The metabolism of human mesenchymal stem cells during proliferation and differentiation
Abstract
Human mesenchymal stem cells (MSCs) reside under hypoxic conditions in vivo, between 4% and 7% oxygen. Differentiation of MSCs under hypoxic conditions results in inhibited osteogenesis, while chondrogenesis is unaffected. The reasons for these results may be associated with the inherent metabolism of the cells. The present investigation measured the oxygen consumption, glucose consumption and lactate production of MSCs during proliferation and subsequent differentiation towards the osteogenic and chondrogenic lineages. MSCs expanded under normoxia had an oxygen consumption rate of ∼98 fmol/cell/h, 75% of which was azide-sensitive, suggesting that these cells derive a significant proportion of ATP from oxidative phosphorylation in addition to glycolysis. By contrast, MSCs differentiated towards the chondrogenic lineage using pellet culture had significantly reduced oxygen consumption after 24 h in culture, falling to ∼12 fmol/cell/h after 21 days, indicating a shift towards a predominantly glycolytic metabolism. By comparison, MSCs retained an oxygen consumption rate of ∼98 fmol/cell/h over 21 days of osteogenic culture conditions, indicating that these cells had a more oxidative energy metabolism than the chondrogenic cultures. In conclusion, osteogenic and chondrogenic MSC cultures appear to adopt the balance of oxidative phosphorylation and glycolysis reported for the respective mature cell phenotypes. The addition of TGF-β to chondrogenic pellet cultures significantly enhanced glycosaminoglycan accumulation, but caused no significant effect on cellular oxygen consumption. Thus, the differences between the energy metabolism of chondrogenic and osteogenic cultures may be associated with the culture conditions and not necessarily their respective differentiation. J. Cell. Physiol. 226: 2562–2570, 2011. © 2010 Wiley-Liss, Inc.
Bone marrow-derived mesenchymal stem cells (MSCs) provide an attractive cell source for musculoskeletal tissue engineering or regenerative medicine therapies, as they have the ability to differentiate into tissues of the mesenchymal lineage, such as cartilage, bone, fat and muscle (Pittenger et al., 1999; Caplan and Bruder, 2001; Pittenger and Marshak, 2001). However, their utilisation in cell therapies may be limited by their limited population doubling during expansion in monolayer culture under 20% oxygen or normoxic culture conditions which does not reflect the oxygen environment in bone marrow in vivo (Moussavi-Harami et al., 2004; Grayson et al., 2006; d'Ippolito et al., 2006).
MSCs reside physiologically under hypoxic conditions within the bone marrow, with oxygen tension reported to lie between 4% and 7% (Grant and Smith, 1963; Kofoed et al., 1985). Studies have shown that culture of MSCs under hypoxia affects both their proliferation and differentiation. MSCs cultured under normoxia exhibit premature senescence and a reduction in population doublings compared with cells cultured under hypoxia (Lennon et al., 2001; Moussavi-Harami et al., 2004; Grayson et al., 2006, 2007; Dos Santos et al., 2010). Moreover, osteogenic differentiation is inhibited under hypoxia, whereas chondrogenesis occurs under both oxygen conditions but may be enhanced under hypoxia (Tuncay et al., 1994; Lennon et al., 2001; Warren et al., 2001; Malda et al., 2004; Salim et al., 2004; Wang et al., 2005; Malladi et al., 2006; d'Ippolito et al., 2006; Markway et al., 2010). These phenomena may be associated with the underlying energy metabolism of the cells, specifically relating to the balance between glycolysis and oxidative phosphorylation and the production of reactive oxygen species (Martin et al., 2004; Moussavi-Harami et al., 2004). Furthermore, understanding the underlying energy metabolism would help to generate suitable growth conditions for MSCs during either proliferation or differentiation, enabling greater cell yield or tissue formation for tissue engineering or regenerative medicine applications.
Studies analysing MSC energy metabolism during proliferation and differentiation have focussed primarily on their glucose consumption and lactate production (Wang et al., 2005; Follmar et al., 2006; Grayson et al., 2006; Mischen et al., 2008). MSCs produce lactate upon consumption of glucose under normoxic conditions. The production of lactate under normoxia, termed the Warburg effect, has been previously demonstrated for other cell types that reside under hypoxia in vivo, such as chondrocytes and tumour cells (Krebs, 1972; Rajpurohit et al., 1996). MSCs increase their rate of glycolysis upon culture under hypoxia, consistent with the Pasteur effect (Krebs, 1972; Wang et al., 2005; Grayson et al., 2006; Mischen et al., 2008). However, MSC oxygen consumption was not measured within these investigations and therefore its contribution to cellular metabolism is unknown.
Few studies have assessed changes in MSC energy metabolism during differentiation toward the osteogenic and chondrogenic lineages. Both Chen et al. (2008) and von Heimburg et al. (2005) reported an increase in oxygen consumption during osteogenic and adipogenic differentiation for MSCs derived from bone marrow and adipose tissue, respectively (Wang et al., 2005; Chen et al., 2008). Wang and co-workers report higher rates of glycolysis following chondrogenic differentiation compared to proliferative MSCs (Wang et al., 2005; Chen et al., 2008). However there are no studies that have compared oxygen consumption during both osteogenic and chondrogenic differentiation. While there is a paucity of data on MSC energy metabolism during differentiation, the behaviour of mature cell phenotypes has been described in more detail. Chondrocytes have been shown to have a high level of glycolysis with minimal oxygen consumption (Rajpurohit et al., 1996; Heywood and Lee, 2008). However, monolayer expansion of chondrocytes results in an increase in oxygen consumption, associated with oxidative phosphorylation (Domm et al., 2002, 2004; Malda et al., 2004; Heywood and Lee, 2008). Osteoblasts have significantly greater oxygen consumption than chondrocytes, and are reported to exhibit a mixed metabolism, utilising both glycolysis and oxidative phosphorylation for their ATP production upon culture under normoxia (Borle et al., 1960a,b; Smith et al., 1973; Komarova et al., 2000).
The hypotheses tested in the current study are first, that human bone marrow-derived MSCs maintain high levels of glycolytic metabolism during expansion under normoxia. Secondly, they maintain their glycolytic metabolism during chondrogenic differentiation but increase levels of oxidative phosphorylation during osteogenic differentiation. In order to address these hypotheses the present study provides a detailed analysis of MSC metabolism during proliferation, osteogenic and chondrogenic differentiation via measurements of oxygen and glucose consumption and lactate production for MSC populations.
Materials and Methods
Culture of MSCs
Human bone marrow-derived MSCs were procured from a commercial source (Lonza, Wokingham, UK) and were cultured to passage two and frozen prior to delivery to host laboratory. Cells were derived from bone marrow aspirated from the iliac crest of three male donors aged 19, 21 and 28. The cells were resuscitated and resuspended in Alpha Minimal Essential Medium (Invitrogen, Paisley, UK) + 8.5% foetal bovine serum (Sigma–Aldrich, Poole, UK), 1 ng/ml basic fibroblast growth factor (Serotec, Oxford, UK), 100 U/ml penicillin/100 µg streptomycin, 2 mM L-glutamine, 25 mM HEPES buffer and 0.2 mM L-ascorbic acid-2-phosphate (all Sigma–Aldrich), referred to hereafter as α-MEM + 8.5% FBS. MSCs were seeded in culture flasks at 2 × 103 cells/cm2 and incubated at 37°C/5% CO2 with medium replenishment every 2–3 days. The cells were trypsinised on reaching 80–90% confluence, phosphate buffered saline (PBS) supplemented with 0.5 mg/ml trypsin and 0.2 mg/ml EDTA (Sigma–Aldrich) solution. The cells were reseeded at 2 × 103 cells/cm2 and cultured for a further three passages. MSC population doubling time was calculated based upon the cumulative cell number for successive passages from P3 to P5.
Measurement of MSC oxygen consumption, glucose consumption and lactate release
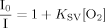 (1)
(1)MSCs were resuspended in oxygen and temperature equilibrated α-MEM (without phenol red; Invitrogen) + 8.5% FBS at four concentrations ranging from 3.85 × 105 to 1.54 × 106 cells/ml that when aliquoted into the biosensor wells at a volume of 130 µl correspond to 0.5 × 105, 1 × 105, 1.5 × 105 and 2 × 105 cells/well. Cell suspension samples at each concentration were added in triplicate, alongside a zero oxygen control (0.1 M sodium sulphite; Sigma–Aldrich) and cell-free oxygen and temperature equilibrated medium samples. The plate was sealed using a sterile plate sealer and the fluorescence intensity was read every 10 min over a period of 2 h using a fluorimeter with excitation set at 485 nm and emission read at 590 nm (Fluostar Galaxy, BMG Labtech, Aylesbury, UK). The plate was maintained at 37°C throughout the period of measurement. The oxygen concentration at each time point measured were calculated as described in a previous study (Heywood et al., 2006). A correction factor for oxygen ingress was applied to the data prior to calculation of the consumption rate and per cell consumption rate, as detailed previously (Heywood et al., 2010). The permeability constant used in the present investigation was 0.23 ± 0.04 (mean ± SD).
At selected time points the medium was aspirated from the wells for glucose and lactate analysis, determined using glucose and lactate assays developed in a previous investigation (Heywood et al., 2006). The rate of glucose consumption and lactate production was derived from the linear portion of the glucose–time and lactate–time curves. These rates were normalised to cell number to obtain glucose consumption and lactate production rates.
Metabolic modulators
In further studies, MSCs were cultured in the presence of modulators that enhance or inhibit specific metabolites or enzymes along the pathways of glycolysis and oxidative phosphorylation (Newsholme and Leech, 1991). The following modulators were used; 2-deoxy-D-glucose, a competitive inhibitor of glucose uptake and metabolism; sodium azide which inhibits complex IV of the mitochondrial electron transport chain; and carbonyl-cyanide-m-chlorophenylhydrazone (CCCP), a potent mitochondrial uncoupler that stimulates oxygen consumption by the electron transport chain to maximal levels (Terada, 1981; Lee and Urban, 1997; Ishihara and Urban, 1999). Cells were resuspended at 1.15 × 106 cells/ml in the presence or absence of 50 mM 2-deoxy-D-glucose, 10 mM sodium azide or 1 µM CCCP. Cell suspensions were aliquoted at a volume of 130 µl into individual wells of either the 384-well oxygen biosensor or 384-well plate, corresponding to 1.5 × 105 cells/well. Oxygen consumption, glucose consumption and lactate production analysis was as described above. MSC viability during culture in the presence of the metabolic modulators was monitored by Calein AM fluorescence (Molecular Probes, Invitrogen, Paisley, UK).
Osteogenic differentiation
For osteogenesis, MSCs were seeded at 2 × 103 cells/cm2 and allowed to attach for 24 h and then a set of flasks were cultured in medium (α-MEM + 10% FBS) containing osteogenic supplements (+0.05 mM L-ascorbic acid-2-phosphate + 10 mM β-glycerophosphate + 0.1 mM dexamethasone all from Sigma–Aldrich). This represented day 0 for MSC osteogenic differentiation with medium replenishments every 2–3 days and cultured for 21 days. Control samples were cultured in α-MEM + 10% FBS for the same period in culture. Furthermore, passage 3 MSCs were seeded at 2 × 103 cells/cm2 onto 6- and 12-well culture plates and utilised for calcium deposition via alizarin red staining and alkaline phosphatase (ALP) activity. Measurements of metabolic parameters for control and osteogenic MSCs were taken on days 0, 1, 7, 14 and 21. Medium was additionally supplemented with 10 mM sodium azide for assessment of oxygen consumption on days 7 and 21 for control and osteogenic MSCs.
ALP activity was measured on days 0, 1, 7, 14 and 21 using an ALP assay kit (Randox Laboratories, Country Antrim, UK) and was performed according to manufacturers' instructions. The ALP concentration was normalised to DNA concentration measured using Quant-it Picogreen assay (Invitrogen), following manufacturers' instructions. Osteogenic cultures were removed after 21 days in culture for assessment of calcium deposition. The cells were washed with PBS, fixed using 70% (v/v) ethanol for an hour at room temperature and then further washed using distilled water. A 2% (w/v) alizarin red solution was added to each well with staining achieved at room temperature for 30 min, prior to washing using distilled water. Cultures were visualised using a light microscope.
Chondrogenic differentiation
For chondrogenesis, MSC pellets containing 2.5 × 105 cells were cultured in the presence of chondrogenic medium (Dulbecco's modified Eagle medium-high glucose (DMEM-HG) + 1 mM sodium pyruvate + 0.35 mM L-proline + 0.17 mM L-ascorbic acid-2-phosphate + 0.1 µM dexamethasone (all Sigma–Aldrich) + 10 nM TGF-β3 (Peprotech, London, UK) + ITS + premix (insulin (6.25 µg/ml), selenious acid (6.25 µg/ml), linoleic acid (5.35 µg/ml), bovine serum albumin (1.25 µg/ml) (BD Biosciences)) for a period of 21 days. A separate set of MSC pellets were cultured in chondrogenic medium without TGF-β3, to act as a control. Measurements of metabolic parameters for control and chondrogenic MSCs were taken on days 0, 1, 7, 14 and 21 with control and chondrogenic pellets transferred into the separate wells of the biosensor plates and each well containing two pellets. The plate was sealed and the oxygen/glucose consumption and lactate production measurements were assessed as described above. Medium was additionally supplemented with 10 mM sodium azide for assessment of oxygen consumption on days 7 and 21. Per cell rates were calculated from the cell number obtained from DNA assays.
At appropriate time points, pellets were digested in papain-digest buffer solution for use in glycosaminoglycan (GAG) and DNA analysis. Total GAG content within the pellet was measured using 1,9-dimethlymethylene blue assay on papain-digested samples taken on days 1, 7, 14 and 21 during culture (Farndale et al., 1986). The GAG content was normalised to the concentration of DNA within the sample. Further pellets were fixed in 3.7% formaldehyde (BDH, Poole, UK) in PBS, embedded in wax and sectioned. The sections were rehydrated and stained with 1% toluidine blue solution. Sections were washed and then dehydrated prior to visualisation under a microscope.
Statistical analysis
The per cell oxygen and glucose consumption and lactate production rates for the cell density data was assessed using a single-factor ANOVA (α = 0.05). If differences were significant between samples then post hoc Student's t-test with Bonferroni correction (P < 0.05) was applied. The same test was applied to the modulator and differentiation studies regarding their oxygen consumption, glucose consumption and lactate production rate data. Alkaline phosphatise activity and GAG/DNA synthesis rates were compared between cultures at each time point using Student's t-test (P < 0.05).
Results
MSC proliferation
Human MSCs were resuscitated at P2 and subsequently cultured for three further passages (P3–5). Population growth curves were generated from the individual cell populations and doubling time was calculated. There was no significant difference in population doubling time between P3 (59.4 ± 8.5 h: mean ± SD) and P4 (65.8 ± 11.7 h). However a significant increase in population doubling time was observed for P5 (88.8 ± 24.8 h) compared to P3 and P4 (Student's t-test; P < 0.05).
MSCs exhibit a mixed metabolism that does not alter during proliferation
Representative data for oxygen, glucose and lactate concentrations within the medium during MSC culture are presented in Figure 1. These concentration measurements were used to calculate per cell oxygen/glucose consumption and lactate release rates for MSCs at P3–5. There were no significant differences between passage for any of the parameters, and therefore data were subsequently combined from the three passages and are presented in Table 1 for cell numbers ranging from 0.5 to 2 × 105 cells/well. In general, there were no significant differences in the per cell rates for oxygen consumption, glucose consumption or lactate production between cell densities (Table 1, ANOVA; P > 0.05), although there were differences in cellular glucose consumption and lactate production rate at 1 × 105 cells/well compared with other cell numbers (Student's t-test; P < 0.05). Furthermore, analysis of the ratio between glucose consumption and lactate production shows that the ratio approached or was, in some cases, greater than the 2:1 stoichiometric ratio for glycolysis.
Example of (A) oxygen consumption, (B) glucose consumption and (C) lactate production measurements for MSCs at passage 3 for 1.5 × 105 cells/well. Data represent mean ± SD of n = 9.
| Cell number (×105) | Oxygen consumption rate/cell (fmol/h/cell) | Glucose consumption rate/cell (fmol/h/cell) | Lactate production rate/cell (fmol/h/cell) |
|---|---|---|---|
| 2 | 105.7 ± 21.7 | 357.3 ± 45.2 | 960 ± 148 |
| 1.5 | 113 ± 22.3 | 327.6 ± 47.8 | 829.1 ± 92.5 |
| 1 | 101.7 ± 18.1 | 272.8 ± 43.3 | 637.5 ± 122.3 |
| 0.5 | 98.2 ± 24.2 | 378.2 ± 127.7 | 741.9 ± 295.5 |
- Data represent mean ± SD of n = 27.
There was a significant increase in oxygen consumption rate in the presence of 2-deoxy-D-glucose when compared with control conditions (Student's t-test; P < 0.05, Table 2). Sodium azide significantly reduced oxygen consumption (Student's t-test; P < 0.05, Table 2), to 25% of untreated values. Sodium azide sensitive oxygen consumption allows us to estimate mitochondrial oxygen consumption to be 73.0 fmol/h/cell. Glucose consumption was completely abolished by 2-deoxy-D-glucose (Student's t-test; P < 0.05, Table 2), while there was no significant difference in the per cell glucose consumption rate between control and sodium azide treated MSCs (Table 2). Lactate production was significantly reduced in the presence of 2-deoxy-D-glucose but was increased in the presence of sodium azide (Student's t-test; P < 0.05, Table 2). The mitochondrial uncoupler, CCCP, induced a significant increase in oxygen consumption rate compared with control samples (Student's t-test; P < 0.05, Table 2). There was found to be a significant reduction in glucose consumption rate (Student's t-test; P < 0.05, Table 2) and no significant difference in lactate production rate between CCCP-treated and control MSC samples (Student's t-test; P > 0.05, Table 2). MSC viability, assessed by Calcein AM fluorescence, was maintained at control levels during culture in the presence of all metabolic modulators (data not shown).
| Inhibitor | Oxygen consumption rate/cell (fmol/h/cell) | Glucose consumption rate/cell (fmol/h/cell) | Lactate production rate/cell (fmol/h/cell) |
|---|---|---|---|
| Control | 97.5 ± 17.7 | 342.1 ± 33.5 | 839.6 ± 79.3 |
| 50 mM 2-deoxy-D-glucose | 137.2 ± 33.9* | 0* | 245.1 ± 16.6* |
| 10 mM sodium azide | 24.5 ± 10.5* | 332.6 ± 61.6 | 1100 ± 157* |
| 1 µM CCCP | 216.1 ± 31.3* | 291.0 ± 25.5 | 775.3 ± 46.5 |
- Significant differences in consumption and production rates upon modulator treatment (*P < 0.05) relative to control were tested using Bonferroni corrected Student's t-test. Data represent mean ± SD of n = 9.
Osteogenic culture suppresses lactate release without alteration in oxygen consumption
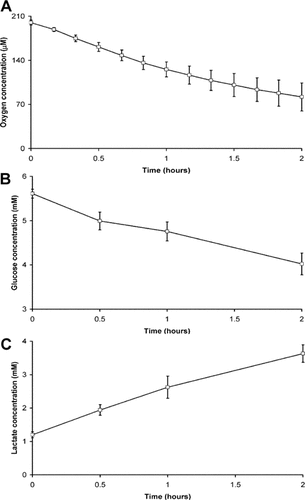
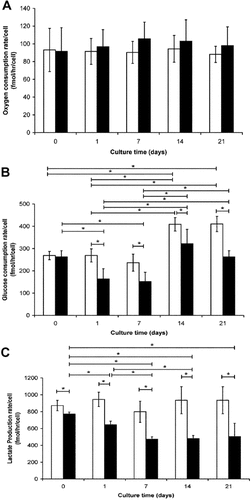
Oxygen/glucose consumption and lactate release data for cells cultured for up to 21 days in osteogenic conditions, compared to proliferative controls are presented in Figure 2. Per cell oxygen consumption rates were maintained throughout the 21-day culture period in both osteogenic and control conditions (Fig. 2A). However, there were significant differences in sodium azide-sensitive oxygen consumption on both day 7 (control—57.8 ± 5.0 fmol/h/cell; osteogenic—80.0 ± 8.4 fmol/h/cell) and day 21 (control—67.3 ± 4.9 fmol/h/cell; osteogenic—81.4 ± 4.4 fmol/h/cell) between osteogenic and control conditions. Furthermore, both glucose consumption (Fig. 2B) and lactate release (Fig. 2C) were inhibited during osteogenic culture compared to control conditions, with significant differences evident throughout for lactate release and from days 1 to 21 for glucose consumption. Osteogenesis was confirmed through enhancement of ALP activity from day 7 onward (Fig. 3A) and the presence of alizarin red staining for calcium deposition in the osteogenic conditions only at day 21 (Fig. 3C).
The change in (A) per cell oxygen consumption, (B) glucose consumption and (C) lactate production rate for MSCs during osteogenic differentiation rate for control and osteogenic MSC samples. The change in per cell. Data represent mean ± SD of n = 6. Control (□) and osteogenic (▪); Bonferroni corrected Student's t-test: *P < 0.05.
A: Alkaline phosphatase activity during osteogenic differentiation of human MSCs. Data represents mean ± SD of n = 6. Control (□) and osteogenic (▪); Bonferroni corrected Student's t-test: *P < 0.05. Alizarin red staining for calcium deposition in (B) control and (C) osteogenic samples within the extracellular matrix. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/jcp]
Oxygen consumption is suppressed during pellet culture but is not affected by the presence of TGF-β3
Oxygen/glucose consumption and lactate release data for cells cultured for up to 21 days in pellet culture in the presence (chondrogenic) or absence (control) of TGF-β3 are presented in Figure 4. The per cell oxygen consumption for chondrogenic and control pellet MSCs was significantly reduced, by approximately 70%, during the initial 24 h in pellet culture for both conditions (Student's t-test; P < 0.05, Fig. 4A). A further significant decrease was observed between days 1 and 7 for both conditions (Student's t-test; P < 0.05), with no further changes between days 7 and 21 (Student's t-test; P > 0.05). There were no significant differences in per cell oxygen consumption between control or chondrogenic MSC pellets at any time point. Sodium azide sensitive oxygen consumption was markedly lower than for osteogenic culture, on day 7 (control—9.4 ± 0.9 fmol/h/cell; chondrogenic—7.1 ± 1.1 fmol/h/cell) and day 21 (control—9.8 ± 2.7 fmol/h/cell; chondrogenic—9.6 ± 2.3 fmol/h/cell). Both glucose consumption (Fig. 4B) and lactate release (Fig. 4C) also decreased during pellet culture compared to day 0 values although the reduction was less marked than for oxygen consumption. There were no significant differences in per cell glucose consumption and lactate release rates between control or chondrogenic MSC pellets, except on day 0, where both parameters were inhibited in the presence of TGF-β3 (Student's t-test; P < 0.05). Chondrogenesis was confirmed through GAG accumulation within pellets cultured in the presence of TGF-β3, assessed both quantitatively (Fig. 5A) and via toluidine blue staining (Fig. 5C).
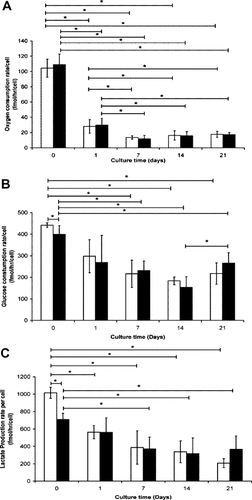
The change in (A) per cell oxygen consumption, (B) glucose consumption and (C) lactate production rate for MSCs during chondrogenic differentiation for MSC pellets cultured in control (−TGF-β3) and chondrogenic (+TGF-β3). Data represent mean ± SD of n = 6: Control (□) and chondrogenic (▪); Bonferroni corrected Student's t-test: *P < 0.05.
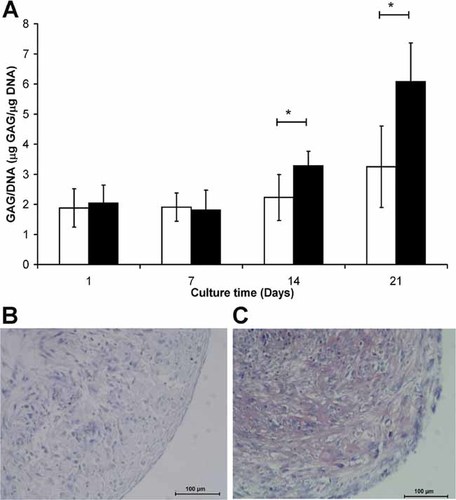
A: The GAG production per DNA during chondrogenic differentiation. Data represents mean ± SD of n = 6: Control (□) and chondrogenic (▪); Bonferroni corrected Student's t-test: *P < 0.05. MSC pellets cultured in (B) control and (C) chondrogenic medium stained for the presence of GAGs using toluidine blue staining. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/jcp]
Discussion
The present investigation assessed the metabolism of bone marrow derived MSCs during monolayer proliferation conditions, that represent the expansion phase for regenerative therapies, and during differentiation towards the osteogenic and chondrogenic lineages. The source of human MSCs, derived from bone marrow aspirated from the iliac crest, and the methods used during both the proliferative phase and differentiation toward the osteogenic and chondrogenic lineages are well established and characterised in the literature. Previous studies, involving clonal expansion, have reported between 70% and 90% of clones exhibit both osteogenic and chondrogenic potential when differentiated using the methods employed in this study (Banfi et al., 2000; Muraglia et al., 2000; Halleux et al., 2001; Russell et al., 2010). In addition, bi-potentiality is reported to be retained for 20 population doublings, which is considerably greater than the maximum population doubling used in the current study (Banfi et al., 2000; Halleux et al., 2001). The methods used to determine oxygen/glucose consumption and lactate release have been validated during a series of similar studies investigating the metabolic phenotype of chondrocytes (Heywood et al., 2006, 2010; Heywood and Lee, 2008, 2010). It should be noted, however, that metabolic parameters were determined for populations of cells that may be heterogeneous in nature. As such there may be individual cell-to-cell variability that cannot be revealed using the current methodologies.
Previous investigations assessing MSC metabolism during proliferation have focussed primarily on glucose consumption and lactate production without assessment of MSC oxygen consumption (Wang et al., 2005; Follmar et al., 2006; Grayson et al., 2006; Mischen et al., 2008; Dos Santos et al., 2010). These studies demonstrated that MSCs exhibit a high level of glycolytic metabolism. The present investigation confirmed these previous observations, as significant amounts of lactate were produced upon consumption of glucose during the period of experimentation (Fig. 1B,C and Table 1). High levels of lactate were produced despite the presence of high oxygen levels within the well, specifically at early time points and lower cell densities. This phenomena has been described as aerobic glycolysis or the Warburg effect (Krebs, 1972). This phenomenon has been reported previously for other cell types that may be adapted to a hypoxic environment in vivo, including chondrocytes and some tumour cells (Krebs, 1972; Rajpurohit et al., 1996). However, in comparison with the previous studies cited, the current study provides an accurate assessment of the oxygen consumption by the cells, and indicates significant levels of oxygen consumption, with per cell consumption rates similar to human adipose-derived MSCs, although significantly lower than adipocytes and hepatocytes, and greater than chondrocytes (Table 3).
| Cell type | Oxygen consumption rate/cell (fmol/h/cell) | Author |
|---|---|---|
| Expanded human bone marrow derived MSCs (1.5 × 105 cells) | 113 | Present study |
| Day 21 chondrogenic MSCs | 12.3 | |
| Day 21 osteogenic MSCs | 98.1 | |
| Rat osteoblasts | 125.8 |
Komarova et al. (2000) |
| Porcine hepatocytes | 324 |
Balis et al. (1999) |
| Bovine chondrocytes | 0.96 |
Heywood and Lee (2008) |
| Human adipose MSCs | 88.7 |
von Heimburg et al. (2005) |
| Adipocytes | 429.8 |
These results suggest that MSCs have a mixed metabolism utilising both glycolysis and oxidative phosphorylation for ATP generation. This suggestion has been further investigated through the use of selected modulators of the metabolic process. Sodium azide inhibits complex IV, a rate limiting enzyme in oxidative phosphorylation. As such, oxygen consumed in its presence (Table 2) is associated with non-mitochondrial processes such as the pentose-phosphate pathway (Alberts et al., 2002). Conversely, azide-sensitive oxygen consumption represents utilisation by the cell mitochondria. Through the co-determination of mitochondrial oxygen consumption during oxidative phosphorylation and glycolytic flux to lactate, the relative proportions of ATP generated via the two mechanisms may be estimated, as described previously (Heywood and Lee, 2008). For each mole of lactate generated there is an equivalent net generation of ATP without oxygen requirement and the ATP yield of the mitochondrial electron transport chain is defined by an ideal mechanistic P/O ratio of 2.5 (Brand et al., 1993). Using these assumptions and based upon the description by Heywood and Lee (2008) and Heywood et al. (2010) we estimate for the current data that oxidative phosphorylation could contribute at most 33% of total ATP generation. We acknowledge that the mitochondrial contribution will in reality be slightly less than 30%, due to the inefficiencies caused by mitochondrial membrane leak. However, mitochondrial leak was determined to account for the minority fraction of total mitochondrial oxygen consumption by articular chondrocytes (Heywood et al., 2010). The use of oxidative phosphorylation for MSCs expanded under normoxia, may be a contributory factor to the induction of premature senescence reported previously, due to the production of ROS from the process of oxidative phosphorylation (Heywood and Lee, 2008).
The data indicating enhanced oxygen consumption in the presence of the potent uncoupler, CCCP (Table 2), illustrates that MSCs do not utilise their full oxidative capacity. CCCP stimulated oxygen consumption by the electron transport chain to maximal levels of 216.1 ± 31.3 fmol/h/cell. Accordingly, it was estimated that during proliferative culture, the MSC mitochondria utilise just 33.7% of the cells full oxidative capacity. The remainder is used for non-mitochondrial processes (11.5%) or kept in reserve (54.8%). Interestingly, the maximal oxidative capacity and proportional breakdown is very similar to that reported for monolayer-expanded chondrocytes (Heywood and Lee, 2008). The high proportion of oxidative capacity kept in reserve may be utilised for oxidative phosphorylation under certain conditions. In the current study, this was demonstrated through suppression of glycolysis, induced by competitive inhibition of glucose uptake by 2-deoxy-D-glucose, inducing increased oxygen consumption, a phenomenon described as the Crabtree effect (Barban and Schulze, 1961; Krebs, 1972; Lee and Urban, 1997; Heywood and Lee, 2008). Thus, MSCs appear readily able to adapt their metabolism depending upon the availability of nutrients and metabolites. Indeed, recent investigations have shown that MSCs are able to survive under severely depleted glucose conditions or adapt their metabolism upon MSC transformation using retrovirus vectors, utilising the described survival mechanism (Follmar et al., 2006; Funes et al., 2007; Mylotte et al., 2008). Furthermore, it has recently been shown that MSCs are able to act as a natural mitochondrial uncoupler and stimulate the Warburg effect when in co-culture with another cell type, such as leukaemia cells via reduction in their mitochondrial oxygen consumption (Samudio et al., 2008).
Differentiation was induced using well-established standard methodologies (Lonza) and the progression of differentiation, indicated via both qualitative and quantitative markers of osteogenesis and chondrogenesis, is consistent with many previous studies (Figs. 3 and 5) (Grigoriadis et al., 1988; Huang et al., 2005; Malladi et al., 2006; Chang et al., 2009; Kupcsik et al., 2010; Russell et al., 2010). Distinct differences were observed in MSC metabolism between osteogenic and chondrogenic culture conditions (Figs. 2 and 4). MSCs cultured under osteogenic conditions, broadly maintained levels of oxygen consumption displayed by proliferating MSCs (Fig. 2). These findings are not consistent with previous studies that have shown a significant increase in oxygen consumption upon differentiation towards the osteogenic lineage compared with undifferentiated MSCs (Chen et al., 2008). While oxygen consumption was maintained during osteogenic culture, there was a greater amount of sodium azide sensitive oxygen consumption compared with control conditions. Furthermore, there was a progressive reduction in lactate production for osteogenic cultures compared with their control, which aligned with the development of osteogenic markers, suggest a suppression in glycolysis during the process of differentiation. Alterations in glucose consumption and lactate production for osteogenic cultures compared to control conditions may be associated with medium components, such as dexamethasone and β-glycerophosphate that have been reported to affect the glucose and oxygen metabolism of the cell (Klein et al., 1993; Roussel et al., 2004; Desquiret et al., 2008). The metabolic phenotype of the differentiating cells was further analysed through estimation of ATP production via glycolysis and oxidative phosphorylation, as outlined above. The data are consistent with 48% of ATP derived from the latter process by day 21 in osteogenic culture, compared to 29% for day 21 controls and 33% for proliferating cells. The proportionate increase in mitochondrial oxygen consumption and suppression of glycolysis during osteogenesis reported in the current study may provide a hypothetical mechanism for inhibited osteogenic differentiation under hypoxia, such that the high levels of glycolysis required for survival under hypoxic conditions are not conducive to osteogenesis (Tuncay et al., 1994; Warren et al., 2001; Salim et al., 2004; Malladi et al., 2006; d'Ippolito et al., 2006). Indeed, Chen et al. (2008) showed that key glycolysis genes are not expressed in osteogenic cultures compared with expanded MSCs, consistent with the current data and suggesting a relationship between the metabolic phenotype of the cells and their differentiation state. Measurements of ALP activity and alizarin red staining (Fig. 3) confirmed the differentiation towards the osteogenic lineage and demonstrated similar results to that shown in previous investigations (Grigoriadis et al., 1988; Malladi et al., 2006; Chang et al., 2009).
By contrast with osteogenic conditions, the formation of MSC pellets associated with chondrogenic culture induced a profound and rapid reduction in oxygen consumption. This phenomenon appeared to be associated with an adaptation to the 3D pellet culture conditions rather than with chondrogenesis per se, as the magnitude of the effect was similar in both the presence and absence of TGF-β3 and the effect preceded the appearance of chondrogenic markers. The co-determination of azide-sensitive oxygen consumption and glycolysis indicates that both chondrogenic cultures only utilise oxidative phosphorylation for at most 13% of their total ATP production on day 21, thereby resulting in a predominantly glycolytic metabolism (89%). The proportionately high levels of glycolysis demonstrated within the pellet culture system may be associated with both the high glucose concentration within the medium and the presence of hypoxia within the pellet (Malladi et al., 2006). Indeed, it has been shown that hypoxia stimulates genes associated with glycolysis (Rajpurohit et al., 1996; Schipani, 2005). Therefore, the culture conditions and the expression of genes associated with glycolysis enable MSCs to develop a glycolytic metabolic phenotype that may be conducive to chondrogenesis on supplementation of differentiation-inducing factors such as TGF-β3. These factors may contribute to the increased level of chondrogenesis reported previously, during culture under hypoxia where a similar highly glycolytic metabolism will be induced (Lennon et al., 2001; Wang et al., 2005; Xu et al., 2007; Markway et al., 2010). Confirmation of MSC chondrogenesis was conducted through GAG/DNA synthesis and toluidine blue staining for GAG deposition within the matrix (Fig. 5). These results were similar to data shown in previous investigations (Huang et al., 2005; Kupcsik et al., 2010).
Interestingly, the progressive reduction in oxidative phosphorylation during MSC culture under conditions conducive for chondrogenesis, mirrors findings reported for monolayer-passaged chondrocytes that show an increased rate of oxygen consumption with time in culture, associated with a loss of chondrocytic phenotype and development of an undifferentiated fibroblast-like phenotype (Heywood and Lee, 2008). This provides further support for a hypothetical link between the metabolic phenotype of MSCs and their differentiated progeny and their differentiation state. Such a link, if proved, could provide novel mechanisms to control differentiation for use in therapeutic strategies.
As mentioned previously the methodology used in this study provides metabolic parameters from a population of MSCs and thus will not reveal cell-to-cell heterogeneity. This is particularly important for the differentiation studies, where there may be considerable heterogeneity in differentiation capacity within the cell population, associated with the underlying cell heterogeneity and the temporal and spatial progression of differentiation. As such the current methodologies are unable to ascertain which cells within the population may be responsible for the alterations in population metabolism. An alternative approach would involve assessment of metabolic parameters on a single cell basis. While this provides an attractive alternative, the methodologies for the measurement of oxygen consumption on a single cell basis remain at a developmental stage and are often associated with significant limitations (Dragavon et al., 2008; Molter et al., 2009). Moreover, measuring the oxygen consumption of single cells in isolation does not necessarily reflect their consumption within more applied tissue engineering type situations, where populations of cells are utilised, typically in a 3-D environment. In the latter situation, the metabolic parameters of each cell are highly dependent on those of the neighbouring cells as utilisation changes the local oxygen/glucose environment, which in turn may alter the consumption rates of the cells. Moreover, the interaction between cells within a population in 3-D may be required for the differentiation process itself, whether via cell/cell contacts, the release of paracrine factors or via alterations in the oxygen microenvironment, associated with cellular consumption. Thus, while the methodology used in the current study does not permit the assessment of cell to cell variation, it remains highly relevant to the development of bioreactor systems that are designed to maintain metabolic activities of MSC populations (Godara et al., 2008; Thorpe et al., 2010) and also research involving the modelling of the developing spatial heterogeneity of oxygen/glucose concentrations within such systems (Sengers et al., 2005; Devarapalli et al., 2009). Ultimately, the development of methodologies that permit the assessment of oxygen consumption by individual cells within a cell population in 3-D will provide the most detailed and relevant data. To this end, the authors are currently developing techniques involving multiphoton fluorescence lifetime imaging (Hosny et al., 2010).
In summary, the results of the investigation indicate that proliferative MSCs maintained under normoxic conditions exhibit a mixed metabolism, with a significant glycolytic component consistent with the Warburg phenomenon. The proportionate contributions of glycolysis and oxidative phosphorylation vary under limiting oxygen and glucose conditions, consistent with the Pasteur and Crabtree effects respectively. Osteogenic differentiation was associated with a suppression of glycolysis, suggesting a proportionate increase in oxidative phosphorylation. 3D pellet culture conditions that are conducive to chondrogenesis strongly suppressed oxidative phosphorylation, but the effect was associated with the culture conditions rather than the chondrogenic state per se. These findings suggest a link between metabolic phenotype of MSCs and their differentiated state that may have therapeutic relevance.
Acknowledgements
The work was funded in part by Wellcome Trust grant (ref. no. 080440/Z/06/Z), Engineering and Physical Sciences Platform grant (ref. no. EP/E046975/1) and via a Queen Mary University of London studentship.




