced with 1 mM IPTG at 30°C for 2 h, and harvested by centrifugation. The cell pellet was resuspended in bacterial lysis buffer (0.2 M NaCl; 25 mM Tris·HCl, pH 7.5; 20% glycerol; 2 mM β-mercaptoethnal; 10 mM imidazole; 10 µg/ml each of aprotinin, leupeptin, pepstain A; and 1 mM PMSF) and sonicated on ice. The cell lysate was centrifuged at 100,000g for 30 min at 4°C and the supernatant was purified under native conditions using a 0.5 ml column of Ni-NTA agarose beads (Qiagen, Toronto, ON), washed three times with 5 ml of Ni-NTA buffer [0.5 M NaCl, 25 mM Tris·HCl (pH7.5), 20% glycerol, 10 mM imidazole, and 1 mM PMSF] and three times with 5 ml of Ni-NTA buffer containing 20 mM imidazole. CaMK-II was eluted sequentially with Ni-NTA buffer containing 50 mM and 100 mM imidazole and stored at −80°C. Before each use, samples were thawed and spun at 4°C at 14,000 rpm in a microcentrifuge for 10 min, to remove aggregates.
Recombinant CaMK-IIδE (5 µg) was resolved on SDS–PAGE and stained with Coomassie Blue. MALDI confirmed that the major 57 kDa band was CaMK-IIδ. A 35 kDa band, probably arising from proteolysis or premature termination, was also CaMK-IIδ sequence. Both bands were recognized by anti-His antibody. A similar result has been reported in an earlier study with CaMK-IIα (Waxham et al., 1990). Autonomous and total CaMK-II activities were measured as described previously (Miralem and Templeton, 1998). Briefly, 100 ng of recombinant protein in 5 µl of 5 mM Tris·HCl, pH 8.0, containing 0.2 mM CaCl2 and 200 µM ATP was incubated with 25 µl of CaMK-II autonomous activity assay buffer (50 mM Hepes, pH 7.5, with 10 mM MgCl2, 100 µM ATP, 1 mM EGTA, 0.02 mM autocamtide peptide, and 1.25 µCi [γ-32p]ATP). For total CaMK II activity, EGTA was replaced with 4 mM CaCl2 and 2 µM calmodulin. Reaction was done at 30°C for 3 min and stopped by 5% TCA. The reaction mixture was spotted onto p81 phosphocellulose paper, washed several times with 75 mM H3PO4, and counted by liquid scintillation. Specific activity of wild type rCaMK-IIδE was approximately 0.1 µmol/min/mg; autonomous activity of the T287D mutant was 0.25 µmol/min/mg, somewhat lower than the specific activity of recombinant CaMK-IIα or CaMK-IIβ isolated from E. coli (1 µmol/min/mg) as reported by Hagiwara et al. (1991).
LC-MS and MALDI
In-gel trypsin digestion was performed on Coomassie blue or silver-stained bands excised from polyacrylamide gels. Coomassie blue-stained bands were destained by sequential washing with 50 mM ammonium hydrogen carbonate and acetonitrile. Silver-stained bands were destained by incubating with 30 mM potassium ferricyanide and 100 mM sodium thiosulfate, following sequential washing with 50 mM ammonium hydrogen carbonate and acetonitrile. The destained gel pieces were reduced with 10 mM dithiothreitol for 30 min at 56°C, and alkylation with 100 mM iodoacetamide for 15 min in the dark at room temperature. Gel pieces were shrunk with 50% acetonitrile and 25 mM ammonium bicarbonate and digested with trypsin (6.6 ng/µl in 50 mM ammonium bicarbonate and 5 mM CaCl2) overnight at 37°C. The peptides were extracted sequentially with 25 mm ammonium hydrogen carbonate, 5% formic acid, and acetonitrile. Pooled extracts were evaporated by SpeedVac and dissolved in 0.1% formic acid for LC-MS without further clean up. For MALDI analysis, samples were further cleaned by C18 Ziptip pipette tips (Millipore; Bedford, MA). Proteins were identified by Mascot peptide mass fingerprinting allowing one missed cleavage and an initial mass tolerance of 50 ppm for MALDI, and by Scaffold 2 proteome software for LC-MS.
Actin polymerization assay
Actin polymerization was analyzed using a kit from Cytoskeleton Inc. (Denver, CO) based on an increase in pyrene flourecence (FLUOstar OPTIMA microtiter plater reader) with λex = 355 nM and λem = 405 nM. For recombinant protein assays, pyrene-labeled G-actin (1 µM) in 5 mM Tris·HCl, pH 8.0, with 0.2 mM CaCl2 and 200 µM ATP was incubated with various concentrations of Ni-NTA-purified CaMK-IIδE for 15 min at room temperature. Polymerization was initiated by addition of polymerization buffer (final concentration: 12.5 mM KCl, 0.5 mM MgCl2, and 0.25 mM ATP). For cell-derived cytoskeletal fractions, 1.5 µM pyrene-labeled actin was incubated with 10 µg of cytoskeletal protein for 10 min at room temperature, and polymerization was initiated by adding polymerization buffer (final concentration: 25 mM KCl, 1.0 mM MgCl2, and 0.5 mM ATP). The change of pyrene fluorescence was measured over 90 min at 37°C for cytoskeletal preparations and at room temperature for recombinant proteins.
CaMK-IIδC siRNA silencing
-
CaMK-IIδ-303:
-
(F) 5′-GGUGAGAAGAUGCAUGAAATT-3′;
-
(R) 5′-UUUCAUGCAUCUUCUCACCTT-3′.
-
-
CaMK-IIδ-510:
-
(F) 5′-CGAACUCUUUGAAGACAUATT-3′;
-
(R) 5′-UAUGUCUUCAAAGAGUUCGTT-3′.
-
-
CaMK-IIδ-577:
-
(F) 5′-CAGAUUCUAGAGAGUGUAATT-3′;
-
(R) 5′-UUACACUCUCUAGAAUCUGTT-3′.
-
-
Negative (scrambled) control:
-
(F) 5′-GCGACGAUCUGCCUAAGAUTT-3′;
-
(R) 5′-AUCUUAGGCAGAUCGUCGCTT-3′.
-
Ten nanomole of siRNA was either forward- or reverse-transfected into RMC in 10 cm culture plates using 20 µl of Lipofectamine RNAiMAX (Invitrogen) for 48 h as recommended by the manufacturer. Cells were starved for 24 h and followed by up to 6 h CdCl2 treatment. Silencing efficiency was followed by anti-CaMK-IIδC western blotting and kinase activity. Five microgram of cell lysate was used for the kinase activity assay, which was performed as described above.
Statistical analyses
Multiple measurements are reported as mean ± S.D. with hypothesis testing for significance reported as a P-value for measurements derived from an unpaired Student's t-test for single comparisons, or by one-way ANOVA followed by Tukey's test when multiple treatment comparisons are considered. Calculations were performed with InStat Software (GraphPad, San Diego, CA).
Results
CaMK-IIδC interacts with monomeric G-actin
Cell cytosolic fractions immunoblotted with anti-CaMK-IIδC antibody show a single band below 55 kDa that appears to be unaffected either by Cd or by the CaMK-II inhibitor, KN93 (Fig. 1A). The same band appears in a pellet from a G-actin pull-down with DNase I. The band was confirmed to be CaMK-IIδC by LC-MS (four peptides identified, 14% coverage). In a similar experiment, immunoblotting with anti-gelsolin antibody (Fig. 1B) a strong full-length gelsolin band is observed at 95 kDa in the pull-down pellet, suggesting that both CaMK-IIδC and gelsolin co-precipitate with G-actin. The intensity of the gelsolin band is independent of Cd and unaffected by the inhibitor of CaMK-II activation, KN93. An additional band recognized by anti-gelsolin antibody is seen at about 50 kDa (Fig. 1B). LC-MS confirmed that both 95 kDa (seven peptides identified, 12% coverage) and 50 kDa (six peptides identified, 13% coverage) bands contained gelsolin sequence. The occurrence of the 50 kDa gelsolin fragment in the pellet is markedly decreased by Cd and restored by KN93 (Fig. 1B).

Interaction of CaMK-IIδC and gelsolin with G-actin. A: Western blot of CaMK-IIδC in cytosolic extracts (first three lanes) and in pellets from G-actin pull-down with DNase I as described in Methods (last three lanes). Cells were held in serum-free medium for 3 h (SF) or in serum-free medium with 40 µM CdCl2 (Cd). Cells were also held for 3 h in serum-free medium with 40 µM CdCl2 in the presence of 10 µM KN93, following a 1 h pretreatment with KN93 (+KN). The experiment was repeated once with identical results each time. B: The blot in (A) was stripped and western blotted for gelsolin. The 95 kDa full-length protein and 50 kDa fragment identified in the text are indicated with arrows. C: Western blot of α-smooth muscle actin on the same membrane as (A) and (B), included as a loading control. D: Cell extracts were immunoprecipitated with anti-α-smooth muscle actin antibody and the resuspended pellet was subjected to western blotting of CaMK-IIδC (first three lanes). Cytosolic extract is included in the last three lanes for comparison. Similar results were obtained in three independent experiments. SF, Cd, and +KN treatments are as in (A).
The interaction of CaMK-IIδC with G-actin was confirmed by α-smooth muscle actin immunoprecipitation (Fig. 1D). CaMK-IIδC is found in the pellet and, as in pull-down by DNase I, is not changed by Cd.
CaMK-IIδC interacts with filamentous F-actin
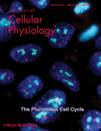
CaMK-IIδC was associated with a Triton X-100-insoluble cytoskeletal fraction separated from cytosol by centrifugation. Although Cd does not significantly deplete the cytosol of CaMK-IIδC, western blotting shows a dose-dependent increase of the protein in the cytoskeletal fraction after cells are treated with Cd (Fig. 2A). KN93 significantly decreases Cd-induced translocation of CaMK-IIδC to the cytoskeletal fraction (Fig. 2B). An increase in phosphorylation of total CaMK-II occurs in both cytosolic and cytoskeletal fractions of Cd-treated cells and is inhibited by KN93 (Fig. 2B). We also verified that CaMK-IIδC is associated with F-actin in an ultracentrifugation co-sedimentation assay according to Chan et al. (2007). Again, its cytoskeletal translocation is increased by Cd (Cd vs. serum-free control, P < 0.01) and significantly prevented by KN93 (Cd vs. Cd + KN, P < 0.05) (Fig. 2C). Gelsolin likewise, and particularly its 50 kDa fragment, is co-sedimented with F-actin, and this association is decreased by KN93 (Fig. 2D).
Association of CaMK-IIδC with F-actin. A: Western blot of CaMK-IIδC in cytosolic extracts (first four lanes) or cytoskeletal fraction (last four lanes) of cells treated for 6 h with increasing concentrations of CdCl2 (0–40 µM). The result is typical of three independent experiments. B: Cells were treated for 6 h in serum-free conditions, without Cd, with CdCl2 (20 µM) alone, or with CdCl2 (20 µM) plus KN93 as described in Figure 1A. Cytosolic extracts (left hand) and cytoskeletal fractions (right side) were subjected to western blotting with antibodies to CaMK-IIδC (top), total phosphorylated CaMK-II (middle), and α-smooth muscle actin (bottom). The experiments was repeated once with similar results. C: Western blots of CaMK-IIδC and α-smooth muscle actin in an F-actin ultracentrifugation co-sedimentation pellet. The histogram depicts the mean ± S.D. of signal intensites of the CaMK-IIδC bands normalized to serum-free alone (SF) as 100%, from six independent experiments. D: Western blot of gelsolin in the ultracentrifugation co-sedimentation pellet, with arrows indicating the 95 kDa full length protein and 50 kDa fragment as in Figure 1B, and actin immunoblotted as a loading control. Results are typical of five experiments.
CaMK-II inhibition prevents a Cd-dependent decrease in actin polymerization in vitro
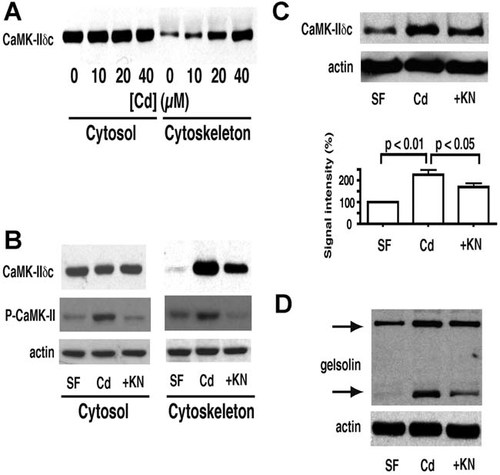
To investigate the effect of actin binding by CaMK-IIδ on actin dynamics, we measured the effect of recombinant CaMK-IIδE (rCaMK-IIδE) in a pyrenyl–actin polymerization assay with rabbit skeletal muscle actin (Fig. 3A). In the presence of 1 µM actin, 1 µM rCaMK-IIδE did not interfere with polymerization, resulting in a polymerization curve which was the same as that of buffer control. A concentration of 5 µM rCaMK-IIδE or higher caused the polymerization curve to plateau earlier than buffer control, suggesting rCaMK-IIδE may sequester actin monomers. There is no difference with respect to inhibition of polymerization between rCaMK-IIδE and a constitutively active mutant rCaMK-IIδE T287D. Thus, inhibitory activity resides in the native protein without need for specific activation at Thr287.
In vitro polymerization of actin. Polymerization was followed as the increase in fluorescence of pyrene-labeled actin as described in Methods. Typical experiments are shown. A: Baseline fluorescence was recorded for 3 min and then polymerization of 1 µM G-actin was initiated at time t = 4 min in the presence of buffer alone or in the presence of the indicated concentration of recombinant protein from 0.1 to 8 µM. WT: wild type rCaMK-IIδE, MT: mutant rCaMK-IIδE (T287D). B: In vitro polymerization of 1.5 µM G-actin in the presence of 10 µg of protein from the cytoskeletal fractions of cells held either in serum-free medium, or in the presence of 40 µM CdCl2 with or without KN93 for 3 h as in Figure 1. The initial slopes of Cd-treated cells and serum-free control differ in the presence and absence of the cytoskeletal fraction (P < 0.01, n = 7), and KN93 prevents the Cd-dependent decrease, as described in the text.
The polymerization of pyrene-labeled G-actin was also measured in the presence of cytoskeletal fractions from control and Cd-treated cells (Fig. 3B). The initial rate of polymerization (within the first 10 min) was significantly decreased in the presence of the cytoskeletal fraction from Cd-treated cells compared to that from serum-free control cells [(80.2 ± 7.9)%, P < 0.01, n = 7]. This result is consistent with our previous observations (Wang and Templeton, 1996b) that this cytoskeletal fraction from Cd-treated cells suppresses filament growth from the barbed end while favoring growth from the pointed end and increasing the degree of capping of the barbed end, suggesting the cytoskeletal fraction contains increased levels of capping activity. Co-treatment with KN93 restored the initial rate of polymerization to the level of Cd-free controls [(99.2 ± 10.0)%, P < 0.05 compared to Cd-treated cells, n = 7)]. The cytoskeletal fraction from Cd-treated cells also decreased the final extent of polymerization, and KN93 prevented the decrease (Fig. 3B).
Cd increases CaMK-IIδE translocation and cytoskeletal extracts from Cd-treated CaMK-IIδE-overexpressing cells decrease actin polymerization
Transfection of CaMK-IIδE into RMC was assessed by anti-Flag western blotting, GFP fluorescence, and CaMK-II kinase activity. CaMK-IIδE plasmid encodes a 57 kDa Flag-tagged protein in a 27 kDa GFP vector. When transfected into RMC, CaMK-IIδE was expressed both in a perinuclear compartment and peripheral to cortical actin (Fig. 4), similar to that previously noted in transfected 3T3 cells (Caran et al., 2001). It has also been reported that in vascular smooth muscle cells, endogenous CaMK-IIδC localizes in the cytoplasm with a perinuclear concentration (Mercure et al., 2008), supporting the relevance of this expression system. The GFP fluorescence signal was increased 1.7-fold in cytosol of cells transfected with CaMK-IIδE and twofold in cells transfected with empty vector, compared with untransfected control cells (data not shown). Consistent with the fluorescence signal, total activity in cytosol lysate from CaMK-IIδE-transfected cells was increased 1.73-fold compared with untransfected and vector controls (relative activities 1.00 and 1.03, respectively).

Transfection of mesangial cells with Flag/GFP-CaMK-IIδE construct. A: GFP fluorescence showing both peripheral (arrows) and perinuclear cytoplasmic staining. B: Rhodamine-labelled phalloidin staining of F-actin showing a predominantly cortical actin pattern of expression. C: overlay of (A) and (B) with nuclear DAPI staining (blue). Magnification ×400.
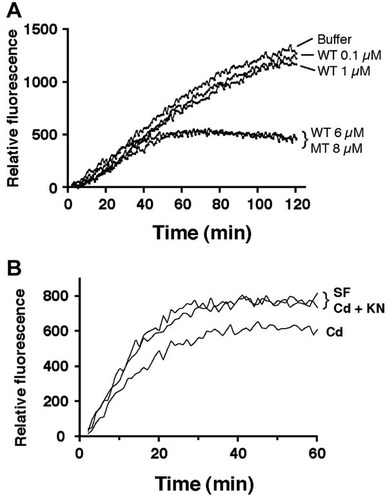
Cadmium-dependent translocation of CaMK-IIδE to the cytoskeleton was demonstrated by anti-Flag western blotting and GFP fluorescence. The 84 kDa Flag-GFP-CaMK-IIδE protein was detectable in cytosolic extracts of the CaMK-IIδE-transfected cells, while Flag-containing vector was seen at 27 kDa in vector-transfected control cells (Fig. 5A). The 84 kDa CaMK-IIδE protein was also detectable in the cytoskeletal fraction of serum-free control cells (Fig. 5B). Cadmium significantly increased the density of the cytoskeletal band (control vs. Cd, P < 0.01, n = 5), and KN93 prevented the Cd-induced change (Cd vs. Cd + KN93, P < 0.05, n = 5) (Fig. 5C). Although vector-transfected cells had a higher level of GFP fluorescence in the cytosol compared with untransfected control and CaMK-IIδE-transfected cells, the fluorescent signal in the cytoskeletal fraction from vector-transfected cells was the same as untransfected control, whereas there was at least a 1.5-fold increase in the fluorescent signal from CaMK-IIδE-transfected cells (data not shown). Cadmium treatment increased GFP expression in the cytoskeleton compared to serum-free control, and KN93 lowered the signal (data not shown).
Translocation of transfected CaMK-IIδE to the cytoskeleton. Cells were transfected with CaMK-IIδE containing both Flag and GFP tags. A: Anti-Flag western blot of cytosol from control (untransfected) cells (first three lanes), CaMK-IIδE construct-transfected cells (middle three lanes), and cells transfected with Flag/GFP vector lacking the CaMK-IIδE-coding insert (right three lanes). In each case cells were either held in serum-free conditions for 3 h, or treated with 40 µM CdCl2 with or without KN93 during that time, as in Figure 1A. Arrows point to the 84 kDa Flag/GFP-tagged CaMK-II protein and the 27 kDa empty Flag/GFP vector. The result is representative of three independent experiments. B: Western blotting of Flag and α-smooth muscle actin in cytoskeletal fractions. Treatments are as in (A). C: The histogram depicts the mean ± S.D. of signal intensities of the transfected Flag-CaMK-IIδE bands of the cytoskeletal fractions shown in (B). Signals are normalized to serum-free alone (SF) taken as 100%, and are from five independent experiments.
We next tested whether CaMK-IIδE overexpression could influence the effect of the cytoskeletal fraction on in vitro actin polymerization. Cytoskeleton from Cd-treated transfected cells significantly decreased the rate of polymerization compared to untreated vector-transfected controls [(75.0 ± 8.1)%, P < 0.001, n = 5]. Again, KN93 eliminated the effect of Cd.
Cytoskeletal extracts from CaMKII-silenced cells increases actin polymerization and abrogates the effect of Cd
CaMK-II silencing was achieved by either forward or reverse transfection with any of several siRNA sequences as described in Methods, and showed significant suppression of CaMK-IIδC expression compared to control cells or cells transfected with scrambled RNA sequence (Fig. 6A). Total CaMK-II activity was decreased by 50% by silencing, whereas scrambled sequence did not affect total activity (93% of control). Although CaMK-II activity was decreased only 50% (probably due to the presence of other endogenous CaMK-II isoforms), silencing nevertheless significantly prevented Cd-induced Erk activation (data not shown), which is mediated through CaMK-II (Xiao et al., 2009). Sequence 303 and reverse transfection were chosen for further studies. Cadmium-induced, KN93-sensitive cytoskeletal translocation of CaMK-IIδC was not observed in silenced cells, whereas transfection with scrambled sequence was without effect on translocation (Fig. 6B). Anti-α-smooth muscle actin antibody precipitated significantly less CaMK-IIδC from cytosol of silenced cells (Fig. 6C).
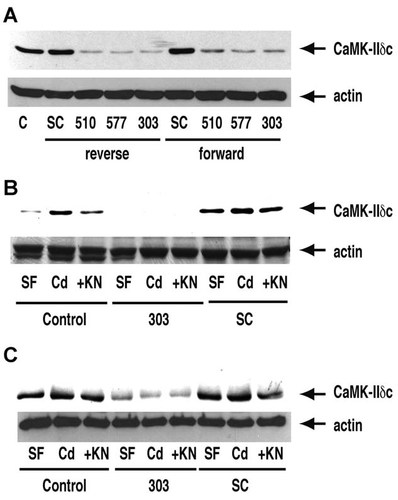
CaMK-IIδC silencing in mesangial cells. A: Cytosolic extracts were prepared from control cells (C) or cells that had been transfected with siRNA sequences designed to target CaMK-IIδ (sequences 303, 510, or 570) or with scrambled sequence (SC) as negative controls, with either forward or reverse transfection protocols as described in Methods. Extracts were western blotted with antibodies to CaMK-IIδC and α-smooth muscle actin. The results are typical of four independent experiments. Sequence 303 and reverse tansfection were chosen for subsequent studies. B: Cells were either untransfected (Control; first three lanes), transfected with siRNA sequence 303 (middle three lanes), or tansfected with scrambled control (SC; last three lanes). Cells in each group were held in serum-free medium for 3 h, or treated with 40 µM CdCl2 with or without KN93 as in Figure 1A. Cytoskeletal fractions were subjected to western blotting with anti-CaMK-IIδC and anti-α-smooth muscle actin antibodies. Again, the results are typical of four independent experiments. C: Cytosol was also prepared from the same cell treatments as in (B) and precipitated with anti-α-smooth muscle actin antibody. The immunoprecipitates were western blotted with anti-CaMK-IIδC and anti-α-smooth muscle actin antibodies as in (B). Similar results were obtained in two independent experiments.
In vitro actin polymerization was performed in the presence of the cytoskeletal fraction from scrambled-sequence control or silenced cells. Silencing significantly increased both the initial rate and the final extent of actin polymerization, compared with scrambled control cells. Cadmium, either with or without KN93, does not effect significantly the initial rate of polymerization in the CaMK-II-silenced cells (Fig. 7), although it does decrease the final extent of polymerization in both silenced and scrambled-transfected cells (data not shown), suggesting that effects of Cd on actin polymerization mediated through CaMK-II are primarily kinetic effects on the initial rate of polymerization.
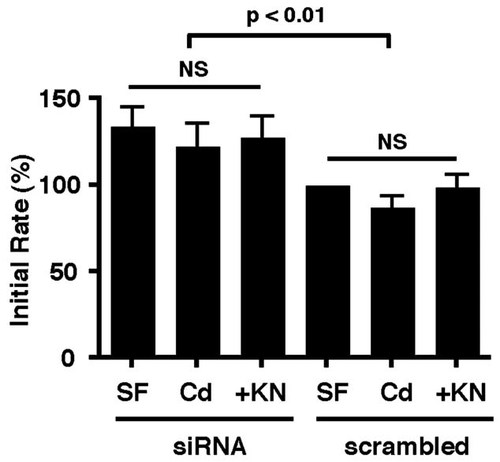
Effect of CaMK-IIδ silencing on actin polymerization. Cytoskeletal fractions were prepared from cells transfected with either siRNA sequence 303 or scrambled sequence prior to treating with Cd or Cd plus KN93 in serum-free conditions as in Figure 6B (40 µM CdCl2 for 3 h). Protein from these fractions (10 µg) was added to the actin polymerization assay as in Figure 3B. The initial rate of polymerization did not differ within trasfectant groups (NS) but was significantly increased in the silenced preparations compared to scrambled-sequence controls (P < 0.01). Values are mean ± S.D. from six independent experiments, normalized to the rate of serum-free controls in scramble-transfected cells taken as 100%.
Silencing CaMK-II decreases gelsolin cytoskeletal translocation
The 50 kDa gelsolin fragment shown in Figure 1B was detectable in cytosol, and translocated to the cytoskeleton upon Cd treatment in a dose-dependent manner (Fig. 8A). The amount of the 95 kDa full-length gelsolin in the cytoskeletal fraction was decreased, and there was a corresponding increase in the 50 kDa fragment as Cd concentration was increased. Both full-length gelsolin and the 50 kDa fragment were increased in the Triton X-100-insoluble cytoskeletal fraction following Cd treatment, and this translocation and cleavage was partially suppressed by the CaMK-II inhibitor KN93 (Fig. 8B). With CaMK-IIδC silencing, translocation of full-length gelsolin, and especially of the 50 kDa fragment, was decreased, supporting a role of CaMK-IIδC in facilitating Cd-dependent complex formation between F-actin and the gelsolin fragment. Cytosolic gelsolin levels were not noticeably affected by silencing (data not shown).
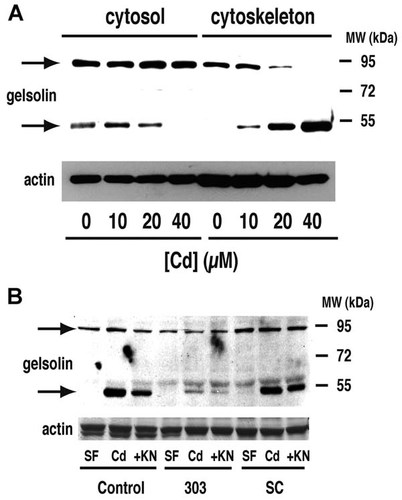
Effect of CaMK-IIδ silencing on gelsolin translocation and cleavage. Arrows indicate the full-length protein and 50 kDa fragment shown in Figure 1B. A: Western blot of gelsolin in cytosolic (first four lanes) and cytoskeletal (last four lanes) fractions of cells treated for 6 h with the indicated concentrations of CdCl2, from 0 to 40 µM. An immunoblot of α-smooth muscle actin is included as a loading control. B: Cells were treated as in Figure 6B,C and cytoskeletal fractions were examined for gelsolin by western blotting. In untransfected cells (first three lanes) Cd causes increased translocation to the cytoskeleton and KN93 diminishes this effect. The effect of Cd is especially marked for the fragment. In scrambled-sequence transfectant (last three lanes), the same pattern with the fragment. Silencing (middle three lanes) diminishes translocation of both full-length protein and fragment, with a more marked effect on the latter. In both panels (A) and (B), the reults are typical of three independent experiments.
Discussion
We showed previously that exposure of mesangial cells to CdCl2 resulted in disruption of the structure of the actin cytoskeleton, and the effect was specific to Cd2+ among a number of divalent metal ions at equimolar concentration (Wang et al., 1996). The effect occurs in vivo, and whereas Cd2+ actually enhances actin polymerization in an in vitro assay, cytoskeletal extracts from Cd-treated cells favor depolymerization in vitro (Wang and Templeton, 1996b). Previous experiments with cytochalasin B suggested that in the presence of Cd2+, preferential suppression of growth from the barbed end of the actin filament occurred, favoring increased capping activity in the cytoskeletal fraction of Cd-treated cells (Wang and Templeton, 1996b). Subsequent studies revealed that disruption of the actin cytoskeleton with cytochalasin D was partially protective against Cd-induced apoptotic cell death, while cytoskeletal stabilization with jasplakinolide was without effect (Liu and Templeton, 2010), implicating the cytoskeleatal effects of Cd in mechanisms of Cd toxicity. Cadmium activates a number of kinases, including Erk, Jnk, and p38 kinase (Thévenod, 2009; Xiao et al., 2009; Templeton and Liu, 2010). In addition, we demonstrated that Cd2+ activates CaMK-II in rat and mouse mesangial cells (Liu and Templeton, 2007, 2008), and a link between CaMK-II and the cytoskeleton was established by showing that inhibition of CaMK-II activation in the presence of Cd2+ protected against apoptosis and prevented disruption of the cytoskeleton (Liu and Templeton, 2010). Here we show that CaMK-IIδ, a prominent isoform in RMC, associates with G-actin and translocates to the cytoskeletal compartment upon treatment with CdCl2, subsequently co-precipitating with actin filaments.
A major isoform of CaMK-II in vascular smooth muscle cells is CaMK-IIδ (Jones et al., 2007), and we have found it to be prominent in mesangial cells. Here we show that it binds to both actin monomer and filament. The observation that rCaMK-IIδE lowers the plateau of polymerization suggests sequestration of G-actin by CaMK-II binding, and Cd-induced translocation of activated kinase to the F-actin cytoskeleton is associated with filament disruption. Capping of the barbed end is another possibility, although electron microscopy did not provide evidence of binding of CaMK-IIβ to the ends of filaments (Sanabria et al., 2009).
The mechanisms by which actin monomer binding proteins regulate actin polymerization dynamics are complex. The CaMK-IIβ isoform binds to G-actin and inhibits actin polymerization in vitro, reducing the rate of polymerization, but also binds to actin filaments, facilitates filament bundling, and increases filament rigidity (Okamoto et al., 2007; Sanabria et al., 2009). These are not necessarily opposing functions; rather, they participate in reorganization and stabilization of the cytoskeleton. Nor are they unprecedented. For example, adenylate cyclase-associated protein-1 (CAP1) is an actin monomer-binding protein that inhibits filament assembly by sequestering monomers in vitro. However functional studies in vivo show that CAP1 promoters stress fiber assembly, probably by acting as an actin monomer-delivery protein (Freeman and Field, 2000). CAP1 appears to promote rapid actin filament depolymerization by directing the function and subcellular localization of cofilin, rather than through its actin monomer-sequestering activity (Bertling et al., 2004). Thus, the association of CaMK-IIδ with actin does not in itself predict its role in cytoskeletal dynamics; rather Cd-dependent activation and translocation appear to set a context for its influence.
That Cd2+ activates CaMK-II is well established (Liu and Templeton, 2007, 2008), and phosphorylation of CaMK-IIδ in both cytosolic and cytoskeletal fractions is increased in Cd-treated cells. Furthermore, both CaMK-IIδ translocation and cytoskeletal effects are inhibited by KN93, which inhibits CaMK-II phosphorylation by binding competitively to its Ca2+/calmodulin binding sites. However, the present study does not address the question of whether CaMK-II phosphorylation is directly responsible for cytoskeletal localization, or whether the resultant kinase activity recruits CaMK-IIδ to the cytoskeleton via activation of other targets. It should be noted that a number of proteins have been shown to bind preferentially to phosphorylated CaMK-II, including Flightless-I (Seward et al., 2008), glutamate receptor (Leonard et al., 2002), and histone deacetylase-4 (Backs et al., 2006). Activation of CaMK-II in the neuron results in the translocation of phosphorylated CaMK-II from the cytosol to bind to the N-methyl-D-aspartate receptor in a post-synaptic compartment (Okamoto et al., 2009). However, CaMK-II also regulates the actin-severing activity of cofilin via other kinases such as the Rho family of small GTPases and LIM kinase-1 in several cell lines (Okamoto et al., 2009; Zhao et al., 2012).
Gelsolin deserves attention as a modulator of the influence of CaMK-II on actin filament dynamics. We showed that Cd2+ shifted gelsolin from a cytosolic distribution to a pattern decorating actin filaments (Apostolova et al., 2006). Whereas gelsolin acted as a nucleating factor for actin polymerization in vitro, and Cd2+ enhances this effect, cytoskeletal extracts from Cd2+-treated cells actually favor depolymerization, and we concluded that gelsolin's actin-severing properties predominate in Cd2+-treated cells (Apostolova et al., 2006). The present study adds additional evidence for a role of gelsolin in the effects of Cd on actin dynamics.
Actin-overlay experiments showed both full-length gelsolin and a 50 kDa fragment (Apostolova et al., 2006) (here confirmed by LC-MS to arise from gelsolin) to associate directly with actin filaments. Indeed, like many actin-binding proteins, gelsolin interacts with both G-actin monomer and F-actin filaments, severing the filaments while also capping the growing barbed end (Sun et al., 1999). Here we present evidence that CaMK-IIδ forms a complex with G-actin that contains gelsolin; when CaMK-II kinase activity is suppressed, in serum-free conditions or KN93-treated cells, gelsolin is retained in the cytosol. Silencing CaMK-II also prevents Cd-induced gelsolin fragmentation and translocation to the cytoskeleton. Our interpretation is that gelsolin is cleaved after translocation to the cytoskeletal fraction in Cd-treated cells. Gelsolin is known to be cleaved by several proteinases such as caspase 3 (Kothakota et al., 1997), granzyme B (Martin et al., 2010), matrix metalloproteases (Mani et al., 2008), and calpain (Wolf et al., 1999). Gelsolin cleaved by caspase severs actin filaments in vitro in a Ca2+-independent manner (Kothakota et al., 1997), and so the appearance of the 50 kDa fragment in Cd-treated cells is suggestive of Cd-dependent generation of an active fragment; indeed, expression of an N-terminal fragment (amino acids 1–352, retaining actin-severing activity) in several cell lines caused a rapid depolymerization of actin filaments and initiated apoptosis (Kothakota et al., 1997).
In summary, CaMK-IIδ associates with G-actin and F-actin in mesangial cells. By activating CaMK-IIδ and enhancing its association with the cytoskeleton, Cd promotes cytoskeletal disruption. Cadmium-dependent effects on gelsolin result in association of gelsolin, and particularly a 50 kDa fragment of gelsolin, in an F-actin-CaMK-IIδ-gelsolin complex. This is the first demonstration that CaMK-II may exert cytoskeletal effects through influencing fragmentation of gelsolin and facilitating its translocation. It provides a new framework for understanding the effects of Cd on cell structure and cell fate.