Abstract
The soluble member of the TNF-R superfamily osteoprotegerin (OPG) is abundantly released under basal conditions by both mesenchymal stem cells (MSC) and fibroblasts and by endothelial cells upon stimulation with inflammatory cytokines. Since MSC, fibroblasts and endothelial cells represent key elements of the normal and tumor microenvironment and express detectable levels of surface TRAIL receptors, we investigated the effect of TRAIL on OPG release. Unexpectedly, recombinant TRAIL decreased the spontaneous OPG release in all cell types examined. Moreover, TRAIL decreased OPG release also in stromal cells co-cultured with lymphoma cells and counteracted the OPG induction by 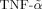 in HUVEC and MSC. Such down-regulation was not due to a masking effect in the ELISA quantification of the OPG released in the culture supernatants due to binding of OPG to its ligands (TRAIL and RANKL), as demonstrated by competition experiments with recombinant TRAIL and by the lack of RANKL release/induction. In addition, OPG down-regulation was not due to induction of cytotoxic effects by TRAIL, since the degree of apoptosis in response to TRAIL was negligible in all primary cell types. With regards to the possible molecular mechanism accounting for the down-regulation of OPG release by TRAIL, we found that treatment of MSC with TRAIL significantly decreased the phosphorylation levels of p38/MAPK. There is a suggestion that this pathway is involved in the stabilization of OPG mRNA. In this respect, the ability of TRAIL to decrease the release of OPG, in the absence of cell cytotoxicity, was mimicked by the p38/MAPK inhibitor SB203580. J. Cell. Physiol. 226: 2279–2286, 2011. © 2010 Wiley-Liss, Inc.
in HUVEC and MSC. Such down-regulation was not due to a masking effect in the ELISA quantification of the OPG released in the culture supernatants due to binding of OPG to its ligands (TRAIL and RANKL), as demonstrated by competition experiments with recombinant TRAIL and by the lack of RANKL release/induction. In addition, OPG down-regulation was not due to induction of cytotoxic effects by TRAIL, since the degree of apoptosis in response to TRAIL was negligible in all primary cell types. With regards to the possible molecular mechanism accounting for the down-regulation of OPG release by TRAIL, we found that treatment of MSC with TRAIL significantly decreased the phosphorylation levels of p38/MAPK. There is a suggestion that this pathway is involved in the stabilization of OPG mRNA. In this respect, the ability of TRAIL to decrease the release of OPG, in the absence of cell cytotoxicity, was mimicked by the p38/MAPK inhibitor SB203580. J. Cell. Physiol. 226: 2279–2286, 2011. © 2010 Wiley-Liss, Inc.
Osteoprotegerin (OPG) is a soluble member of the tumor necrosis factor (TNF) receptor super-family, which regulates bone homeostasis by neutralizing the TNF family member receptor activator of NF-kB ligand (RANKL) [Simonet et al., 1997]. OPG acts as a neutralizing decoy receptor for RANKL. In addition, OPG binds also to another TNF family member: TNF-related apoptosis inducing ligand (TRAIL) [Zauli and Secchiero, 2006]. The ability to bind TRAIL and RANKL with comparable affinity [Vitovski et al., 2007] places OPG in the unique position of regulating two different fundamental aspects of tumor biology: apoptosis and osteoclastogenesis. In particular, since TRAIL is considered a key player in the immune surveillance of the host against cancer [Zauli and Secchiero, 2006], the ability of OPG to inhibit TRAIL cytotoxicity is likely to represent an important blocking mechanism against the tumoricidal activity of TRAIL [Malyankar et al., 2000; Holen et al., 2002; Mirandola et al., 2004; Neville-Webbe et al., 2004; Pritzker et al., 2004; Pettersen et al., 2005]. In this respect, it has been demonstrated that OPG derived from bone marrow stromal cells promotes the survival of myeloma cells [Shipman and Croucher, 2003] and protects breast and prostate cancer cells from TRAIL-induced apoptosis [Neville-Webbe et al., 2004; Nyambo et al., 2004]. Other studies have shown that OPG is produced in vitro by different cell types, including breast and prostate cancer cell lines and have demonstrated that OPG released in the culture supernatants is able to neutralize TRAIL induced apoptosis [Holen et al., 2002, 2005; Pritzker et al., 2004; Cross et al., 2006; Kobayashi-Sakamoto et al., 2006; Secchiero et al., 2006a]. Finally, elevated levels of serum OPG have been detected in patients affected by different types of malignancies [Lipton et al., 2002].
Since stromal and endothelial cells have been shown to actively contribute to the growth and invasiveness of solid tumors [Albini et al., 2005], the aim of this study was to investigate the potential interplay between TRAIL and OPG in primary mesenchymal stem cells (MSC), fibroblasts and vascular endothelial cells, which represent key elements of the tumor microenvironment.
Materials and Methods
Cell cultures
Human umbilical vein endothelial cells (HUVEC) were purchased from BioWhittaker (Walkersville, MD) and grown on 0.2% gelatin-coated tissue culture plates in M199 endothelial growth medium supplemented with 20% FBS, 10 µg/ml heparin, and 50 µg/ml ECGF (all from BioWhittaker) as previously described [Secchiero et al., 2005]. Neonatal human dermal fibroblasts were purchased from Lonza (Verviers, Belgium) and grown in DMEM containing 20% FBS, L-glutamine and penicillin/streptomycin (Gibco-BRL; Grand Island, NY). Bone marrow (BM) derived MSC were purchased from Lonza. Cell preparations used for the experiments described below were homogenously CD105+, CD90+, CD34−, CD45−, CD14−, which is a typical MSC surface antigen profile. MSC were routinely cultured in MSC-Growth Medium (MSC-GM; Lonza). In all experiments, primary cells were used between the 2nd and 6th passage in vitro.
The MOLM and SKW6.4 cell lines were obtained from DSMZ (Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany) or purchased from the American Type Culture Collection (ATCC, Manassas, VA), and cultured in RPMI-1640 containing 10% FBS, L-glutamine and penicillin/streptomycin. For co-culture experiments, BM-MSC, HUVEC, and fibroblasts were seeded in 6 well-plates and the following day SKW6.4 cells were added to the stromal cell monolayers. After 96 h the culture supernatants were collected for ELISA assays and cell viability and apoptosis levels were examined by Trypan blue dye exclusion and Annexin V/PI staining, respectively.
Flow cytometric analyses for surface antigens
The purity of BM-MSC stock cultures was determined by analysis of different antigens after staining with fluorochrome conjugated (FITC- or PE-) mAbs anti-human CD105, CD90 (Beckman Coulter, Marseille, France) CD31, CD106 (Ancell Corp., Bayport, MN), CD29, CD14, CD34, and CD45 (DakoCytomation; Glostrup, Denmark) and analyzed by FACScan [Secchiero et al., 2008]. Surface TRAIL-receptor expression in cell cultures was analyzed by using PE-conjugated mAbs anti-human TRAIL-R1, TRAIL-R2, TRAIL-R3, TRAIL-R4 (all from R&D Systems; Minneapolis, MN) [Secchiero et al., 2008]. Non-specific fluorescence was assessed using irrelevant isotype-matched conjugated antibodies.
Cell treatments
For the evaluation of OPG release and for the evaluation of potential cytotoxic effects, cells were seeded and grown to subconfluence (defined as 70–75% of the optimal confluence for each cell population used) before treatment with the following cytokines: recombinant TRAIL, prepared and purified as previously described [Secchiero et al., 2006b] and recombinant TNF-α (10 ng/ml), purchased from R&D Systems. In selected experiments, cells were treated with SB203580 (10 µM; Calbiochem, La Jolla, CA), a cell-permeable pharmacological inhibitor of the p38/MAPK pathway. The optimal concentrations of TNF-α as well as of SB203580 were determined in preliminary dose–response experiments. TRAIL was used at the concentrations in the range of 0.01–1 µg/ml, as specified for each set of experiments.
Enzyme-linked immunosorbent assays (ELISA)
Human OPG and RANKL levels were measured in cell culture supernatants using sandwich-type enzyme-linked immunosorbent assay (ELISA) kits, purchased from Apotech (Epalinges, Switzerland), used according to the manufacturer's instructions. Measurements were done in duplicates and the results were read at an optical density of 450 nm using an Anthos 2010 ELISA reader (Anthos Labtec Instruments Ges.m.b.H; Salzburg, Austria). Sensitivity of the OPG assay was 2.8 pg/ml, the intra- and inter-assay coefficients of variation (CV) were 9% and <10%, respectively. Sensitivity of the RANKL assay was 1.56 pg/ml, the intra- and inter-assay CV were <5% and <10%, respectively.
For evaluation of potential masking effect of OPG detection by TRAIL, the OPG standard samples (used for the assay standard curve) and cell culture supernatants were pre-incubated for 1 h at 37°C with 1 µg/ml of TRAIL before addition to the anti-OPG Ab coated wells.
Assessment of cell viability and apoptosis
At different times post-treatments, cell viability was monitored by microscopically analysis of the cell monolayer, followed by quantitative examination by means of Trypan blue dye exclusion. Induction of apoptosis was quantified by Annexin V-FITC/propidium iodide (PI) staining (Immunotech, Marseille, France) followed by flow cytometry analysis, as previously detailed [Milani et al., 2003]. To analyze the degree of apoptosis in the entire cell population of fibroblast, HUVEC, and BM-MSC cultures, substrate-attached cells were harvested by trypsin treatment and pooled with floating cells for the staining. To avoid non-specific fluorescence from dead cells, live cells were gated tightly using forward and side scatter.
In apoptosis neutralization assays, recombinant TRAIL was pre-incubated for 1 h at 37°C with recombinant OPG (used at different OPG/TRAIL ratio), before addition to the TRAIL sensitive MOLM cells for 24 h.
Real-time reverse transcription-PCR analysis
Total RNA was extracted from cells by using the Qiagen RNeasy Plus mini kit (Qiagen, Hilden, Germany) according to the supplier's instructions. The quality of the total RNA preparation was verified by agarose gel before transcription into cDNA, using the QuantiTect Reverse Transcription kit (Qiagen). For analysis of OPG gene expression, in either untreated or treated cultures, we have used the SYBR Green real-time PCR detection method, using the SABiosciences RT2 Real-Time™ gene expression assay, that include specific validated primer sets and PCR master mixes (SABiosciences, Frederick, MD). The PCR primer set for human OPG/TNFRSF11B give a 157 bp amplicon (Reference Position: 1373, given on the Refseq Accession #NM_002546), while the primer sets for the housekeeping gene POLR2A give a 183 bp amplicon (Reference Position: 2781, given on the Refseq Accession #NM_000937) as reported at the SABiosciences web site. All samples were run in triplicate with the Real Time thermal analyzer Rotor-Gene™ 6000 (Corbett, Cambridge, UK). Expression values were normalized to the housekeeping gene POLR2A amplified in the same sample.
Western blot analysis
For the analysis of MAPK-pathway, cells were subjected to partial serum reduction (3% FBS) for 18 h prior to the addition of TRAIL ± TNF-α. Cells were harvested in lysis buffer containing 1% Triton X-100, Pefablock (1 mM), aprotinin (10 mg/ml), pepstatin (1 mg/ml), leupeptin (10 mg/ml), NaF (10 mM), and Na3VO4 (1 mM), as previously described [Secchiero et al., 1997]. Protein determination was performed by Bradford assay (Bio-Rad, Richmond, CA). Equal amounts of protein (50 µg) for each sample were migrated in acrylamide gels and blotted onto nitrocellulose filters. Blotted filters were probed with Abs (from Cell Signaling Technology, Beverly, MA) for the phosphorylated form of p38/MAPK and for the respective total protein kinase content for verifying loading evenness, as described [Gibellini et al., 1998]. For the analysis of OPG detection by Western blot, scalar doses (500–0.2 ng, corresponding to 7,500–3 pg/ml) of recombinant OPG (R&D Systems) were migrated in acrylamide gels and blotted onto nitrocellulose filters before probing with the anti-OPG Ab (R&D Systems).
After incubation with peroxidase-conjugated anti-mouse or anti-rabbit IgG, specific reactions were revealed with the ECL detection kit (Amersham Pharmacia Biotech, Buckinghamshire, UK). Densitometric values were estimated by the ImageQuant TL software (Amersham Pharmacia Biotech). Multiple film exposures were used to verify the linearity of the samples analyzed and avoid saturation of the film.
Statistical analysis
Descriptive statistical were calculated. For each set of experiments, values are reported as means ± SD. Data were analyzed by Student's t test. Statistical significance was defined as P < 0.05.
Results
Comparison of the expression and release of OPG among human fibroblasts, BM-MSC, and endothelial cell cultures
In the first group of experiments, we have compared the levels of OPG released by human primary MSC, fibroblasts and endothelial cell cultures. The release of OPG, calculated as ng/ml of protein released in 24 h by 106 cells, showed a great variability, with MSC and fibroblasts producing significantly higher levels compared to endothelial cells (Fig. 1A). The differences in term of OPG protein release were correlated to the levels of OPG mRNA expression, evaluated by quantitative RT-PCR (Fig. 1B). OPG released in the culture supernatants was documented also by Western blotting analysis, but most of the experiments described in this study were performed using ELISA, taking into account that ELISA was markedly more sensitive than Western blot (lower detection limit of ELISA: 2.8 pg/ml, lower detection limit of Western blot: 300 pg/ml, Supplementary Fig. 1) and allowed for better quantification.
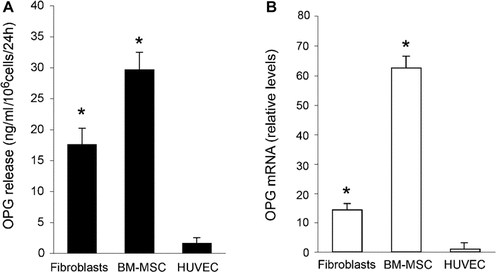
Comparison of expression and release levels of OPG among human fibroblasts, BM-MSC, and endothelial cell cultures. Primary human fibroblasts, BM-MSC, and endothelial cell (HUVEC) cultures were comparatively analyzed for the levels of OPG released in the supernatants, assayed by ELISA (A), and for levels of OPG mRNA, measured by RT-quantitative PCR (B). In A, ELISA results are expressed as ng/ml of OPG released in 24 h by 106 cells. In (B), results from quantitative RT-PCR, done in triplicate, are expressed as relative RNA levels calculated, after normalization for the housekeeping gene, respect to HUVEC, which were set to 1 for a better comparison. Data are expressed as means ± SD of results from four independent experiments, each performed in duplicate. *P < 0.05, compared to HUVEC.
Differential modulation of OPG expression/release by TRAIL and TNF-α
In order to evaluate the potential interplay between recombinant TRAIL and native OPG released by the cells of the stromal microenvironment [Zauli et al., 2009], we next investigated the effect of recombinant TRAIL on the release of OPG. Unexpectedly, treatment with recombinant TRAIL promoted a significant (P < 0.05) down-regulation of OPG release in the culture supernatants in all primary cell type examined (Fig. 2A). On the other hand, treatment of the same cultures with TNF-α revealed a cell-type dependent ability of TNF-α to modulate OPG release. TNF-α exhibited a limited effect on fibroblast OPG release, a significant induction of OPG release in BM-MSC (approximately 2-fold) and a marked induction of OPG release in endothelial cells (more than 10-fold) (Fig. 2A). Quantitative RT-PCR analysis of HUVEC revealed opposite effects of TRAIL and TNF-α in modulating OPG mRNA levels (Fig. 2B), consistently with the data of OPG protein release in the culture supernatant (Fig. 2A).
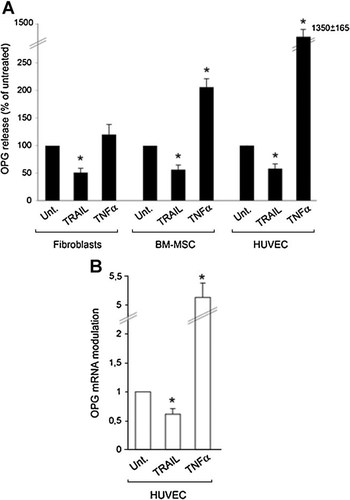
Differential modulation of OPG by TRAIL and TNF-α. In (A), primary human fibroblasts, BM-MSC and HUVEC cultures were left untreated (Unt.) or treated either with TRAIL (1 µg/ml) or with TNF-α (10 ng/ml) for 72 h, before assessing OPG levels in culture supernatants. Results are expressed as percentage of OPG release calculated with respect to the untreated cultures, which were set to 100. In (B), OPG mRNA modulation in HUVEC, exposed for 24 h to either TRAIL (1 µg/ml) or TNF-α (10 ng/ml) was evaluated by RT-quantitative PCR. Results are expressed as relative RNA levels calculated, after normalization for the housekeeping gene, respect to control untreated cultures, which were set to 1. Data are expressed as means ± SD of results from at least four independent experiments, each performed in duplicate. *P < 0.05, compared to untreated.
The possibility that the decline of OPG observed in culture supernatants of primary cells might be due to a masking effect, as result of its binding to recombinant TRAIL, was ruled out by carrying out competitive ELISA. These assays were performed by incubating the standard OPG samples (12.5–500 ng/ml) or the cell culture supernatants with recombinant TRAIL (1 µg/ml). Indeed, pre-incubation with recombinant TRAIL showed minor effects on the detection of OPG, inducing a decrease that was always <10%, compared to OPG alone (Fig. 3A). These findings are relevant since the quantity of TRAIL largely exceeded that of OPG. On the other hand, pre-incubation of recombinant TRAIL with scalar doses of OPG induced a dose-dependent inhibition of TRAIL-induced apoptosis in a sensitive leukemic cell line (Fig. 3B). Taken together, these data indicate that, although TRAIL binds effectively to OPG, it shows little interference with the OPG determination by ELISA also when present in large excess.
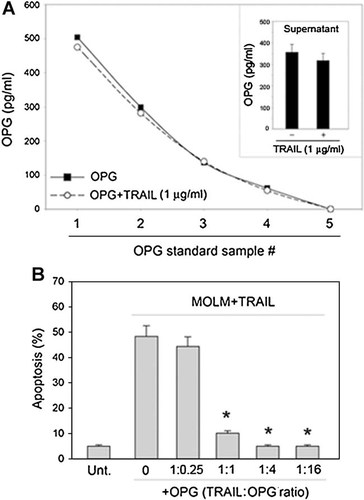
Binding of recombinant TRAIL to OPG does not affect the OPG ELISA detection. In (A), for evaluation of potential masking effect of OPG ELISA detection by TRAIL, the OPG standard samples (used for the assay standard curve) and fibroblast culture supernatants (inset) were pre-incubated for 1 h at 37°C in the absence or presence of 1 µg/ml of TRAIL before addition to the anti-OPG Ab coated wells. Data, representative of three independent experiments, demonstrating that the presence of an excess of TRAIL does not significantly interfere with detection of OPG are shown. In (B), inhibitory effect of OPG on TRAIL-induced apoptosis in leukemic cells. TRAIL (1 µg/ml) was incubated for 1 h at 37°C with recombinant OPG used at the indicated TRAIL/OPG ratio, before addition to MOLM leukemic cells. Data are expressed as means ± SD of results from three independent experiments, each performed in duplicated. *P < 0.01 compared to TRAIL alone (OPG = 0).
Since RANKL shows a comparable but higher affinity for OPG compared to TRAIL [Vitovski et al., 2007], we also considered the possibility that the down-modulation of OPG release might be due to the concomitant presence/induction of soluble RANKL in TRAIL-treated cultures. However, the possible masking effect of RANKL on OPG detection was ruled out since soluble RANKL was undetectable (below the assay detection limit) in all cell cultures and in all the conditions assayed in this study (data not shown).
Down-modulation of OPG release by TRAIL in the absence of cell cytotoxicity
Having observed that TRAIL down-regulates OPG release, in a time- (Fig. 4A) and dose- (Fig. 4B) dependent manner, reaching approximately 50% inhibition at concentration 1 µg/ml after 72 h of culture, we investigated whether exposure to TRAIL at a concentration of 1 µg/ml and 72 h of exposure affected the viability of MSC, fibroblasts or endothelial cells. As shown in Figure 4C, all primary stromal cells appeared resistant to TRAIL, which instead induced marked cytotoxicity in a leukemic cell model already after 18 h of treatment. The lack of toxicity of TRAIL when added to primary stromal cells was not due to the absence of surface TRAIL receptors in these cells, since both MSC and fibroblasts (Fig. 4D), as well as HUVEC [Secchiero and Zauli, 2008] expressed detectable amounts of TRAIL-R2, which is one of the two receptors able to transduce intracellular signals [Di Pietro and Zauli, 2004]. These experiments rule out also the possibility that OPG down-modulation is the indirect consequence of the cytotoxic effect of TRAIL.
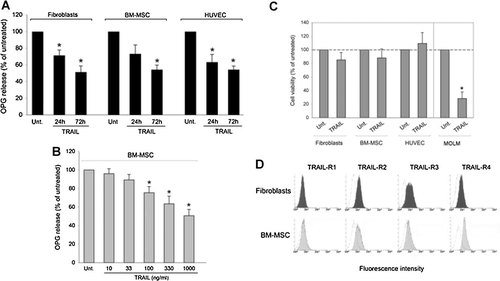
Down-modulation of OPG release by TRAIL in the absence of cell cytotoxicity. The indicated cell cultures were left untreated (Unt.) or treated with TRAIL (used at 1 µg/ml in A,C). OPG levels were measured in culture supernatants harvested at both 24 and 72 h, in (A), or only at 72 h, in (B). Cell viability was evaluated after 72 h of TRAIL treatment (C); cell cytotoxicity by TRAIL in leukemic MOLM cells is also shown as internal control of TRAIL activity. Data are expressed as means ± SD of results from four independent experiments, each performed in duplicate. *P < 0.05, compared to untreated. In (D), surface expression of TRAIL-receptors was evaluated by flow cytometry. Shaded histograms represent cells stained with mAbs specific for indicated TRAIL-receptors (TRAIL-R1, TRAIL-R2, TRAIL-R3, and TRAIL-R4), and unshaded histograms represent background fluorescence obtained by staining the same cells with isotype-matched control Ab. A representative of four separate experiments is shown.
In addition, in order to mimic potentially relevant in vivo pathological conditions, we assessed the effect of TRAIL on OPG release in co-culture systems of stromal cells (MSC, fibroblasts and HUVEC) with lymphoma cells (SKW6.4). While OPG was not detectable in the culture supernatants of the lymphoma cell cultures, its release by stromal cells was not significantly affected (with a trend to increase) by the concomitant presence of lymphoma cells (Fig. 5). Interestingly, the ability of TRAIL to decrease OPG levels was preserved also in the in vitro MSC/lymphoma, HUVEC/lymphoma, and fibroblast/lymphoma co-cultures (Fig. 5).
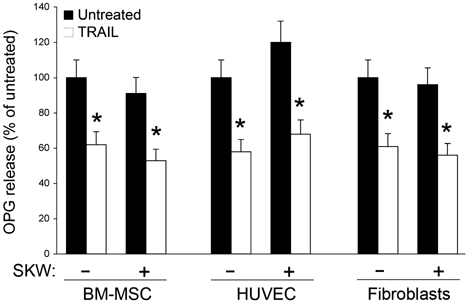
The in vitro co-culture with lymphoma cells does not affect the ability of TRAIL of down-regulating OPG release by stromal cells. MSC, HUVEC, and fibroblast were cultured in the absence or presence of SKW6.4. TRAIL (1 µg/ml) was added at the co-cultures as indicated. After 96 h of culture (72 h of TRAIL treatment), OPG levels were measured in the culture supernatants by ELISA. Data are reported as mean ± SD of four independent experiments, each performed in duplicate. *P < 0.05 compared to untreated.
TRAIL inhibits the basal and TNF-α-induced activation of the p38/MAPK pathway
Considering that chronic inflammation represents an important risk factor in the promotion of neo-angiogenesis and cancer development [Albini et al., 2005] and that OPG can directly contribute to inflammation by promoting the adhesion of leukocytes to endothelial cells [Mangan et al., 2007; Zauli et al., 2007], we assessed whether TRAIL was able to modulate the release of OPG induced by TNF-α. In this set of experiments, we used MSC and endothelial cells which respond to TNF-α with a significant increase of OPG expression and release (Fig. 2A). Exposure to recombinant TRAIL significantly (P < 0.05) reduced (approximately 50%) the TNF-α-induced increase in OPG production (Fig. 6A). The analysis of the 1,106-bp OPG promoter sequence (according to the sequence in GenBank accession no. AB008821) by applying computer programs PROMO and TFSEARCH (prediction algorithm) identified a great variety of potential transcription factor binding sites, including sites which bind factors regulated by the p38-MAPK pathway (such as Max and CRE-BP1 transcription factors). Previous studies have indicated that aside from the transcriptional regulation, there is a significant role of post-transcriptional mechanism(s) in the modulation of OPG protein release [Pantouli et al., 2005; Lambert et al., 2007; Kondo et al., 2008]. In the effort to elucidate the intracellular mechanism(s) underlying the inhibitory activity of TRAIL, we next focused our attention on the MAPK family member p38/MAPK, which is known to play a major role also in stabilizing the mRNA of OPG. To investigate the potential role of p38/MAPK in our cell models, MSC (Fig. 6B) and HUVEC (data not shown) were treated with SB203580, a cell permeable pharmacological inhibitor of the p38/MAPK pathway. As shown in Figure 6B,C, rapid inhibition of p38 phosphorylation (as evaluated by Western blot, Fig. 6B) was accompanied by reduction of OPG release in the absence of cell cytotoxicity (Fig. 6C), confirming the key role of the p38/MAPK in modulating OPG release. Similarly, exposure to TRAIL rapidly reduced baseline levels of p38/MAPK phosphorylation, while TNF-α induced an upregulation of p38/MAPK phosphorylation (Fig. 6B). Notably, treatment with TRAIL attenuated TNF-α-induced p38/MAPK phosphorylation (Fig. 6B). Overall, these observations indicate a role of p38/MAPK pathway in TRAIL-mediated down-modulation of OPG in stromal cells.
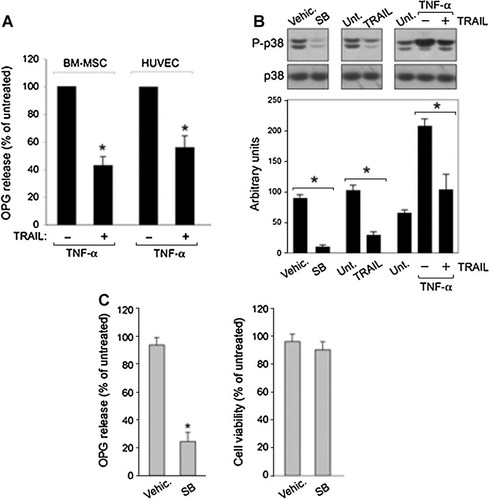
TRAIL inhibits the basal and TNF-α-induced activation of the p38/MAPK pathway. In (A), BM-MSC and HUVEC were treated with TNF-α (10 ng/ml) in the presence or absence of TRAIL (1 µg/ml), which were added to the cultures at the same time. OPG levels in culture supernatants were assessed by ELISA and expressed as percentage with respect to TNF-α-treated cultures. Data are expressed as means ± SD of results from three independent experiments, each performed in duplicate. *P < 0.05. In (B), MSC cultures were left untreated (Unt.) or exposed for 15 min to TRAIL (1 µg/ml) and TNF-α (10 ng/ml), used either alone or in combination. In parallel, as control, cells were treated with SB203580 (SB, 10 µM) or with the control vehicle (Vehic.). After the indicated treatments, equal amounts of cell lysates were analyzed by Western blot using antibodies specific for the native form of the kinase (p38) and for residues that are phosphorylated upon activation (P-p38). One of three experiments with similar results is shown. Protein bands were quantified by densitometry and level of P-p38, expressed as arbitrary units, were calculated for each treatment after normalization to total p38. Data are reported as means ± SD of results from three experiments. Decreased levels of P-p38 in cell lysates confirm the activity of the SB203580 pharmacological inhibitor. In C, the effect of SB203580 (10 µM) on OPG release and cell viability was assessed after 72 h of treatment, in comparison to the control vehicle. Data are expressed as percentage with respect to the untreated cultures (set to 100%). Data are reported as means ± SD of results from three experiments performed in triplicates.
Discussion
A number of studies have demonstrated that OPG supports the survival of tumor cells and also of tumor-associated endothelial cells [Holen et al., 2002, 2005; Lipton et al., 2002; Shipman and Croucher, 2003; Neville-Webbe et al., 2004; Nyambo et al., 2004; Pettersen et al., 2005; Cross et al., 2006; De Toni et al., 2008; Rachner et al., 2009]. In particular, concerning the role of OPG on endothelial cell survival, it has been shown that engagement of integrins on the endothelial cell surface triggers a NF-kB-dependent expression of OPG that mediates the anti-apoptotic activity of NF-kB in both endothelial and epithelial cells [Shipman and Croucher, 2003]. Moreover, the significant up-regulation of OPG expression and release in response to inflammatory cytokines [Pantouli et al., 2005; Toruner et al., 2006] supports a potential biological role for OPG in tumor progression. Taking into account that therapy with repeated injections of high concentrations of recombinant TRAIL (8–10 mg/kg/die) is currently being investigated in clinical trials for different types of solid tumors and hematological malignancies [Herbst et al., 2010], we found of interest to investigate the expression and the release of OPG, as well as its modulation, in primary cells, which represent key elements in the tumor microenvironment, such as endothelial cells, BM-MSC, and fibroblasts. In this context, our data showing that the activation of the TRAIL pathway attenuates the release of OPG in a variety of stromal cells, including MSC, fibroblasts and endothelial cells, represents a completely novel finding with potential important clinical implications. In fact, although a causative role of OPG in tumor progression has not been conclusively established [Zauli et al., 2009], different potential pro-tumoral effects have been proposed: (i) recruitment of macrophages in the tumor tissue; (ii) induction of angiogenesis; (iii) inhibition of TRAIL-mediated apoptosis of tumor cells. Concerning this last point, in vitro experiments have demonstrated that native OPG produced by a variety of cancer cells inhibits the pro-apoptotic activity of recombinant TRAIL in multiple myeloma, prostate, breast and colon cancer cell lines, acting in an autocrine/paracrine manner [Holen et al., 2002, 2005; Lipton et al., 2002; Shipman and Croucher, 2003; Neville-Webbe et al., 2004; Nyambo et al., 2004; Pettersen et al., 2005; Cross et al., 2006; De Toni et al., 2008; Rachner et al., 2009]. As mentioned above, however, it should be noted that OPG displays biological activities which are independent of its TRAIL or RANKL neutralizing effects, such as the promotion of the adhesion of both normal and leukemic cells to endothelial cells [Zauli et al., 2007]. OPG expression is regulated by a variety of mechanisms, as can be predicted by sequence analysis of the 1,106-bp OPG promoter region. Notably, a key role for NF-kB was originally suggested by the presence of several NF-kB binding sites (nucleotide positions: 36–45, 156–165, 782–791, 793–802; numbered according to the sequence in GenBank accession no. AB008821) and confirmed by the ability of TNF-α (and other inflammatory cytokines) to induce OPG through activation of NF-kB. Although it has been reported that TRAIL is able to activate the NF-kB pathway in some tumor cell models [Zauli and Secchiero, 2006], we have previously documented that recombinant TRAIL does not activate NF-kB in primary normal cells, such as HUVEC [Secchiero et al., 2005], primary vascular smooth cells [Secchiero et al., 2004], and primary MSC [Secchiero et al., 2008], excluding the involvement of the NF-kB pathway in the modulation of OPG expression/release in response to recombinant TRAIL.
Although the molecular mechanism involved in the down-regulation of OPG expression by TRAIL requires further investigation, we showed that TRAIL can down-regulate the baseline and the TNF-α-induced p38/MAPK phosphorylation levels. On the other hand, previous studies performed on pancreatic [Siegmund et al., 2007], prostatic [Son et al., 2010] and colon [Zhang et al., 2004] cancer cell models have shown that TRAIL can activate the p38/MAPK pathway, mainly in cells resistant to TRAIL-mediated apoptosis. It is uncertain whether the differential ability of TRAIL to either promote or suppress p38/MAPK is cell-type dependent or might rather reflect the (normal vs. transformed) status of the cells. We and other authors were unable to document induction of p38 phosphorylation in response to TRAIL in primary human vascular smooth muscle cells, MSC, and fibroblasts [Secchiero et al., 2004, 2008; Steele et al., 2006; Audo et al., 2009].
The ability of TRAIL to down-regulate OPG release was not confined to endothelial cells but was also observed in BM-derived MSC and primary fibroblasts. Thus, besides exerting a direct cytotoxic effect in various cancer cell types, TRAIL may also act indirectly against cancer cells by attenuating the release of OPG, by endothelial cells, fibroblasts, and/or MSC, which represent key elements in the tumor microenvironment. Furthermore, TRAIL inhibited OPG release also in MSC/lymphoma cocultures, which mimick pathological in vivo conditions. Since specific agonistic antibodies for TRAIL-R1 and TRAIL-R2 are currently being tested as alternatives to recombinant TRAIL [Ichikawa et al., 2001, 2003], it will be interesting to find out whether also these agonistic antibodies are able to down-regulate OPG.
Acknowledgements
This work was supported by grants from the Italian Association for Cancer Research (AIRC) and from Beneficientia Foundation.