Histone deacetylase inhibitors induce mitochondrial elongation
Abstract
Although various stimuli-inducing cell demise are known to alter mitochondrial morphology, it is currently debated whether alteration of mitochondrial morphology is per se responsible for apoptosis execution or prevention. This study was undertaken to examine the effect of histone deacetylase (HDAC) inhibitors on mitochondrial fusion–fission equilibrium. The mechanism underlying HDAC inhibitor-induced alteration of mitochondrial morphology was examined in various cells including primary cultured cells and untransformed and cancer cell lines treated with seven different HDAC inhibitors. Suberoylanilide hydroxamic acid (SAHA)-induced mitochondrial elongation in both Hep3B and Bcl-2-overexpressing Hep3B cells, apart from its apoptosis induction function. SAHA significantly decreased the expression of mitochondrial fission protein Fis1 and reduced the translocation of Drp1 to the mitochondria. Fis1 overexpression attenuated SAHA-induced mitochondrial elongation. In addition, depletion of mitochondrial fusion proteins, Mfn1 or Opa1, by RNA interference also attenuated SAHA-induced mitochondrial elongation. All of the HDAC inhibitors we examined induced mitochondrial elongation in all the cell types tested at both subtoxic and toxic concentrations. These results indicate that HDAC inhibitors induce mitochondrial elongation, irrespective of the induction of apoptosis, which may be linked to alterations of mitochondrial dynamics regulated by mitochondrial morphology-regulating proteins. Since mitochondria have recently emerged as attractive targets for cancer therapy, our findings that HDAC inhibitors altered mitochondrial morphology may support the rationale for these agents as novel therapeutic approaches against cancer. Further, the present study may provide insight into a valuable experimental strategy for simple manipulation of mitochondrial morphology. J. Cell. Physiol. 227: 2856–2869, 2012. © 2011 Wiley Periodicals, Inc.
Mitochondria, which are strategically distributed to meet cellular needs and receive signals from outside, continuously divide and fuse to form small individual units or interconnected networks within the cell (Bereiter-Hahn, 1990). The number, size, and overall shape of steady-state mitochondria are maintained by balancing the opposing processes of mitochondrial fusion and fission events (Karbowski and Youle, 2003; Cerveny et al., 2007). Unbalanced fission or fusion results in mitochondrial elongation or fragmentation. Under certain conditions, the mitochondria in mammalian cells can be physically interconnected (De Giorgi et al., 2000). This mitochondrial network can effectively deliver energy or channel calcium between different areas of the cell (Jeong and Seol, 2008). Mitochondrial fission can be controlled in response to a variety of cellular events, including cell division, metabolic flux, death, and differentiation (Cerveny et al., 2007).
Mitochondrial fusion and fission events are complex processes that are precisely regulated by numerous mitochondrial morphology-regulating proteins (Karbowski and Youle, 2003; Cerveny et al., 2007; Benard and Karbowski, 2009). In mammalian cells, mitochondrial fission machinery is controlled by Fis1 (Yoon et al., 2003) and dynamin-related protein 1 (Drp1) (Smirnova et al., 2001). Drp1, a large GTPase, translocates to the puncta on mitochondria, where it couples GTP hydrolysis with mitochondrial membrane constriction and fission (Smirnova et al., 1998; Smirnova et al., 2001). Fis1, anchored to the mitochondrial outer membrane via its C-terminal region (James et al., 2003), contributes to mitochondrial fission by participating in the recruitment of Drp1 to the mitochondria (Yoon et al., 2003). Mitochondrial fusion machinery requires at least three large GTPases: mitofusin (Mfn) 1 and 2 and Opa1 (Chen et al., 2003; Olichon et al., 2003). Mfn1 and Mfn2 are located on the mitochondrial outer membrane. Opa1 is a protein found in the intermembrane space in pools that are either soluble or closely associated with the inner membrane (Arnoult et al., 2005). At least eight splicing isoforms of Opa1 have been reported (Delettre et al., 2001). In general, disruption of fusion induces the alteration of the normal tubular network of mitochondria into short rods or spheres. Conversely, disruption of fission generates elongated, interconnected tubules that often cluster in the perinucleus (Cerveny et al., 2007).
Previous studies showed that mitochondrial network connectivity decreases in cells undergoing apoptosis (Frank et al., 2001; Pinton et al., 2001; Karbowski et al., 2002; Breckenridge et al., 2003; James et al., 2003; Karbowski and Youle, 2003). Mitochondrial fragmentation occurs in most forms of apoptosis and is due to the activation of mitochondrial fission machinery with or without the inhibition of fusion (Frank et al., 2001; Breckenridge et al., 2003). Mitochondrial fusion and fission machineries are involved in the regulation of some steps of this process (Frank et al., 2001; Jeong and Seol, 2008). Recently, the apoptosis-regulating Bcl-2 family of proteins has been implicated in the regulation of mitochondrial dynamics. Bcl-2 family members can perturb mitochondrial fission and fusion dynamics. These proteins have the capacity to regulate mitochondrial morphology by binding to the mitofusins in healthy cells (Cleland et al., 2010). Upon induction of apoptosis, Drp1 translocates to the potential scission sites of the mitochondria (Frank et al., 2001). Bcl-xL has been suggested to positively regulate Drp1 to alter mitochondrial function in a manner that stimulates synapse formation (Li et al., 2008). Bax translocates from the cytosol to the mitochondrial scission sites (Karbowski et al., 2002). An elaborate study elucidated that acetylation of Ku70 blocks its ability sequestrating Bax from mitochondria, leading to Bax-mediated apoptosis (Cohen et al., 2004). Bak may block mitochondrial fusion to induce fragmentation (Brooks and Dong, 2007). The collaboration of Bax and Bak promotes the permeabilization of the mitochondrial outer membrane, leading to the release of apoptogenic factors. Although numerous previous studies demonstrated mitochondrial fragmentation in cells undergoing apoptosis, whether mitochondrial fragmentation is indispensible for apoptosis execution is currently debated (Arnoult, 2007).
Histone deacetylase (HDAC) inhibitors are chemotherapeutic drugs that inhibit deacetylase activity, thereby increasing acetylation of many proteins, including histones (Atadja, 2011). Previous studies demonstrated that HDAC inhibitors acetylates Ku70 and releases Bax, allowing it to translocate to mitochondria and trigger cytochrome c release, leading to caspase-dependent apoptotic cell death (Cohen et al., 2004; Subramanian et al., 2005). However, to date the effect of HDAC inhibitors on mitochondrial fusion and fission has not been documented.
This study was undertaken to examine the effect of HDAC inhibitors on mitochondrial fusion–fission equilibrium. As shown here, HDAC inhibitors induce mitochondrial elongation irrespective of the induction of apoptosis, which may be linked to alterations of mitochondrial dynamics regulated by mitochondrial morphology-regulating proteins.
Materials and Methods
Materials
The following reagents were obtained commercially: rabbit polyclonal anti-human caspase-3, caspase-9, Mfn1, Mfn2, Tom20, and GAPDH antibodies from Santa Cruz Biotechnology (Santa Cruz, CA); mouse monoclonal anti-human poly(ADP-ribose) polymerase (PARP) antibody from Oncogene (Cambridge, MA); rabbit polyclonal anti-human Bcl-2, acetyl histones H2A, H3, and H4, caspase-6 and -7, and acetyllysine antibodies from Cell Signaling (Danvers, MA); mouse monoclonal anti-DLP1/Drp1, Calnexin, and Opa1 antibodies from BD Transduction Laboratories (Lexington, KY); FITC-conjugated goat anti-rabbit and Texas Red-conjugated horse anti-mouse IgGs from Vector (Burlingame, CA); HRP-conjugated donkey anti-rabbit and sheep anti-mouse IgGs from Amersham Pharmacia Biotech (Piscataway, NJ); mouse monoclonal anti-human phospho-serine and β-actin antibodies, Hoechst 33342, dimethyl sulfoxide (DMSO), RNase A, proteinase K, leupeptin, propidium iodide (PI), apicidin, sodium butyrate (NaB), trapoxin A, trichostatin (TsA), valproic acid (VPA), resveratrol, etoposide, staurosporine, and nocodazole from Sigma–Aldrich (Irvine, CA); caspase inhibitor I zVAD-fmk and neomycin sulfate (G418) from Calbiochem (San Diego, CA); 3,3′-dihexyloxacarbocyanine iodide (DiOC6) and mitotrackers from Molecular Probes (Eugene, OR); rabbit polyclonal anti-human Fis1 antibody, SAHA and MS-275 from Alexis Biochemicals (San Diego, CA); SuperSignal West Pico enhanced chemiluminescence Western blotting detection reagent from Pierce (Rockford, IL); rabbit polyclonal anti-human Mff antibody from Abcam (Cambridge, UK).
Cells
Hep3B, ARPE19, T98G, U118MG, U87MG, U373MG, PC3, and ZR-75-1 cells were obtained from the American Type Culture Collection (ATCC; Rockville, MD). KAT-18 was kindly provided by Dr. K.B. Ain (University of Kentucky Chandler Medical Center, Lexington, KY). Rat articular chondrocytes for primary culture were obtained and cultured as described previously (Lee et al., 2008).
Cell culture and establishment of Bcl-2- and Fis1-overexpressing Hep3B cells
Hep3B cells were cultured in complete Dulbecco's modified Eagle's medium (DMEM; GibcoBRL, Gaithersburg, MD) supplemented with 10% heat-inactivated fetal bovine serum (FBS; GibcoBRL) and 100 U/ml penicillin in 5% CO2 at 37°C. Mammalian expression vector encoding Flag-tagged Bcl-2 was kindly provided by Prof. A. Strasser (The Walter and Eliza Hall Institute of Medical Research, Melbourne, Vic., Australia). Hep3B cells were transfected with the expression vector encoding Flag-tagged Bcl-2. Stable Hep3B cell lines overexpressing Bcl-2 were selected with changes of fresh medium containing puromycin (4 µg/ml). Fis1-overexpressing Hep3B cells were generated using a pcDNA3 vector containing the human FIS1 gene and maintained in medium containing 200 µg/ml of G418. Hep3B cells were transfected using FuGENE6 reagent (Roche, Mannheim, Germany) according to the manufacturer's instructions. The transfected cells were incubated for 2 days, and stable cell lines were then selected with changes of fresh medium containing puromycin (4 µg/ml) or G418 (500 µg/ml) for 4 weeks. Single-cell clones were isolated by limiting dilutions and subsequently analyzed for an increase of Bcl-2 and Fis1 protein expression relative to identically cloned empty vector controls.
Treatment with HDAC inhibitors and other pharmacological agents
Forty-eight hours after Hep3B cells were subcultured, the original medium was removed. Cells were washed with PBS and then incubated in the same fresh medium. NaB and VPA were stocked in PBS, and apicidin, trapoxin A, TsA, SAHA, and MS-275 were stocked in DMSO. SAHA from a stock solution was added to the medium to obtain indicated dilutions (0–16 µM) of the drug for 0–48 h. Other HDAC inhibitors from stock solutions were added to the medium to obtain the indicated dilution of each drug for 48 h. The concentration of PBS or DMSO used in this study had no effect on Hep3B cell proliferation in our preliminary studies. To examine the effect of SAHA on apoptosis-inducing agents or the effect of pharmacological agents on the efficacy of SAHA, cells were incubated in the presence or absence of resveratrol, HS-1793 (kindly provided by Dr. H. Suh, Pusan National University, Busan, Korea), etoposide, staurosporine, nocodazole, or zVAD-fmk and further exposed to 8 µM SAHA for 48 h.
Cell viability assay
Cell viability was determined by the Vi-Cell cell counter (Beckman Coulter, Miami, FL), which performs an automated trypan blue exclusion assay.
Nuclear morphology
Cell suspensions were cytospun onto clean fat-free glass slides using a cytocentrifuge. Centrifuged samples were fixed in 4% paraformaldehyde for 10 min and stained in 4 µg/ml Hoechst 33342 for 30 min at 4°C. Cells were observed and photographed under an epifluorescence microscope by an observer who was blinded to the experimental group.
Quantification of DNA hypoploidy and cell cycle phase analysis by flow cytometry
Ice-cold 95% ethanol with 0.5% Tween 20 was added to the cell suspension to a final concentration of 70% ethanol. Fixed cells were pelleted and washed in 1% BSA–PBS solution. Cells were re-suspended in 1 ml PBS containing 11 Kunitz U/ml RNase, incubated at 4°C for 30 min, washed once with BSA–PBS, and re-suspended in PI solution (50 µg/ml). After cells had been incubated at 4°C for 30 min in the dark and washed with PBS, DNA content was measured on an Epics XL (Beckman Coulter), and data were analyzed using Multicycle software, which allowed a simultaneous estimation of cell cycle parameters and apoptosis.
Western blot analysis
Equal amounts of proteins were run on 7.5–15% sodium dodecylsulfate–polyacrylamide gel electrophoresis (SDS–PAGE). The proteins were transferred to a nitrocellulose membrane (Amersham Pharmacia Biotech) and reacted with each antibody. Immunostaining with antibodies was performed using SuperSignal WestPico enhanced chemiluminescence substrate and detected with LAS-3000 Plus (Fuji Photo Film, Tokyo, Japan).
Immunocytochemistry and confocal microscopy
Cells grown in two-well chamber slides were treated as indicated, fixed with 4% paraformaldehyde for 10 min, permeabilized with 0.2% Triton X-100 for 15 min, and then blocked with 2% bovine serum albumin for 1 h at room temperature (RT). Cells were probed with rabbit polyclonal anti-Mfn1 (1:75) and mouse monoclonal anti-Opa1 (1:75) overnight at 4°C, followed by staining with goat anti-mouse Alexa Fluor 488 antibody (1:100; Molecular Probes) or goat anti-rabbit Alexa Fluor 488 antibody (1:100; Molecular Probes) for 1 h at RT. After washing, cells were mounted with SlowFade Light antifade reagent (Molecular Probes) and analyzed by confocal microscopy. To visualize the mitochondria in living cells, 20 nM mitotracker CMXRos was added and incubated for 20 min. Fluorescent images were observed and analyzed under Zeiss LSM 700 laser-scanning confocal microscope.
RNAi and shRNA
Bcl-2 expression was silenced by using siRNA duplexes against human BCL2 transcripts purchased from Dharmacon (Thermo Fisher Scientific, Lafayette, CO). Sequences were: Sense: GGGAGAACAGGGUACGAUAUU-3′ and Antisense: 5′-PUAUCGUACCCUGUUCUCCCUU-3′. As a negative control, the same nucleotides were scrambled to form a non-targeting combination. siRNAs were transfected using siPORT Amine (Ambion, Austin, TX) in Opti-MEM (GibcoBRL). For knockdown of the mitochondrial morphology-regulating genes, OPA1, MFN1, and FIS1, the pREP4-based plasmids containing shRNAs were used (Lee et al., 2007). Sequences for the target genes were as follows: 5′-GATGAAGTTATCAGTCTGAGCCAGGTTAC-3′ for OPA1, 5′-TCCTGGCATCCAGGAGTTAGAGATTGAGCGGT-3′ for MFN1, and 5′-GAGACGCGGGAGCCCACGGAGAACGCTCC-3′ for FIS1. The target sequence for the control shRNA was 5′-TCGTACTCATAATCAGCTCTGCATACATC-3′. One day after the transfection of the shRNA plasmid constructs with FuGENE 6, the Hep3B cells were grown in DMEM containing 150 µg/ml hygromycin B for 2 days, followed by 3–4 days in DMEM containing 50 µg/ml hygromycin B for the selection of transfectants.
Observation of mitochondrial morphology
Prewarmed (37°C) staining solution containing 20 nM mitotracker red was added to cells growing on coverslips inside a Petri dish, and cells were incubated at 37°C for 20 min in experimental growth conditions. The staining solution was washed with fresh prewarmed media, and the fixed cells were identified using Zeiss LSM 700 laser-scanning confocal microscope.
Transmission electron microscopy
Forty-eight hours after treatment, cells were harvested, pelleted, and fixed in 2.5% glutaraldehyde (Sigma–Aldrich) in phosphate buffer. After rinsing with phosphate buffer, the samples were post-fixed in 1% osmium tetroxide (Sigma–Aldrich) for 1 h, rinsed with water, dehydrated in a graded series of ethanol followed by propylene oxide (Sigma–Aldrich) and kept overnight in Epon812 (Sigma–Aldrich). The samples were embedded in Epon812 and cured in an oven at 60°C. Ultrathin sections were obtained with a Reichert Ultracut E microtome. The sections were stained with uranyl acetate and lead citrate and observed using a transmission electron microscope (Hitachi, Tokyo, Japan).
Subcellular fractionation
Hep3B cells (107 cells/well) were washed in Tris-based Mg2+/Ca2+ -free buffer (135 mM NaCl, 5 mM KCl, and 25 mM Tris–HCl pH 7.6) and allowed to swell for 10 min in ice-cold hypotonic CaRSB buffer (10 mM NaCl, 1.5 mM CaCl2, 10 mM Tris–HCl pH 7.5, and protease inhibitor cocktail). Cells were disrupted by dounce homogenization with 60 strokes on ice. Mitochondria stabilization (MS) buffer (210 mM mannitol, 70 mM sucrose, 5 mM EDTA, and 5 mM Tris–HCl pH 7.6) was then added to stabilize the mitochondria (2 ml of 2.5 × MS buffer per 3 ml of homogenate). After collecting the nuclei by centrifugation twice at 800 × g for 15 min, the supernatant was spun at 16,000 × g for 20 min at 4°C. The pellet and the supernatant were collected separately to obtain mitochondrial and cytoplasmic fractions, respectively.
Co-immunoprecipitation (Co-IP)
Cell extracts that were incubated with antibodies were precipitated with protein A-Sepharose beads. Immunoprecipitated proteins were separated on SDS–PAGE, and Western blot analysis was performed as described. Each Co-IP experiment was confirmed via reciprocal IP.
Statistical analysis
Four independent experiments were conducted in vitro. The results are expressed as the means ± SD from four experiments, each performed in triplicate. Statistical significance of differences was determined by the paired Kruskal–Wallis non-parametric test. A P-value <0.05 was considered significant.
Results
SAHA induces mitochondrial elongation in Hep3B cells, regardless of their susceptibility to cell death
SAHA treatment for 48 h significantly reduced the viability of Hep3B cells and vector-expressing Hep3B cells (Hep3B/vector) in a concentration-dependent manner. However, SAHA did not reduce the viability of Bcl-2-overexpressing Hep3B cells (Supplementary Fig. S1A). Because the concentration of SAHA required for half-maximal inhibition of the viability of Hep3B and Hep3B/vector cells was approximately 8 µM, this concentration was used in further studies. To investigate whether the reduced viability of SAHA-treated Hep3B cells resulted from the induction of apoptosis, we conducted various apoptosis assays. All of these results demonstrated that SAHA induced the apoptotic cell death of Hep3B cells and that this phenomenon was substantially attenuated by Bcl-2 overexpression (Supplementary Fig. S1B–E).
Because we observed through apoptosis assays that Bcl-2-expressing SAHA-treated Hep3B cells (Hep3B/Bcl-2) had elongated mitochondria, we first hypothesized that Bcl-2 overexpression contributes to prevent the apoptosis of Hep3B cells that is induced by SAHA through mitochondrial elongation. To test our hypothesis, we investigated the effect of Bcl-2 depletion in Hep3B/Bcl-2 cells. The siRNA against the Bcl-2 gene efficiently downregulated the expression of Bcl-2 protein in Hep3B/Bcl-2 cells and augmented the death of Hep3B/Bcl-2 cells treated with SAHA (Fig. 1A). However, in contrast to our hypothesis, the Bcl-2 siRNA did not prevent the mitochondrial elongation induced by SAHA in Hep3B/Bcl-2 cells (Fig. 1A). Because these data invalidated our hypothesis, we next examined whether SAHA itself is associated with the mitochondrial elongation in Hep3B cells. Surprisingly, confocal microscopy using the mitochondrial marker Mitotracker and transmission electron microscopy demonstrated that SAHA induced mitochondrial elongation in Hep3B and Hep3B/vector cells, as observed in the Hep3B/Bcl-2 cells, indicating that SAHA induces mitochondrial elongation in Hep3B cells irrespective of Bcl-2 overexpression (Fig. 1B). We further conducted a time-sequenced assay to examine the correlation between cell death and mitochondrial fusion in these cells. SAHA-induced mitochondrial elongation in a time-dependent manner in both Hep3B/Bcl-2 and Hep3B cells. The population of cells with elongated mitochondria at each time point was similar in both cell types (Fig. 2A,B). Noticeably, mitochondrial elongation preceded the decrease in viability of Hep3B cells (Fig. 2A). The pan-caspase inhibitor zVAD-fmk, which efficiently inhibited SAHA-induced apoptosis, did not prevent the mitochondrial elongation (Fig. 2C). These data indicate that the mitochondrial elongation induced by SAHA in Hep3B cells is not correlated with the prevention of apoptosis.
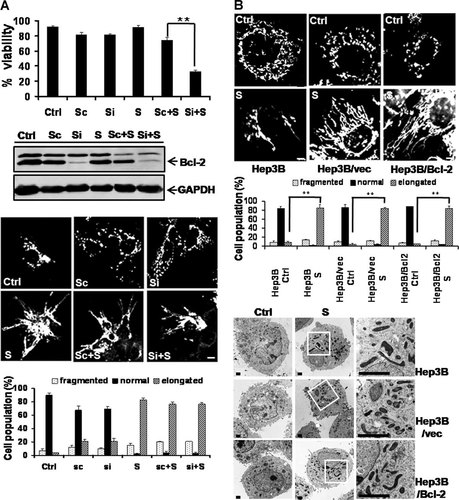
SAHA induces mitochondrial elongation in Hep3B cells irrespective of Bcl-2 overexpression. Cells were treated with 8 µM SAHA for 48 h. To visualize the mitochondrial morphology, cells were directly stained with Mitotracker CMSRos (red) for 20 min before harvesting and observed under a confocal microscope. Ctrl, control. S, SAHA. Si, siRNA against the Bcl-2 gene. Sc, scrambled. The percentage of cells with fragmented (dotted), normal (solid), or elongated (striped) mitochondria are depicted in the diagram. At least 100 cells in several fields were counted in each experiment. Data represent the mean ± SD of at least four independent experiments. A: Viability assay in the upper panel: siRNA against the Bcl-2 gene augmented the reduction by SAHA of Hep3B/Bcl-2 cell viability. **P < 0.01. Western blot assay in the middle panel: siRNA against the Bcl-2 gene efficiently downregulated the expression of Bcl-2 protein in Hep3B/Bcl-2 cells. GAPDH, a loading control. Confocal microscopy showing mitochondrial morphology and quantification in the lower panel. siRNA against the Bcl-2 gene did not significantly prevent the mitochondrial elongation induced by SAHA in Hep3B/Bcl-2 cells. B: Confocal microscopy showing mitochondrial morphology and quantification in the upper panel. Transmission electron microscopy in the lower panel. SAHA-induced mitochondrial elongation in Hep3B as well as in Hep3B/vector and Hep3B/Bcl-2 cells. Bar, 5 µm. **P < 0.01. The asterisks indicate a significant difference compared with the each control cell.
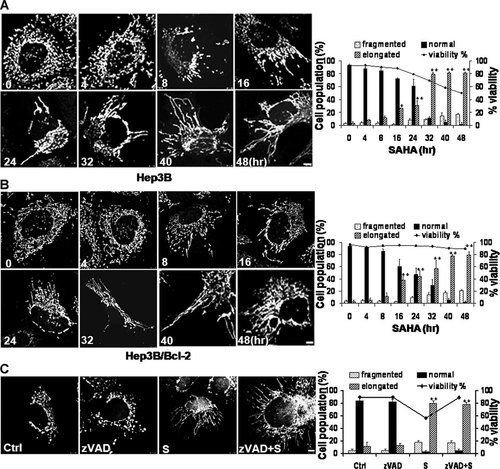
SAHA induces mitochondrial elongation in Hep3B cells, although the elongation is not correlated with the prevention of cell death. Cells were treated with 8 µM SAHA and harvested 0–48 h after treatment. To visualize the mitochondrial morphology, cells were directly stained with Mitotracker CMSRos (red) for 20 min before harvesting and observed under a confocal microscope. The percentage of cells with fragmented (dotted), normal (solid), or elongated (striped) mitochondria and viability (solid line) are depicted in the diagram. At least 100 cells in several fields were counted in each experiment. Data represent the mean ± SD of at least four independent experiments. Effect of SAHA treatment on mitochondrial morphology in (A) Hep3B cells and (B) Hep3B/Bcl-2 cells. The increase in elongated mitochondria was time-dependent, and the appearance of cells with elongated mitochondria preceded the decrease of viability. *P < 0.05, **P < 0.01. The asterisks indicate a significant difference compared with the untreated control. C: Effect of pan-caspase inhibitor zVAD-fmk pretreatment on SAHA-induced elongation of mitochondria. Confocal microscopy shows that zVAD-fmk pretreatment did not prevent mitochondrial elongation induced by SAHA. **P < 0.01. The asterisks indicate a significant difference compared with the experimental control treated with zVAD alone. See Figure 1 for other definitions. Bar, 5 µm.
SAHA treatment reversed the mitochondrial fragmentation resulting from treatment with each of the anticancer agents
We next examined whether other types of apoptosis-inducing agents also have mitochondria-elongating effects similar to SAHA in Hep3B cells. We tested resveratrol, the resveratrol analog HS-1793 (Jeong et al., 2009), staurosporine, and etoposide. All four agents reduced cell viability to approximately 50%, but in contrast to SAHA, they induced mitochondrial fragmentation in Hep3B cells (Fig. 3A). Although resveratrol, HS-1793 and staurosporine did not efficiently reduce the viability of Hep3B/Bcl-2 cells, these agents induced mitochondrial fragmentation (Fig. 3B). Etoposide, which bypassed the resistance to cell death induced by Bcl-2 in Hep3B/Bcl-2 cells, also induced mitochondrial fragmentation in Hep3B/Bcl-2 cells (Fig. 3B). These data are consistent with previous reports that apoptosis-inducing agents in general induce mitochondrial fragmentation (Frank et al., 2001; Karbowski et al., 2002; Breckenridge et al., 2003; Karbowski and Youle, 2003). Intriguingly, SAHA treatment reversed the mitochondrial fragmentation resulting from treatment with each of the anticancer agents (Fig. 3C).
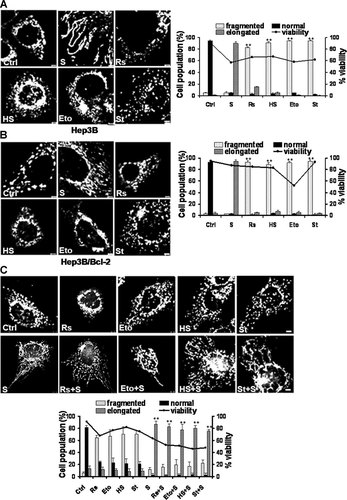
Several apoptosis-inducing agents induce mitochondrial fragmentation in Hep3B cells. Cells were treated with five different apoptosis-inducing agents for 48 h. To reduce cell viability by approximately 50%, SAHA (S), resveratrol (Rs), HS-1793 (HS), etoposide (Eto), and staurosporine (St) were used at 8, 200, 80, 40, and 200 nM, respectively. See Figure 2 for the confocal microscopy procedure used to observe mitochondrial morphology and the procedure for quantifying the data. Induction of mitochondrial fragmentations in (A) Hep3B cells and (B) Hep3B/Bcl-2 cells were induced by apoptosis-inducing agents. In contrast to SAHA treatment, treatment with any of the tested apoptosis-inducing agents induced mitochondrial fragmentation in Hep3B cells and Hep3B/Bcl-2 cells. **P < 0.01. The asterisks indicate a significant difference compared with the untreated control. C: Reversal of the mitochondrial fragmentation in Hep3B cells treated with apoptosis-inducing agents. Hep3B cells were pretreated with apoptosis-inducing agents for 24 h and subsequently treated with 8 µM SAHA for 24 h. SAHA treatment significantly reversed the mitochondrial fragmentation resulting from treatment with each of the anticancer drugs. **P < 0.01. The asterisks indicate a significant difference compared with the each experimental control pretreated with apoptosis-inducing agent alone. See Figure 1 for other definitions. Bar, 5 µm.
All tested HDAC inhibitors induce mitochondrial elongation in Hep3B cells and other types of cells
We next examined the effect of other HDAC inhibitors on mitochondria elongation in Hep3B cells. We tested six other HDAC inhibitors: TsA, VPA, NaB, trapoxin A, apicidin, and MS-275. Noticeably, all the HDAC inhibitors tested at the concentration that induced apoptosis in approximately 50% of the cells induced mitochondrial elongation in Hep3B cells (Fig. 4A,B). To examine whether the induction of mitochondrial elongation by these HDAC inhibitors is cell type-specific, we tested the effects of these HDAC inhibitors in various cancer cell lines (glioma, prostate, breast, and thyroid cancers), the untransformed human retinal pigment epithelial cell line ARPE19, and primary cultured rat chondrocytes. Importantly, all the HDAC inhibitors induced mitochondrial elongation in all types of cells tested (Fig. 5A,B and Supplementary Fig. S2A–G).
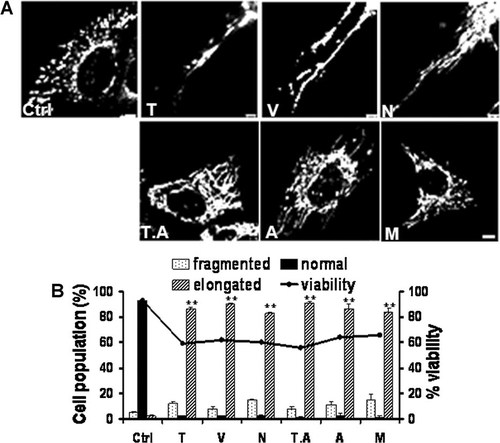
Six different HDAC inhibitors induce mitochondrial elongation in Hep3B cells. Cells were treated with six different HDAC inhibitors for 48 h. To reduce cell viability by approximately 50%, trichostatin A (T), valproic acid (V), sodium butyrate (N), trapoxin A (T.A), apicidin (A), and MS-275 (M) were used at 40 µM, 30 mM, 10 mM, 10 nM, 1 µg, and 15 µM, respectively. A: Confocal micrographs showing mitochondrial elongation in Hep3B cells treated with each HDAC inhibitor. See Figure 2 for the confocal microscopy procedure used to observe the mitochondrial morphology. B: The percentage of cells with elongated mitochondria increased after treatment with each of the HDAC inhibitors. See Figure 2 for the procedure for quantifying the data. See Figure 1 for other definitions. Bar, 5 µm. **P < 0.01. The asterisks indicate a significant difference compared with the control.
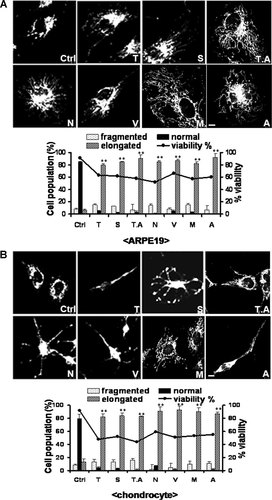
HDAC inhibitors induce mitochondrial elongation in an untransformed ARPE19 cell line and primary cultured rat chondrocytes. Cells were treated with six different HDAC inhibitors for 48 h. To reduce cell viability by approximately 50%, trichostatin A (T), valproic acid (V), sodium butyrate (N), trapoxin A (T.A), apicidin (A), and MS-275 (M) were used at 40 µM, 30 mM, 10 mM, 10 nM, 1 µg, and 15 µM, respectively. See Figure 2 for the confocal microscopy procedure used to observe mitochondrial morphology and the procedure for quantifying the data. Induction of mitochondrial elongation by HDAC inhibitors in (A) the human retinal pigment epithelial cell line ARPE-19 and (B) primary cultured rat articular chondrocytes. All HDAC inhibitors tested at the concentration inducing apoptosis in approximately 50% of cells induced mitochondrial elongation and increased the percentage of cells with elongated mitochondria. See Figure 1 for other definitions. Bar, 5 µm. **P < 0.01. The asterisks indicate a significant difference compared with the control.
Mitochondrial fusion and fission machineries may be involved in SAHA-induced mitochondrial elongation
Because previous reports have shown that drug-induced mitochondrial elongation is abolished by pretreatment with the microtubule-disrupting agent nocodazole (Bowes and Gupta, 2005, 2008), we examined whether pretreatment with nocodazole could also abolish the mitochondrial elongation by SAHA. Pretreatment with nocodazole did not prevent SAHA-induced mitochondrial elongation (Supplementary Fig. S3). We next examined whether mitochondrial fusion and fission machineries are involved in the mitochondrial elongation by SAHA. Our Western blot analysis showed that SAHA substantially reduced the level of Fis1, whereas SAHA did not substantially influence the expression levels of the other mitochondrial morphology-regulating proteins Drp1, Mff, Mfn1, Mfn2, and Opa1 (Fig. 6A and Supplementery Fig. S4A).
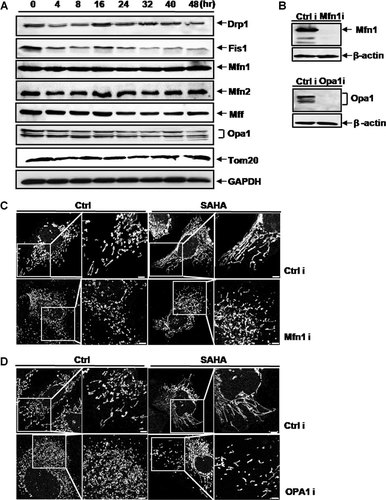
Mitochondrial fusion and fission machineries are involved in the SAHA-induced mitochondrial elongation. A: Western blot assay of the mitochondrial morphology-regulating proteins. Hep3B cells were treated with 8 µM SAHA for 0–48 h. GAPDH, a loading control. Tom 20, a loading control for mitochondrial proteins. B: Total cell lysates from the Mfn1 or Opa1 RNAi-treated or control RNAi cells were prepared. The expression levels of Mfn1 and Opa1 were analyzed by Western blot analysis. β-actin was also analyzed as a loading control. Mfn1 or Opa1 was efficiently depleted in Hep3B cells. C,D: To observe the mitochondrial morphology, cells were stained with Mitotracker CMSRos (red) for 20 min before fixation. In the Mfn1-depleted (C) and Opa1-depleted (D) Hep3B cells, mitochondria were fragmented irrespective of the presence of SAHA, although mitochondrial length was slightly longer with SAHA treatment. Bar, 5 µm.
We examined whether SAHA-induced mitochondrial elongation depended on the fusion-regulating proteins Mfn1 and Opa1. We used shRNAs to deplete Mfn1 and Opa1 in Hep3B cells (Fig. 6B). In control RNAi cells, we observed mitochondrial elongation as a result of treatment with SAHA. In both RNAi cells depleted of Mfn1 and Opa1, mitochondria were fragmented irrespective of the presence of SAHA, although mitochondrial length was slightly greater with SAHA treatment (Fig. 6C,D). These results indicate that the mitochondrial fusion-regulating proteins Mfn1 and Opa1 are important in SAHA-induced mitochondrial elongation, although the mechanisms remain to be clarified.
Because Fis1 was downregulated in SAHA-treated Hep3B cells, we examined the role of mitochondrial fission-regulating protein Fis1 in SAHA-induced mitochondrial elongation. We first examined whether depletion of Fis1 is linked with the elongation of mitochondria in Hep3B cells. Transient knock-down of Fis1 by shRNA also prominently induced mitochondrial elongation. SAHA had no effect on a further increase in the mitochondrial elongation by Fis1 depletion (Supplementary Fig. S4B), indicating an involvement of Fis1 downregulation in the SAHA-induced mitochondrial elongation. We next established Fis1-overexpressing Hep3B cells, which did overexpress the Fis1 protein. Most Fis1-overexpressing Hep3B cells had fragmented or punctate mitochondria (Fig. 7A). SAHA slightly decreased the expression amount of Fis1 also in Fis1-overexpressing Hep3B cells, which might cause the moderate mitochondrial elongation in cells overexpressing Fis1 (Fig. 7A). Alternatively, the moderate mitochondrial elongation by SAHA treatment in cells expressing Fis1 may be caused by the unknown effects of SAHA on Fis1 and/or other mitochondrial morphology-regulating proteins via modulating their functions or stabilities. By comparison between the SAHA-treated cells with and without overexpressing Fis1, the relative length of the mitochondria in Hep3B/Fis1 cells treated with SAHA was shorter than in Hep3B/Vector cells treated with SAHA (Fig. 7A). Quantification data also showed that SAHA-treated Hep3B/Fis1 cells predominantly contained the tubular mitochondria (<10 µm in length), while SAHA-treated Hep3B/Vector cells predominantly contained the elongated mitochondria (≥10 µm in length) (Fig. 7A). These results suggested that overexpression of Fis1 attenuates the SAHA-induced mitochondrial elongation.
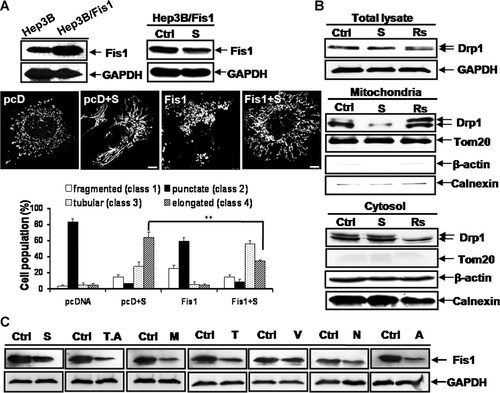
Reduced expression of Fis1 and reduced translocation of Drp1 to the mitochondria by HDAC inhibitors play a pivotal role in the induction of mitochondrial elongation. A: Overexpression of Fis1 attenuated the SAHA-induced mitochondrial elongation. The established Fis1-overexpressing Hep3B cells (Hep3B/Fis1) did overexpress the Fis1 protein (Upper left). Treatment with SAHA for 48 h slightly decreased the expression amount of Fis1 also in Fis1-overexpressing Hep3B cells (Upper right). GAPDH, a loading control. Confocal microscopy (middle panel) and quantification of the data (lower panel) showed that overexpression of Fis1 attenuated the SAHA-induced mitochondrial elongation. Whereas most Fis1-overexpressing Hep3B cells have fragmented (class 1) or punctate (class 2) mitochondria, SAHA treatment induced the mitochondrial elongation in Fis1-overexpressing Hep3B cells in that most Fis1-overexpressing Hep3B cells treated with SAHA had tubular (class 3) or elongated (class 4) mitochondria. However, SAHA treatment significantly decreased the proportion of Hep3B/Fis1 cells with elongated (class 4) mitochondria compared to Hep3B/vector cells (**P < 0.01), indicating that overexpression of Fis1 attenuated the SAHA-induced mitochondrial elongation. The percentage of cells with fragmented (white), punctate (solid), tubular (<10 µm in length, dotted), or elongated (≥10 µm in length, striped) mitochondria are depicted in a diagram. At least 100 cells in several fields were counted in each experiment. Data represent the mean ± SD of at least four independent experiments. GAPDH is loading controls. B: Western blot assay showing the amount of Drp1 in the mitochondrial and cytosolic fractions and whole cell lysate. The amount of Drp1 in the mitochondrial fraction of cells treated with mitochondrial fission-inducing resveratrol at 200 µM was substantially increased compared to the control. Notably, the amount of Drp1 in the mitochondrial fraction of the SAHA-treated cells was substantially reduced. The expression level of Drp1 in whole cell lysate and cytosolic fraction was not altered by treatment of SAHA, whereas the expression level of Drp1 in cytosolic fraction of cells treated with resveratrol was substantially reduced. GAPDH and β-actin are loading controls. Tom20 and calnexin are mitochondria and endoplasmic reticulum (ER) markers, representatively. C: Reduction of Fis1 expression by treatment with six different HDAC inhibitors. Similar to treatment with SAHA, six other different HDAC inhibitors reduced the expression of Fis1 protein. Cells were treated with 8 µM SAHA, 40 µM trichostatin A (T), 30 mM valproic acid (V), 10 mM sodium butyrate (N), 10 nM trapoxin A (T.A), 1 µg apicidin (A), or 15 µM MS-275 (M) for 48 h. All HDAC inhibitors tested reduced the expression of Fis1. GAPDH, a loading control. See Figure 1 for other definitions. Bar, 5 µm.
Because Fis1 recruits cytosolic Drp1 to the mitochondrial membrane, we further tested whether SAHA could affect Drp1 translocation. In the control experiment, enhanced translocation of Drp1 to mitochondria was observed in cells treated with resveratrol. Noticeably, the amount of Drp1 in the mitochondrial fraction was reduced in SAHA-treated cells compared to the control cells, while the expression level of Drp1 in whole cell lysate was not altered by treatment of SAHA (Fig. 7B). However, the expected increase of the cytosolic Drp1 by treatment of SAHA was not detected (Fig. 7B). This may be due to the presence of an abundant amount of Drp1 in the cytosolic fraction, which can lead to making it difficult to detect small changes in the cytosolic Drp1 level by Western blotting. We next examined whether SAHA directly induces the acetylation of mitochondrial morphology-regulating proteins, but we did not observe any acetylation of Drp1, Fis1, Mfn1, or Opa1 in SAHA-treated Hep3B cells (Supplementary Fig. S5). We further examined whether other HDAC inhibitors also reduced the expression level of the Fis1 protein. Notably, all six HDAC inhibitors tested reduced Fis1 expression (Fig. 7C).
Mitochondrial elongation was observed in cells treated with HDAC inhibitors at doses inducing histone acetylation
Finally, we examined whether mitochondrial elongation by HDAC inhibitors is correlated with histone acetylation. For this assay, Hep3B cells were treated with different concentrations of HDAC inhibitors. Mitochondrial elongation was observed in cells treated with concentrations of the HDAC inhibitors required to initiate histone acetylation (Fig. 8A–E and Supplementary Fig. S6).
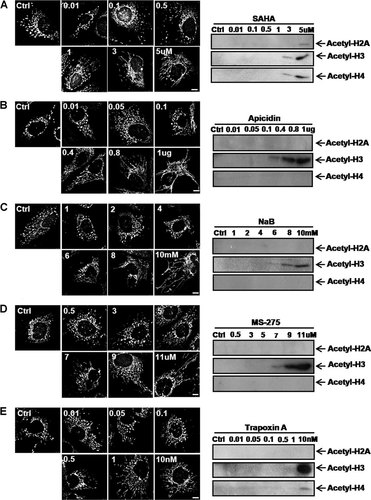
Mitochondrial elongation was observed in cells treated with HDAC inhibitors at doses inducing histone acetylation. Hep3B cells were treated with each HDAC inhibitor at the indicated concentrations, and cells were harvested 48 h after treatment. Left panel, confocal microscopy. To visualize the mitochondrial morphology, cells were directly stained with Mitotracker CMSRos (red) for 20 min before harvesting. Right panels, Western blot assay using acetylated histone H2A, H3, and H3 antibodies. Confocal microscopy and Western blot analysis revealed that both mitochondrial fusion and histone acetylation were induced by concentrations of (A) above 3 µM for SAHA, (B) above 0.4 µg for apicidin (C), over 8 mM for sodium butyrate (D), over 7 µM for MS-275, or (E) over 10 nM for trapoxin A. See Figure 1 for other definitions.
Discussion
Acetylation and deacetylation of histones, which are core proteins of the nucleosome, play a role in the regulation of gene expression. The extent of acetylation is determined by the opposing activities of two classes of enzymes: histone acetyl tranferases (HATs) and HDACs. Altered HAT or HDAC activity is associated with cancers. In the search for cancer treatments, much effort has been put forth to discover novel HDAC inhibitors to modulate histone acetylation. To date, a number of structurally dissimilar HDAC inhibitors have been identified. HDAC inhibitors belong to several chemical structural categories, including (i) hydroxamic acids, e.g., trichostatin A, oxamflatin and SAHA; (ii) cyclic tetrapeptides containing the epoxyketone structure (2S,9S)-2-amino-8-oxo-9,10-epoxy-decanoyl (Aoe) moiety, e.g., trapoxin A and trapoxin Cyl-1 and Cyl-2, HC-toxin, WF-3161 and chlamydocin; (iii) cyclic peptides not containing an Aoe moiety, e.g., depsipeptide (FK228) and apicidin; (iv) benzamides, e.g., MS-275; (v) short-chain and aromatic fatty acids, e.g., sodium butyrate and sodium phenylbutyrate; and (vi) miscellaneous compounds, e.g., depudecin. HDAC inhibitors exert their activity by activating the transcription of a defined set of genes through chromatin remodeling (Atadja, 2011). Numerous novel HDAC inhibitors were found to have anti-cancer function. Among them, varinostat has been approved by FDA for treating cutaneous T-cell lymphoma (CTCL) for patients with progressive, persistent, or recurrent disease. Furthermore, other HDAC inhibitors such as PXD101, PCI24781, ITF2357, MGCD0103, and LBH589 have also demonstrated therapeutic potential as monotherapy or combination with other anti-tumor drugs in CTCL and other malignancies (Tan et al., 2010).
However, modification of the epigenetic histone code is not sufficient to account for the efficacy of HDAC inhibitors. HDAC inhibitors can modulate the acetylation of non-histone proteins. The counteracting modifications of acetylation and deacetylation affect the activity non-histone proteins. A steadily growing number of non-histone proteins are reportedly subject to acetylation by HATs and HDACs. The non-histone targets include transcription factors, hormone receptors, signal transducers, chaperones, and the proteins of the cytoskeleton (Yang and Seto, 2008). Reversible lysine acetylation of non-histone proteins modulates their functions by altering their stability, the stability of their mRNAs, protein localization and degradation, and protein–protein and protein–DNA interactions (Singh et al., 2010). In addition, HDAC inhibitors facilitate the dephosphorylation of signaling kinases, including Akt (Chen et al., 2005).
HDAC inhibitors not only induce cultured tumor cells to undergo growth arrest, differentiation, and/or apoptotic cell death but inhibit the growth of cancer cells in animal models (Glick et al., 1999; Kim et al., 1999). HDAC inhibitors are considered a new group of agents that regulate gene expression and induce apoptosis and cell cycle arrest in cancer cells by altering the acetylation status of chromatin and other non-histone proteins.
Based on our data showing that mitochondrial elongation occurred in cells treated with concentrations of HDAC inhibitors that induced the acetylation of histones, we presume that the modulation of mitochondrial morphology-regulating proteins by HDAC inhibitors is correlated with their histone- or non-histone-acetylating activity. Although our data indicate that the HDAC inhibitors we employed did not directly increase the acetylation of mitochondrial morphology-regulating proteins, HDAC inhibitors could modify post-translationally mitochondrial morphology-regulating proteins. In addition, we cannot exclude the possibility that HDAC inhibitors induce mitochondrial elongation by modulating signaling kinases. Thus, deciphering the detailed molecular mechanism by which HDAC inhibitors modulates mitochondrial morphology-regulating proteins via multiple pathways is an important and challenging task.
To date, few previous studies have reported on drug-induced mitochondrial elongation (Bowes and Gupta, 2005; Bowes and Gupta, 2008; Cassidy-Stone et al., 2008; Mishra et al., 2010). One previous study demonstrated that the cysteine-alkylators ethacrynic acid and N-ethylmaleimide induce mitochondrial elongation by forming a large reticulum and that these drugs induce rapid mitochondrial fusion at near-toxic concentrations approximately 30 min after treatment (Bowes and Gupta, 2005). Furthermore, another study that employed the microtubule-disrupting agent nocodazol found that drug-induced mitochondrial elongation coincides with a cessation of fast mitochondrial movement, which depends on microtubules, indicating that mitochondrial elongation by these drugs results from inhibition of mitochondrial fission combined with continued application of motile force by microtubule-dependent motor complexes (Bowes and Gupta, 2008). However, mitochondrial elongation induced by HDAC inhibitors can be distinguished from the fusion induced by cysteine-alkylators. Here, HDAC inhibitors induced mitochondrial fusion at subtoxic as well as toxic concentrations. Elongated mitochondria were not observed until 4 h after treatment, but a substantial increase in the proportion of cells exhibiting elongated mitochondria was attained until 16 h after treatment. Pretreatment with nocodazole did not prevent the mitochondrial elongation by SAHA, indicating that HDAC inhibitor-induced mitochondrial elongation is not correlated with microtubule-dependent mitochondrial movement. We demonstrated that HDAC inhibitors induced downregulation of Fis1 and decrease of mitochondrial translocation of Drp1 in Hep3B cells. Depletion of Fis1 or Drp1 by RNAi is known to induce mitochondrial fusion (Lee et al., 2004). Previous studies also showed that Drp1 recruitment to the mitochondria occurs via interaction with Fis1 (Yoon et al., 2003) and is regulated by post-translational modulations including protein phosphorylation, sumoylation, ubiquitination, and S-nitrosylation (Cereghetti et al., 2008; Chang and Blackstone, 2010). Therefore, it is highly probable that a decreased level of Fis1 in SAHA-treated cells might subsequently lead to decreased Drp1 recruitment to the mitochondria, which causes inhibition of mitochondrial fission and promotion of fusion and finally results in disequilibration of mitochondrial morphology. A recent study demonstrated that Mff is anchored in the mitochondrial outer membrane (MOM) and controls mitochondrial and peroxisomal fission in mammalian cells. The study further suggested that Mff and Fis1 play different roles in the fission process although both are anchored in MOM (Gandre-Babbe and van der Bliek, 2008). Another study elicited that Mff knockdown releases the Drp1 foci from MOM and Mff-dependent mitochondrial fission proceeds independent of Fis1, suggesting that Mff functions as an essential factor in mitochondrial recruitment of Drp1 (Otera et al., 2010). Although our results show that the expression level of Mff was not substantially altered after SAHA treatment, the molecular role of Mff in HDAC-induced mitochondrial elongation is still remained to be elucidated. Our results also suggested that the mitochondrial fusion-regulating proteins Mfn1 and Opa1 were essential for SAHA-induced mitochondrial elongation. On the basis of the data obtained from the present study, HDAC inhibitors seem to induce mitochondrial elongation by decreasing Fis1 expression and mitochondrial fusion machinery may participate in this phenomenon, although the molecular mechanisms remain to be clarified.
A previous study identified a chemical inhibitor of Drp1, called mdivi-1 (Cassidy-Stone et al., 2008). Mdivi-1 causes mitochondrial fission deficit, resulting in excessively interconnected mitochondria due to ongoing fusion. Because this small-molecule inhibitor not only induces mitochondrial elongation but also prevents Bax-mediated mitochondrial membrane permeabilization during apoptosis, mdivi-1 offers a new approach to explore the role of mitochondrial fission in apoptosis. Unlike mdivi-1, our HDAC inhibitors induced mitochondrial elongation by modulating Fis1 expression rather than Drp1 expression. Furthermore, HDAC inhibitors induced mitochondrial elongation at subtoxic and toxic concentrations that can induce apoptosis. Thus, HDAC inhibitors may be useful for exploring the roles of mitochondrial fusion in response to a myriad of cellular functions and in cell fate in both the non-apoptotic steady-state and during apoptosis.
The present study may provide insight into a valuable experimental strategy for simple manipulation of mitochondrial morphology. Although numerous studies have examined mitochondrial dynamics, studies on the artificial induction of mitochondrial elongation are limited. Numerous apoptosis-inducing chemicals, such as staurosporine, actinomycin D and etoposide, induce mitochondrial fragmentation. In contrast, drugs that induce mitochondrial elongation by modulating mitochondrial morphology-regulating proteins without toxicity are limited. RNAi approach manipulating the expression of fission proteins Drp1 and Fis1 is currently used to develop cells bearing elongated mitochondria. However, this method is not completely useful because of its experimental limitations, such as low-transfection efficiency, non-specific gene targeting, and difficulty of turning on/off. Thus, the simple application of HDAC inhibitors may replace this complicated experimental procedure. The present study provides an easily approachable experimental tool for mitochondrial investigators who study mitochondrial morphology and function.
In conclusion, the present study shows that HDAC inhibitors induce mitochondrial elongation. Although the detailed molecular mechanism by which HDAC inhibitors modulate mitochondrial morphology-regulating proteins via multiple pathways remains to be deciphered, our results provide insight into the role of HDAC inhibitors in mitochondrial morphology.
Acknowledgements
This study was supported by National Research Foundation of Korea Grant by the Korean government, grant number 2010-0001942.