Functional role of rho-kinase in ameloblast differentiation
Abstract
During tooth development, inner enamel epithelial (IEE) cells differentiate into enamel-secreting ameloblasts, a polarized and elongated cellular population. The molecular underpinnings of this morphogenesis and cytodifferentiation, however, are not well understood. Here, we show that Rho-associated coiled-coil-containing protein kinase (ROCK) regulates ameloblast differentiation and enamel formation. In mouse incisor organ cultures, inhibition of ROCK, hindered IEE cell elongation and disrupted polarization of differentiated ameloblasts. Expression of enamel matrix proteins, such as amelogenin and ameloblastin, and formation of the terminal band structure of actin and E-cadherin were also perturbed. Cultures of dental epithelial cells revealed that ROCK regulates cell morphology and cell adhesion through localization of actin bundles, E-cadherin, and β-catenin to cell membranes. Moreover, inhibition of ROCK promoted cell proliferation. Small interfering RNA specific for ROCK1 and ROCK2 demonstrated that the ROCK isoforms performed complementary functions in the regulation of actin organization and E-cadherin-mediated cell–cell adhesion. Thus, our results have uncovered a novel role for ROCK in amelogenesis. J. Cell. Physiol. 226: 2527–2534, 2011. © 2010 Wiley-Liss, Inc.
During tooth development, ameloblasts undergo several differentiation processes to form dental enamel (Fincham et al., 1999). In the rodent incisor, dental epithelial stem cells are localized in the apical bud (Harada et al., 1999; Harada and Ohshima, 2004). These cells produce inner enamel epithelium (IEE) cells, a transit-amplifying progenitor population that further differentiates into pre-secretory ameloblasts. Differentiation from pre-secretory ameloblasts into highly polarized secretory ameloblasts involves cytoplasmic growth and elongation, changes in nuclear and organelle polarities, and development of a complex cytoskeleton (Slavkin, 1974). The processes of morphogenesis and cytodifferentiation are regulated by sequential and reciprocal epithelial–mesenchymal interactions, which are mediated by a number of soluble proteins (Jernvall and Thesleff, 2000; Thesleff et al., 2001; Thesleff and Mikkola, 2002). In addition, cytoskeletal components and cell–cell junctional complexes have been implicated in the regulation of histomorphogenesis (Lesot et al., 1982; Fausser et al., 1998). Although a number of paracrine signal molecules that mediate these interactions have been identified, intracellular signaling in ameloblasts is poorly understood.
The small GTPase Rho is a molecular switch that regulates several steps during cytoskeletal dynamics and contributes to various cellular processes, including cell polarity, adhesion, migration, cytokinesis, proliferation, secretion, and transformation (Bar-Sagi and Hall, 2000; Schmitz et al., 2000; Etienne-Manneville and Hall, 2002). In addition, recent studies have highlighted a potential regulatory role for Rho-mediated signaling in amelogenesis (Hatakeyama et al., 2009; Biz et al., 2010).
Rho activity is transduced by downstream Rho effectors, including Rho-associated coiled-coil-containing protein kinase (ROCK). Two ROCK isoforms have been identified in mammals: ROCK1 and ROCK2. Each contains a highly conserved N-terminal serine/threonine kinase domain (>90% homology between the two isoforms), whereas the carboxyl terminals differ (Nakagawa et al., 1996). Previous studies showed that ROCK1-deficient mice were born with open eyelids and an omphalocele phenotype (Shimizu et al., 2005), whereas loss of ROCK2 resulted in placental dysfunction, intrauterine growth retardation, and fetal death (Thumkeo et al., 2003).
ROCK reorganizes the actin cytoskeleton by phosphorylating several substrates, such as myosin regulatory light chain and LIM kinase, each of which contributes to actin filament assembly and contractility (Maekawa et al., 1999; Amano et al., 2000). Therefore, ROCK plays important roles in cell morphology (Ridley et al., 2003; Riento and Ridley, 2003). In addition, ROCK is involved in cell–cell adhesion. ROCK and myosin-based contraction of the actin cytoskeleton are required for cadherin-mediated adhesion and maintenance (Shewan et al., 2005; Martinez-Rico et al., 2009). Potential roles for ROCK in tooth development, in particular amelogenesis, have not been elucidated, however. Here, we addressed a functional role of ROCK in amelogenesis using mouse incisor organ cultures and a dental epithelial cell line.
Materials and Methods
Reagents
Antibodies: Anti-ROCK1 rabbit monoclonal antibodies were obtained from Abcam (Cambridge, MA). Anti-ROCK2 goat polyclonal antibodies and anti-β-catenin mouse monoclonal antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-E-cadherin mouse monoclonal antibodies were obtained from BD Biosciences (San Jose, CA). Monoclonal rat anti-Ki67 antibodies were obtained from DakoCytomation (Glostrup, Denmark). Hoechst33342, Alexa Fluor 488, and Alexa Fluor 546 secondary antibodies were purchased from Invitrogen (Carlsbad, CA). Anti-amelogenin and anti-ameloblastin rabbit polyclonal antibodies were kindly gifted from Dr. Uchida (Uchida et al., 1991, 1997). Inhibitors: Y27632 (50 µM), fasudil (10 µM), and H-1152 (1 µM) were purchased from Merck (Tokyo, Japan). Latrunculin A (Lat-A; 1 µM) was obtained from Invitrogen.
Mouse incisor organ culture
The mouse incisor organ culture has been previously described (Harada et al., 1999). Briefly, incisors were dissected from the lower jaws of ddY mice (Japan SLC, Shizuoka, Japan) on post-natal day 1–3. The apical bud regions were mechanically removed from the incisors using an 18-gauge needle and cultured for 7 days in culture medium. The tissues were fixed in 4% paraformaldehyde in PBS, and decalcified in Osteosoft (Merck). For hematoxylin and eosin (H&E) and immunostaining, the samples were dehydrated in a graded ethanol series, embedded in paraffin wax, and sectioned (thickness, 6 µm). All experiments were conducted in accordance with the Protocols for the Humane Treatment of Animals of Iwate Medical University.
Cell culture
mHAT9a cells are a dental epithelial cell line derived from the apical bud of a mouse incisor. Culture medium consisted of Dulbecco's modified Eagle's medium/F12 (Invitrogen) containing B-27 supplement (Invitrogen), bFGF (20 ng/ml; R&D Systems, Minneapolis, MN), and EGF (20 ng/ml; R&D Systems). Cell numbers were determined using the trypan blue dye exclusion method.
Immunostaining and F-actin staining
After adding blocking serum (5% normal horse serum in PBS), paraffin-embedded sections were incubated with primary antibodies overnight at room temperature. A VECTASTAIN ABC kit (Vector Laboratories, Burlingame, CA), and Alexa Fluor 488- and Alexa Fluor 546-conjugated secondary antibodies were used to detect target labeling. DAB (Vector Laboratories) was used as the chromogen, and sections were counterstained with hematoxylin. Cultured mHAT9a cells grown on glass coverslips were fixed with 4% paraformaldehyde and permeabilized in 0.1% Triton X-100 (Sigma–Aldrich, Tokyo, Japan). After adding blocking serum, cells were incubated with primary antibodies for 1 h at room temperature. Alexa Fluor 488- or 546-conjugated secondary antibodies were then added. To detect actin, sections and cells were stained using Alexa Fluor 546-conjugated phalloidin.
Fluorescent images were obtained under a fluorescence microscope (IX71, Olympus, Tokyo, Japan), or laser-scanning confocal microscope (FV300, Olympus). Image analyses were carried out using software provided with confocal microscope or standard image analysis software (Metamorph, Universal Imaging, Universal Imaging Corporation, Downingtown, PA).
Real-time actin imaging
To detect actin dynamics in living cells, Cellular Lights actin RFP (Invitrogen) was introduced into mHAT9a cells according to the manufacturer's instructions. Cells cultured in glass bottom dishes were transferred to a culture chamber installed on the stage of a confocal microscopy, and maintained at 37°C with moisturized 5% CO2 gas during image acquisition. Images were taken every 5 min.
Small interfering RNA
Three 25-mer duplex Small interfering RNAs (siRNAs) that targeted ROCK1 and ROCK2 were obtained from a commercial source (Stealth select RNAi; Invitrogen). All siRNA duplexes (10 nmol/L) were transfected into cells using Lipofectamine RNAiMAX (Invitrogen) for 72 h at 37°C in a CO2 incubator according to the manufacturer's instructions. Stealth siRNA negative control duplexes (Invitrogen) were used as a control for sequence-independent effects following siRNA delivery. Transfection efficiency, which was monitored using a fluorescent oligonucleotide (BLOCK-iT fluorescent oligonucleotide; Invitrogen), was estimated to be 80–90%. Inhibitory effects were observed with all siRNA duplexes and siRNA that induced the highest degree of inhibition was used in three independent experiments.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was extracted using an RNeasy mini Kit (Qiagen, Tokyo, Japan) according to the manufacturer's protocol. Reverse transcription of total RNA (1 µg) was performed using a PrimeScript RT reagent kit (Takara Bio, Siga, Japan). For quantitative analysis of gene expression, cDNA amplification was performed in qRT-PCRs using SYBR® Premix Ex Taq™ (Takara Bio) and specific oligonucleotide primers for target sequences (Table 1) with a Thermal Cycler Dice (Takara Bio) according to the manufacturer's protocol. Amplification conditions included 30 sec at 95°C, 40 cycles of 95°C for 5 sec and 60°C for 30 sec, followed by dissociation for 15 sec at 95°C, and 30 sec at 60°C. Target gene expression levels were normalized based on GAPDH expression. The relative gene expression levels were calculated based on levels in negative control cultures using the comparative Ct (2−ΔΔCt) method. Experiments were carried out in triplicate.
| mRNA | Orientation | Sequence |
|---|---|---|
| ROCK1 | Forward | 5′-GGTATCGTCACAAGTAGCAGCATCA-3′ |
| Reverse | 5′-TAAACCAGGGCATCCAATCCA-3′ | |
| ROCK2 | Forward | 5′-TTGCCAACAGTCCCTGGGTAG-3′ |
| Reverse | 5′-CGCCTGGCATGTACTCCATC-3′ | |
| E-cadherin | Forward | 5′-CGTCCTGCCAATCCTGATGA-3′ |
| Reverse | 5′-ACCACTGCCCTCGTAATCGAAC-3′ | |
| β-Catenin | Forward | 5′-TGCAGATCTTGGACTGGACATTG-3′ |
| Reverse | 5′-GGCCGTATCCACCAGAGTGAA-3′ | |
| GAPDH | Forward | 5′-TGTGTCCGTCGTGGATCTGA-3′ |
| Reverse | 5′-TTGCTGTTGAAGTCGCAGGAG-3′ |
Statistics
All data are reported as means ± SD. Statistical significance was assessed using two-tailed Student's t-tests for two groups or analysis of variance Tukey's test for more than two groups. Significance was defined as P < 0.05.
Results
ROCK expression in mouse incisors
Expression of ROCK was immunohistochemically examined in 1-day-old mouse incisors. The expression gradually increased in the process of dental epithelial differentiation. In the apical bud, low levels of both ROCK1 and ROCK2 were detected in basal epithelial cells, IEE cells and pre-secretory ameloblasts, whereas those proteins were not detected in stellate reticulum cells (Fig. 1A-c, B-f). ROCK1 and ROCK2 were strongly expressed in the cytosol of secretory and mature ameloblasts and the underlying stratum intermedium (Fig. 1A-a and b, B-d and e). ROCK1 and ROCK2 were also detected in differentiated odontoblasts (Fig. 1A-a and b, B-d and e). These findings suggested that ROCK is involved in ameloblast differentiation.
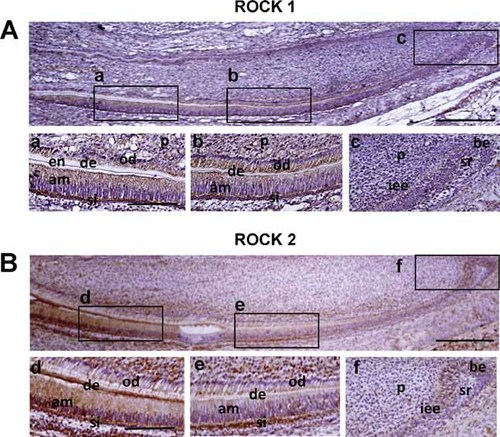
Expression of ROCK in mouse incisors. Images show immunohistochemistry for ROCK1 (A) and ROCK2 (B) in lower incisors from 1-day-old mice. The upper images are lower magnification views of the incisors. Bar = 250 µm. Lower images are high magnification views of the boxed areas in the upper images. (a–f). Bar = 150 µm. ab, apical bud; am, ameloblasts; be, basal epithelium; de, dentin; en, enamel; iee, inner enamel epithelium; od, odontoblasts; p, dental pulp; si, stratum intermedium; sr, stellate reticulum.
ROCK mediates ameloblast differentiation by regulating cell polarity and proliferation
Loss-of-function experiments were carried out using a mouse incisor organ culture system and the ROCK-specific inhibitor Y27632. We prepared incisors without the apical bud, including dental epithelial stem cells, to allow us to observe the differentiation process from IEE cells to mature ameloblasts in culture (Harada et al., 1999). In control samples, H&E tissue staining revealed new production of enamel and dentin after 7 days of culture (Fig. 2A, B). IEE cells changed from cuboidal to columnar morphology. Immunohistochemical analysis found that ameloblasts secreted both amelogenin and ameloblastin on the distal side of the cells (Fig. 2C), indicating that the IEE cells differentiated into ameloblasts. Emergence of the stratum intermedium layer was also observed.
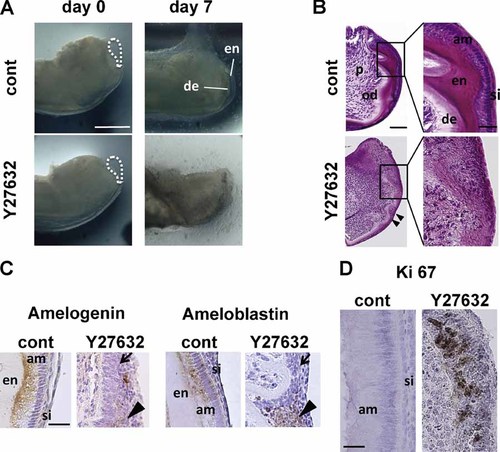
Effects of a ROCK inhibitor on mouse incisors in organ cultures. A: Stereomicroscope images show development of dissected apical ends of untreated (cont) and Y27632-treated (Y27632) mouse incisors that have been cultured for 7 days. Apical buds (dashed oval) were removed before culture (day 0). Bar = 500 µm. The experiments were replicated seven times. B: Left: Lower magnification images of H&E-stained sections of untreated (cont) and Y27632-treated incisors on day 7. Bar = 50 µm. Right: Higher magnification images of the boxed areas on the left. Bar = 20 µm. C: Immunostaining for amelogenin and ameloblastin in untreated (cont) and Y27632-treated incisors cultured for 7 days. Arrows indicate cells that were not expressing enamel matrix proteins. Arrowheads indicate cells secreting enamel matrix proteins in all directions. Bar = 25 µm. D: Ki-67 immunostaining in control and Y27632-treated incisors on day 7. Bar = 25 µm.
In contrast, treatment with Y27632 resulted in morphologic degeneration of the incisors. The layers of enamel and ameloblasts, and dentin and odontoblasts were not clearly observed (Fig. 2A, B). IEE cells remained cuboidal and changes in cell polarity were not observed. Further, ameloblasts that had already differentiated at the start of the culture lost their polarity, and the original layers of ameloblasts and stratum intermedium disappeared (Fig. 2B, arrowheads). Other ROCK inhibitors, such as fasudil and H-1152, produced similar results (data not shown). In Y27632-treated incisors, IEE cells did not differentiate into amelogenin- and ameloblastin-expressing ameloblasts (Fig. 2C arrows). Although already differentiated ameloblasts lost their cellular polarity, expression of these proteins was observed (Fig. 2C, arrowheads). The results suggested that ROCK does not drive the expression of amelogenin and ameloblastin in ameloblasts, but may regulate the direction in which the proteins are secreted. Interestingly, ameloblasts that differentiated from IEE cells in control samples were Ki67-negative, whereas IEE cells in Y27632-treated incisors were Ki-67 positive (Fig. 2D). Additionally, apoptotic cells were not detected in both control and Y27632-treated samples (data not shown). Therefore, the results suggested that Y27632 inhibited only differentiation of IEE cells into ameloblasts.
Effects of ROCK on the cytoskeleton and cell–cell adhesion during amelogenesis
We examined the effects of ROCK on the arrangement of actin and proteins in cell adherence junctions. In control samples, a thick actin bundle—the so-called terminal band—was observed at both the apical and basal ends of the ameloblasts (Fig. 3A, arrows). E-cadherin expression was also restricted to both the apical and basal ends (Fig. 3B, arrows). β-catenin was prominently expressed at the ameloblast basal end (Fig. 3C, arrows). In Y27632-treated incisors, however, terminal band structures were not seen, actin filaments were diffusely distributed in cytoplasm, and fluorescent signals were weak. E-cadherin and β-catenin were evenly distributed across the cell membrane and the intensity of labeling was weak. These results suggested that the ROCK inhibitor disrupted cell polarization by affecting localization of cell–cell adhesion molecules.
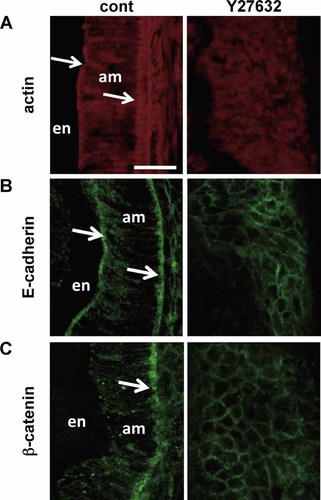
ROCK-mediated regulation of actin organization and localization of cell–cell adhesion molecules in mouse incisors. Images show apical labeling of phalloidin (A), E-cadherin (B), and β-catenin (C) in mouse incisors cultured for 7 days. Left: Untreated (cont). Right: Y27632-treated. Arrows indicate the terminal band. Bar = 20 µm.
To elucidate the function of ROCK in dental epithelium further, we performed cell culture experiments using a cell line established from the mouse incisor apical bud (mHAT9a cells) (Kawano et al., 2002). First, we confirmed that ROCK1 and ROCK2 were expressed in mHAT9a cells. Immunostaining showed that mHAT9a cells expressed ROCK1 and ROCK2 predominantly around the nuclei (Fig. 4A), and ROCK2 was localized at junctional membranes adjacent to neighboring cells (Fig. 4A). We next confirmed the effects of ROCK inhibitors on mHAT9a cells. In control samples grown on regular culture dishes, mHAT9a cells grew as monolayer, which was characterized by cell–cell junctions and square-shaped epithelial cell morphology. After 24 h of treatment with Y27632 and fasudil, cell–cell adhesions were lost and the cells changed from a flattened epithelial phenotype to fibroblast-like cells. Moreover, the cells no longer coalesced in a colony and instead migrated peripherally (Fig. 4B).
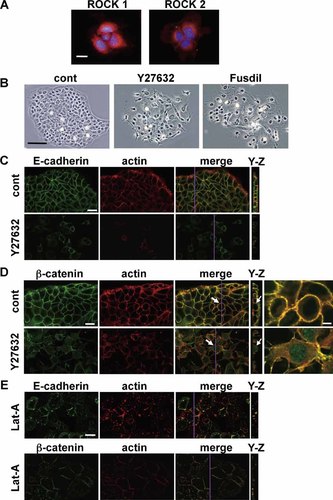
Effects of a ROCK inhibitor in mHAT9a cells. A: Images show immunofluorescence labeling of ROCK1 and ROCK2 in mHAT9a cells (red). The nucleus is stained with Hoechst 33342 (blue). Bar = 20 µm. B: Phase contrast images show mHAT9a cells in untreated (cont), Y27632-treated, and fasudil-treated cultures after 24 h. Bar = 100 µm. C,D: Confocal microscopic images of untreated (cont) and Y27632-treated mHAT9a cell monolayers labeled for E-cadherin or β-catenin, and actin after 24 h of culture. The panels labeled as Y–Z depict reconstructions from confocal z-stacks in the Y–Z direction. Bar = 20 µm. Arrows in D indicate the cells magnified in the rightmost images. Bar = 5 µm. E: Images show fluorescent labeling of E-cadherin, β-catenin, and actin after 6 h of culture with latrunculin A (Lat-A). Bar = 20 µm. All imaging data obtained from experiments that were replicated at least five times.
To examine the relationship between actin and E-cadherin in mHAT9a cells, we carried out three-dimensional analyses of their distributions using confocal microscopy. In control samples, midplane images of the mHAT9a cell monolayer showed that E-cadherin colocalized at cell membranes with actin bundles (Fig. 4C, left). Y–Z merged images showed that E-cadherin and actin were distributed in both the apical and basolateral membranes (Fig. 4C, right). β-catenin was also expressed at the membrane (Fig. 4D). In contrast, in Y27632-treated cells, E-cadherin and actin were distributed diffusely in the cytoplasm, and the fluorescence signals were weaker than those observed in control samples (Fig. 4C). Expression of β-catenin at the membrane was also weak, and faint signals were detected in the cytosol and nucleus (Fig. 4D, arrows). Additionally, treatment with the actin polymerization inhibitor latrunculin A disrupted E-cadherin and β-catenin localization at the cell membrane (Fig. 4E), suggesting that the distributions of E-cadherin and β-catenin are directly regulated by the actin network. Further, to evaluate the effects of ROCK on actin kinetics, we performed real-time imaging of actin in single mHAT9a cells. At baseline, short actin bundles (stress fibers) were observed in the cytoplasm. After Y27632 was added, the actin bundles time-dependently degraded and disappeared in 6 h (Fig. 5 and Supplementary online material). Additionally, we examined proliferation of mHAT9a cells that were treated with a ROCK inhibitor. Y27632 increased the growth rate of mHAT9a cells compared with results from control culture conditions, indicating that ROCK inhibits cell proliferation (Fig. 6).
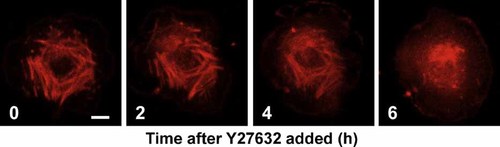
Effects of a ROCK inhibitor on actin stress fibers in mHAT9a cells. mHAT9a cells containing Cellular Lights actin RFP were used. Confocal fluorescence images taken at 2-h intervals show degradation of actin stress fibers in single mHAT9a cells after exposure to Y27632. Bar = 10 µm. Each experiment was replicated three times.
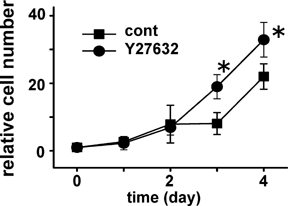
ROCK inhibition promotes cell proliferation. mHAT9a cells cultured with or without Y27632 were counted using the trypan blue exclusion method. Results are the means ± SD (n = 3 for each group). *P < 0.05 relative to the untreated group.
Effects of knocking down ROCK expression on actin organization and cell–cell adhesion
To determine which ROCK isoform affected cytoskeletal organization and cell–cell adhesion, we transfected mHAT9a cells with siRNAs specific for either ROCK1, ROCK2, or both, each of which reduced RNA levels by at least 50% (Fig. 7A). The double knockdown cells assumed a spindle shape and cell–cell adhesion was not observed. Fluorescent signals for E-cadherin and actin decreased at the membrane, and E-cadherin was diffusely and weakly observed in the cytosol. This result mirrored those observed with ROCK inhibitors (Fig. 7B). When either ROCK1 or ROCK2 expression was knocked down, however, the cells' shapes and distributions of actin and E-cadherin were not changed (data not shown). Furthermore, quantitative RT-PCR revealed that knocking down both ROCK1 and ROCK2 expression reduced the expression of E-cadherin and β-catenin mRNA, whereas no specific effect was noted with either ROCK1 or ROCK2 knockdown (Fig. 7C). Together these findings indicate that one isoform can compensate for the loss of the other to regulate cell morphology and cell–cell adhesion in dental epithelial cells.
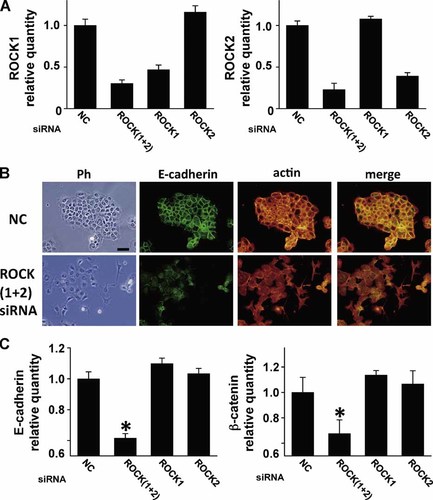
Effects of knocking down ROCK1 and ROCK2 expression in mHAT9a cells. A: Expression of ROCK1 (left panel) or ROCK2 (right panel) mRNA in mHAT9a cells transfected with siRNA specific for ROCK1, ROCK2, or both. Results are the means ± SD (n = 3 in each group). B: Images show phase contrast (Ph) E-cadherin- and phalloidin-specific fluorescence in mHAT9a cells transfected with siRNA specific for both ROCK1 and ROCK2 (ROCK(1+2) siRNA). Bar = 50 µm. C: E-cadherin (left) and β-catenin (right) mRNA expression after knocking down the expression of ROCK1, ROCK2, or both. Data are expressed as the means ± SD (n = 3 in each group). NC, non-specific control siRNA. *P < 0.05 relative to the NC-treated group.
Discussion
During amelogenesis, ameloblasts change from cuboidal to columnar, re-orient their overall polarity with the formation of Tomes process, and begin to secrete enamel proteins toward the nearby dentin. Our results have shown that ROCK contributes to the polarity, proliferation, and differentiation of these cells by regulating organization of the actin cytoskeleton and cell–cell adhesion.
ROCK regulates ameloblast polarity and differentiation
ROCK expression in mouse incisors was examined immunohistochemically. Although ROCK expression in other tissues has been previously reported (Riento and Ridley, 2003; Schmandke and Strittmatter, 2007), we have detected it for the first time in ameloblasts and odontoblasts. High expression levels of the ROCK1 and ROCK2 isoforms were detected in highly polarized ameloblasts and odontoblasts compared with non-polarized epithelial and mesenchymal cells (Fig. 1). These findings suggested a role for ROCK in ameloblast and odontoblast differentiation, and was consistent with previously published data that showed increased expression of ROCK1 and ROCK2 mRNA during rat molar development (Biz et al., 2010).
Establishing and maintaining epithelial cell polarity is critical for development in multicellular organisms. Members of the Rho family of GTPases play essential roles in these processes (Drubin and Nelson, 1996; Van Aelst and Symons, 2002; Fukata et al., 2003). Using organ culture experiments, we showed that activation of ROCK was required for ameloblast polarization. Specific inhibitors of ROCK clearly disrupted ameloblast polarity and enamel formation (Fig. 2B). Consistent with these findings, Hatakeyama et al. (2009) showed that expression of RhoGDI, an inhibitor of Rho GTPase, was down-regulated in polarized ameloblasts, indicating that Rho signaling was activated in these cells. Additionally, the authors demonstrated that enamel matrix proteins, such as amelogenin and ameloblastin, regulated ameloblast polarization via Rho signaling. Our results indicated that ROCK regulated the expression of enamel matrix proteins and ameloblast polarization (Fig. 2C, arrows), and that Rho signaling regulated amelogenin expression in molar tooth germ cells (Biz et al., 2010). We, therefore, hypothesize that Rho signaling and enamel matrix proteins reciprocally regulate each other during ameloblast differentiation.
Interestingly, in response to ROCK inhibition, already differentiated ameloblasts lost their polarized phenotype, and amelogenin and ameloblastin were no longer directionally secreted (Fig. 2C, arrowheads). Because Rho signaling regulates granule secretory pathways (Stowers et al., 1995; Pinxteren et al., 2000; Chiang et al., 2001; Kanzaki and Pessin, 2001), and amelogenin and ameloblastin are synthesized together and contained within the same secretory granules (Zalzal et al., 2008), ROCK-mediated cellular polarization may also direct distal secretion of enamel matrix proteins.
Regulatory mechanisms for ameloblast polarization and differentiation
Dynamic rearrangement of the cytoskeleton allows cells to establish cell polarity and change morphology during development. ROCK phosphorylates various substrates to mediate actin organization (Fukata et al., 2001; Vaezi et al., 2002; Riento and Ridley, 2003). We demonstrated that ROCK regulates actin polarization in ameloblasts. In organ cultures of incisors, ROCK inhibition disrupted formation of the actin terminal band structure and establishment of polarized cells (Fig. 3). In amelogenesis, changes in the apical terminal band have been shown to precede alterations in enamel secretion (Nishikawa et al., 1988), suggesting that the polarized actin structure is involved in amelogenesis. Moreover, polarization of actin bundles at apical and basolateral membranes was impaired in culture cells (Figs. 4 and 7). Consistent with our data, Vaezi et al. (2002) reported that ROCK plays an important role in the formation of polarized actin networks during epithelial stratification. ROCK-mediated polarization of actin, therefore, may be indispensable for ameloblast differentiation and subsequent enamel formation.
In addition to actin cytoskeletal dynamics, epithelial polarity depends on E-cadherin-mediated cell–cell adhesion. This process requires association between the cytoplasmic tail of E-cadherin and the actin cytoskeleton, which is mediated by interactions with such intracellular proteins as β-catenin (Adams et al., 1996). We assessed the potential role of ROCK in cadherin-mediated adhesion in ameloblasts. In both organ and cell cultures, ROCK inhibitors markedly affected E-cadherin and β-catenin localization in ameloblasts. The perturbed distributions colocalized with actin in the cytosol, implying direct associations among these proteins. This result was confirmed using an inhibitor of actin polymerization, which completely disrupted E-cadherin and β-catenin localization in the cell membrane (Fig. 4E). Thus, cell–cell adhesion is dependent on ROCK-mediated actin organization. Consistent with our findings, Rho is required for establishing cadherin-mediated cell–cell adhesion in keratinocytes, and actin re-organization is necessary to stabilize receptors at intercellular junctions (Braga et al., 1997).
Because pharmacologic inhibition blocked both isoforms of ROCK, we used siRNA to determine the specific functional roles of ROCK1 and ROCK2 in ameloblasts. Only knocking down expression of both ROCK1 and ROCK2 produced a marked effect on actin organization and cell–cell adhesion (Fig. 7), which implies that the two isoforms can compensate functionally for each other. Knocking out ROCK1 and ROCK2 in mice demonstrated that the two isoforms cooperatively regulate actin bundle assembly during eyelid and ventral body wall closure (Shimizu et al., 2005; Thumkeo et al., 2005). Both ROCK1 and ROCK2 are required to prevent myoblast differentiation (Castellani et al., 2006). Although tooth phenotypes in ROCK1/ROCK2 double knockout mice have not yet been reported, these mice may provide further insights into the roles of this kinase family in amelogenesis.
It should be noted that β-catenin translocated from the cell membrane to the nucleus when ROCK was inhibited (Fig. 4). A recent study showed that Rho signaling promoted accumulation of stabilized/mutated β-catenin in the nucleus (Esufali and Bapat, 2004). β-catenin is a key mediator of Wnt signaling, and its translocation to the nucleus results in transcriptional activation to regulate the development of a broad range of epithelial appendages, including teeth (Gat et al., 1998; Andl et al., 2002; Liu et al., 2007). During tooth development, high levels of nuclear β-catenin are observed at the early cap stage, and β-catenin mRNA expression is up-regulated in IEE cells and enamel knots (Obara and Lesot, 2004; Obara et al., 2006). Although not addressed here, it would be interesting to know how ROCK regulates ameloblast differentiation via β-catenin-mediated signaling.
Acknowledgements
This work was supported, in part, by KAKENHI (20890208 to K. O.) and an Open Research Project (2007–2011) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.




