Expression of members of the miRNA17–92 cluster during development and in carcinogenesis
Abstract
The six microRNAs (miRNA) encoded by the miR-17–92 cluster, also named oncomir-1, have been associated with carcinogenesis and typically exhibit-increased expression in tumors. Despite the well-established role for the miR-17–92 cluster in an oncogenic network, the physiological function of these miRNAs in normal tissues remains unresolved. In order to investigate whether there are similar patterns of miR-17–92 expression during embryogenesis and carcinogenesis, we have preformed a systematic study of the expression in cultured carcinoma cells, cultured primary human keratinocytes (KC), and during development of some murine tissues. Both levels of expression of the primary transcript (pri-miRNA) and levels of expression of the individual members of the cluster were monitored. Irrespectively of tissue examined we found that the level of expression decreased markedly during development. With cultured primary human KCs their levels of expression of some of these microRNAs decreased as the number of cell passages increased. Their levels of expression in cultured carcinoma cells, in contrasts, increased, or remained unchanged, with increasing number of cell passages. The results suggest these microRNAs are involved in the regulation of foetal development and that they may promote proliferation and inhibit differentiation during embryogenesis and carcinogenesis. Additionally, the six microRNAs exhibit variable tissue expression, suggesting selective processing of these microRNAs. J. Cell. Physiol. 226: 2257–2266, 2011. © 2010 Wiley-Liss, Inc.
MicroRNAs are small non-coding RNAs of approximately 22 nucleotides (nt) that negatively regulate messenger RNA (mRNA) translation and stability at a post-transcriptional level (Krol et al., 2010). These small molecules have been suggested to be constituents of a major digital-style regulatory network (Mattick, 2007) which may be of major significance both during development (Stefani and Slack, 2008) and disease (Callis et al., 2007).
MicroRNA transcription occurs via RNA polymerase II. These transcripts, referred to as primary (pri-) miRNAs, can be several kilo bases long and containing monocistronic or polycistronic stem–loop structures. The monocistronic transcripts encode individual miRNAs, while the polycistronic transcripts contain a number of miRNAs that are coordinately expressed, so called miRNA clusters. These clusters are processed into individual miRNAs (Olena and Patton, 2010). Studies have shown that the level of expression of matured miRNAs belonging to the same cluster is not equivalent (Yu et al., 2006).
The mature, single-stranded microRNA (either sense, antisense, or occasionally both sequences) is subsequently incorporated into the RNA-induced silencing complex (RISC), which uses the miRNA as a guide strand to identify target mRNA. One criteria used to identify the mRNA target is the “seed” sequence, referring to nucleotide 2–7 of the 5′-end of the miRNA (Olena and Patton, 2010). Altered translation and stability of the target mRNA is caused by imperfect base-paring of the “seed” region to a target sequence in the 3′ untranslated region of the mRNA (Bagga et al., 2005; Bartel, 2004). MiRNAs with similarities in the “seed” sequence can, therefore, target analogous mRNAs. Consequently, based on the “seed” sequence, miRNAs are grouped into families.
MiRNA encoding genes are scattered throughout the genome of both plants and animals. They are located in almost every region of the genome (Bartel, 2004). Some 36% and 45% miRNAs are found in clusters in human and mouse (Griffiths-Jones et al., 2008). To date, 55 different miRNA clusters have been identified in the human genome, and 51 in the murine genome. The clusters often express miRNA belonging to different families, enabling a cluster to target unrelated mRNAs (Olena and Patton, 2010).
The miR-17–92 miRNA cluster is encoded by a polycistronic gene located on human chromosome 13q31, and on murine chromosome 14 (van Haaften and Agami, 2010). The primary miR-17–92 transcript is the precursor of seven miRNA molecules (miR-17–3p, miR-17–5p (miR-17), miR-18a, miR-19a, miR-20a, miR-19b-1, and miR-92a-1). The sequence and the organization of these miRNAs are conserved in all vertebrates (Mendell, 2008). They are grouped in to four families based on their “seed” sequences (Bonauer and Dimmeler, 2009).
Several studies have shown that miRNAs of the mir-17–92 cluster are highly expressed in cancer cells, and has also been shown to be subjected to frequent amplification and over-expression (Mendell, 2008; Ventura et al., 2008). On the other hand, in ovarian-, breast-, and skin cancer the miR-17–92 cluster is deleted (Bonauer and Dimmeler, 2009).
A number of studies have implicated the miR-17–92 clusters in development. The miR-17–92 cluster have a critical role in lung and heart development [for review see Bonauer and Dimmeler (2009)]. Using mouse, Ventura et al. (2008) showed that deficient expression of miR-17–92 is not compatible with post-natal survival. Another study show the miR-17–92 members to be highly expressed in lung epithelial progenitor cells, their levels of expression decreasing during development of the lung (Lu et al., 2007). These findings were confirmed by Carraro et al. (2009) demonstrating that over-expression of miR-17 leads to proliferation of epithelial cells. Results also point toward miR-17–92 being an important player in aging (Grillari et al., 2010).
So far, about 30 mRNAs targets for the members of the miR-17–92 cluster have been confirmed, several of which are involved in carcinogenesis and/or regulation cell cycle (Grillari et al., 2010).
In an earlier study we mapped the miRNA expression profiles of murine tooth germ, submandibular salivary gland, and liver at different stages of development (Jevnaker and Osmundsen, 2008). The result revealed that miR-17–5p, miR-18a, miR-19b, miR-20a, and miR-92a were highly expressed in embryonic organs and that their expression decreased during development.
Here we expand our earlier results. To this end we, therefore, report levels of expression of all members of the cluster (miR-17–3p, miR-17–5p, miR-18a, miR-19a, miR-20a, miR-19b-1, and miR-92a-1) in a range of mouse embryonic and young adult tissues, i.e., liver, lung, salivary gland, tooth germ, kidney, heart, small intestine, skin, and cerebral hemisphere at an extensive range of developmental stages (E11.5, E13.5, E15.5, E17.5, P0, P5, and P25). We also measured the level of expression of members of the miR-17–92 cluster in mouse embryos at E8.5 and E11.5.
Further, to illustrate a potential association to proliferation, we monitored levels of expression of all members of the cluster at selected passages of cultured, primary, oral keratinocytes (KCs; P2 and P7) and at selected passages of cultured oral carcinoma cells (P6, P15, and P25).
Additionally, we measured the level of expression of the primary transcript, pri-miR-17–92, in mouse liver, lung, and salivary gland (E11.5 E13.5, E15.5, E17.5, P0, P5, and P25), cultured primary oral KCs (P2 and P7) and cultured oral carcinoma cells (P6, P15, and P25) to investigate whether the level of expression of the pri-miRNA transcript reflects those of the resulting mature miRNAs.
Materials and Methods
Experimental animals
The liver and the lungs were isolated from CD-1 mouse embryos, newborns, and young adults at embryonic day 11.5 (E11.5), E13.5, E15.5, E17.5 and at postnatal day 0 (P0), P5, and P25. The right submandibular salivary gland were isolated from CD-1 mouse embryos, newborns, and young adults at E13.5, E15.5, E17.5 and at postnatal day 0 (P0), P5, and P25. The right first molar mandibular tooth germ were isolated at E15.5, P0, and P5. The kidney, the small intestine, the heart, the right cerebral hemisphere, and skin were isolated from CD-1 mouse embryos and young adults at embryonic day 13.5 (E13.5), E15.5 and postnatal day 25 (P25). Embryos were collected at E8.5 and E11.5. The day of appearance of a vaginal plug was set to 0.5 day post coitum. Embryos were staged according to the Theiler criteria (Kaufman, 1994). All experiments were carried out using embryos from at least three pregnant mice at each developmental stage.
The pregnant mice were sacrificed by cervical dislocation, embryos, and newborns by decapitation. Embryos were immediately immersed in RNALater™ (Ambion Inc., TX, USA) diluted 1:2 with PBS. Heads were isolated and, while still immersed in PBS/RNA-Later, the right first mandibular molar tooth germ, the right submandibular salivary gland, and the right cerebral hemisphere were isolated and transferred into separate 1 ml of undiluted RNALater™. Liver, kidneys, lungs, small intestine, heart, and abdominal skin were removed and immediately transferred into undiluted RNALater™. Decapitated embryos were transferred into undiluted RNALater™.
The animals were kept at a 12 h light: dark cycle, 21 °C and with a relative humidity of 65%. Food and water were supplied ad lib. The animals were kept as defined by the Norwegian Gene Technology Act of 1994.
Cell lines
Three oral squamous carcinoma cell lines (clone C12 (PE/CA-PJ34), clone D2 (PE/CA-PJ41), and clone E10 (PE/CA-PJ49)) were obtained from the European Collection of Cell Cultures (ECACC). The cells were grown in Iscove's Modified Dulbecco's Medium (Sigma-Aldrich, St. Louis, MO), supplemented with 10%FBS, 2 nM L-glutamine, 1% penicillin, 1%streptomycin, and 0.25 µg/ml amphotericin B (all reagents purchased from Lonza, Basel, Switzerland). The cells were split twice weekly at 80% confluence.
Primary human normal oral KCs were isolated from biopsy samples after third molar extraction as described by Costea et al. (Costea et al., 2002). The procedure had been approved by the Ethics Committee in Norway. They were grown on plastic surfaces in KC serum free medium (KSFM) supplemented with 1 ng/ml human recombinant epidermal growth factor and 25 mg/ml bovine pituitary extract (all purchased from GibcoBRL, Dorset, UK). The medium was further added 2 mM L-glutamine, 1%penicillin, 1% streptomycin, and 0.25 mg/ml amphotericin B (all purchased from Lonzo, Basel, Switzerland). The media were replaced every second day and the cells were split at 80% confluence using a ratio of 1:3.
Transfection of mature miR-17–3p molecules into cell line E10
Cell line E10 was incubated in IMDM medium with 5% FBS and transfected with INTERFERin (Polyplus-Transfection, Illkirch, France) according to the manufacturer's protocol using 20 nM Allstar scrambled control (Qiagen, Hilden, Germany) or mature miR-17–3p (GenePharma, Shanghai, China). MicroRNAs were isolated 24 h after transfection using the miRNeasy Mini Kit as described by the manufacturer (Qiagen).
Isolation of RNA
Total RNA were extracted from liver, lung, submandibular gland, and carcinoma cells using the RNeasy Mini Kit as described by the manufacture (Qiagen) and treated with the TURBO DNA-free kit to remove genomic DNA (Applied Biosystems, CA, USA). Fractions of total RNA, enriched with respect to miRNA, were isolated from whole embryos, tooth germ, submandibular salivary gland, liver, kidney, lung, small intestine, heart, cerebral hemisphere, skin, squamous carcinoma cell, and primary oral KCs using the miRNeasy Mini Kit as described by the manufacturer (Qiagen).
The first molar mandibular tooth germs were isolated from ten phenotypical embryos/newborns from each of at least three pregnant mice at E15.5, P0, and P5. RNA was extracted from each batch of ten tooth germs, the RNA resulting from one batch of ten tooth germs representing one sample of miRNA for subsequent analysis. The submandibular salivary gland (at E13.5, E15.5, E17.5, P0, P5, and P25), lungs (at E11.5, E13.5, E15.5, E17.5, P0, and P5), kidneys, small intestine, heart, right cerebral hemisphere, and skin (at E13.5 and E15.5) were removed from three to five embryos/newborns obtained from at least three mothers. RNA isolated from a batch of three to five samples of tissue represented one of at least three replicate samples. At P25, RNA was isolated from each of three separate tissue samples/organs, constituting the three replicate samples.
Livers were removed at E11.5, E13.5, E15.5, E17.5, P0, and P5 from two to four embryos/pups derived from each of three mice. RNA isolated from a batch of two to four livers represented one of at least three replicate samples at E11.5, E13.5, E15.5, E17.5, P0, and P5. RNA isolated from young adult (P25) liver was obtained from at least three samples of tissue isolated from at least three separate mice.
Embryos were collected from three separate mice at E8.5 and E11.5. RNA isolated from one embryo represented one of at least three replicate samples at E8.5 and E11.5.
The quality and composition of solutions of RNA were assessed using the Agilent Bioanalyzer (Agilent, Palo Alto, CA, USA). Concentrations of solutions of purified RNA were assayed using the Nanodrop ND-1000 spectrophotometer.
Reverse transcription
The High-Capacity cDNA Reverse Transcription Kit was used to reverse transcribe 130 µg DNase-treated total RNA to cDNA (Applied Biosystems). Each 20 µl reverse transcription reaction was performed according to the manufactures protocol and run in an Eppendorf MasterCycler (Eppendorf GmBh, Hamburg, Germany).
TaqMan® MicroRNA Assay (hsa_miR_17–3p, hsa_miR_17–5p, hsa_miR_18a, hsa_miR_19a, hsa_miR-19b-1, hsa_miR_20a, and hsa_miR_92a-1) were used to reverse transcribe 40 ng of total RNA, enriched in miRNA, to cDNA (Applied Biosystems). Each 15 µl reverse transcription reaction was performed according to the manufactures protocol and run in an Eppendorf MasterCycler (Eppendorf GmBh).
The snoRNA135 TaqMan® Assay was used as control for RNA isolated from murine tissues and the RNU48 TaqMan® Assay was used as a control for RNA isolated from human cells. Neither snoRNA135 nor RNU48, however, was well suited as controls. The level of expression of RNU48 varied both between cultures and between cells assayed at different number of passages, suggesting that RNU48 should be applied with caution. The level of expression of snoRNA135 was found to vary considerably with, e.g., time of development. This is a consistent problem when studying gene expression in developing tissues. Although snoRNA135 has been claimed to be a “control” for tissues from mice our results showed this is not the case with developing tissues. Instead, an internal control was used to normalize between plates and samples.
Quantitative real-time PCR
Quantitative real-time PCR was carried out using TaqMan® MicroRNA Assay (hsa_miR_17–3p, hsa_miR_17–5p, hsa_miR_18a, hsa_miR_19a, hsa_miR-19b-1, hsa_miR_20a, and hsa_miR_92a-1), the TaqMan® Pri-miRNA assay protocol (hsa-pri-miR-17, hsa-pri-miR-92a-1, mmu-pri-miR-17, and mmu-pri-miR-92a-1), and TaqMan® Universal PCR Master Mix (Applied Biosystems). RNA isolated at the various time points was analyzed using both technical and biological triplicates. An internal control was used to normalize between samples and PCR-plates. Assays were carried out using the Stratagene MX 3005 PCR system (Stratagene, CA, USA). All results were analyzed using multiple experiment analysis applying a common threshold.
Statistical analysis
The GenEx program (MultiD Analysis AB, Gothenburg, Sweden) was used to calculate the fold-change and to perform statistical analysis. The fold-change was calculated using a reference sample specific for each tissue. The non-parametric Mann–Whitney's test was used to test differences between groups. Two-sided P-values < 0.05 were considered statistically significant. Results are presented as mean and SD.
Results
Expression of miRNAs coded by the miR-17–92 miRNA cluster during development of murine liver.
Results presented in Figure 1 show levels of expression of the various miRNAs of the miR-17–92 miRNA cluster in liver isolated at E11.5, E13.5, E15.5, E17.5, P0, P5, and P25 (adult mouse). All miRNAs of the cluster was detected at all time-points investigated. In general, their levels of expression decreased during development. However, the lower level of expression of miR-19a, miR-19b, and miR-20a were found at E17.5, their levels subsequently increasing from E17.5 to P0. From P0 their levels of expression progressively declined, reaching a lower level of expression in adult liver (P25).
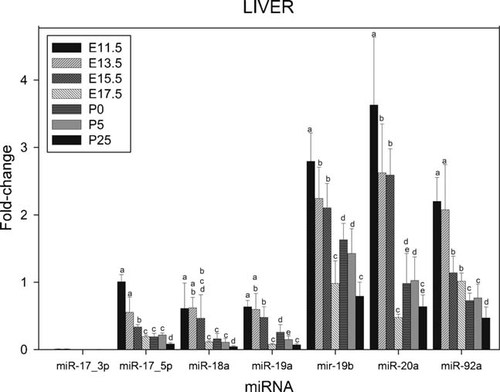
Relative levels of expression of the microRNAs of the miR-17–92 cluster in murine liver at various stages during development. Fractions of RNA enriched with respect to microRNA were isolated from murine livers at various the stages of development shown in Figure. The various microRNAs of the miR-17–92 cluster (shown in the Fig.) were assayed by real-time RT-PCR as described in Experimental Section. Fold changes in expression and statistical parameters were calculated using the GenEx program. The plotted data represents mean values ± SD derived from at least three separate samples of tissue (derived from three separate embryos/pups/young adults). For each specific miRNA, the mean values with the same letter are not significantly different at P < 0.05. All other values are significantly different.
Based on their relative levels of expression, the seven miRNAs of the miR-17–92 cluster can be organized into three groups. The higher levels of expression were found with miR-19b, miR-20a, and miR-92, followed by those of miR-17–5p, miR-18a, and miR-19a. The level of expression of miR-17–3p was barely detectable. A positive control experiment was carried out to confirm the consistently negative results observed with the miR-17–3p assay (Fig. 8c).
Changes in expression of miRNAs encoded by the miR-17–92 miRNA cluster in developing murine lung
Results presented in Figure 2 show the levels of expressions of the constituents of the miR-17–92 cluster in lung at E11.5, E13.5, E15.5, E17.5, P0, P5, and P25. The level of expression of miR-17–5p is higher at early stages of development, in agreement with Carraro et al. (2009). From E11.5 to E17.5 miR-17–5p exhibit a high, relatively stable, level of expression. Following a decline at birth (P0), the level expression subsequently increases at P5. The lower level of expression is observed at P25. The temporal expression of miR-18a is similar to that of miR-17–5p, except for the decreased level of expression at P0.
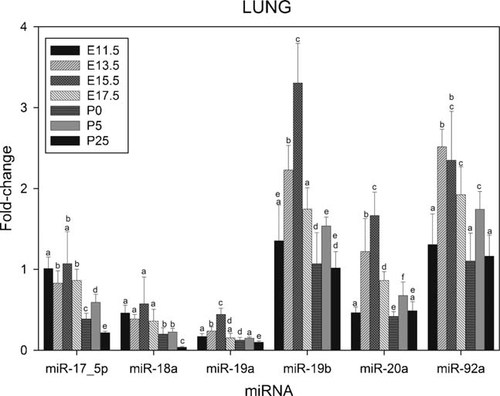
Relative levels of expression of the microRNAs of the miR-17–92 cluster in murine lung at various stages during development. Fractions of RNA enriched with respect to microRNA were isolated from murine lung at the various stages of development shown in Figure. Experimental details are otherwise as given in legend to Figure 1.
The low level of expression at P0 is also observed for mir-19b, miR-20a, and miR-92a. Another major difference between these miRNAs and miR-17–5p, apart from their higher overall level of expression, is the significant up-regulation of miR-19b, miR-20a, and miR-92a from E11.5 to E15.5. The higher level of expression of these miRNAs was observed at E15.5. The lower levels of expression are found at E11.5, P0, and P25.
Mir-19a exhibits a pattern of expression analogous to that of miR-19b, miR-20a, and miR-92a. The level of expression of miR-19a increases from E11.5 to E15.5, and then decreases to a minimum level at P25.
Based on their relative level of expression, the seven miRNAs of the miR-17–92 cluster can be organized into four groups; the levels of expression of miR-19b and miR-92 were higher than those of miR-17–5p and miR-20a, while miR-18a and miR-19a exhibit the lower level of expression. The level of expression of miR-17–3p was barely detectable (results not shown). The results for miR-18a, miR-19a, miR-19b, and miR-20a are in agreement with previous studies of the miR-17–92 cluster of embryonic and adult lung (Lu et al., 2007).
Expression of miRNAs coded by the miR-17–92 cluster during development of murine submandibular salivary gland
Figure 3 presents results showing the pattern of expression of miRNAs coded by the miR-17–92 cluster in salivary gland at E13.5, E15.5, E17.5, P0, P5, and P25. Here the six miRNAs may be clustered into two groups; miR-18a, miR-19a, and miR-92a exhibited high, and stable, levels of expression during the earlier stages of development, progressively declining toward a minimum level at P25, resembling hepatic expression found with miR-17–5p, miR-18a, and miR-92a.
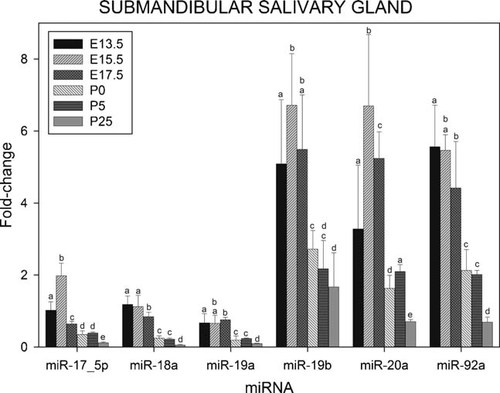
Relative levels of expression of the microRNAs of the miR-17–92 cluster in murine submandibular salivary gland at various stages during development. Fractions of RNA enriched with respect to microRNA were isolated from murine submandibular salivary gland at the various stages of development shown in Figure. Experimental details are otherwise as given in legend to Figure 1.
The levels of expression of mir-17–5p, miR-19b, and miR-20a, on the other hand, are significantly increased from E13.5 to E15.5. From E15.5 onwards the changes in expression are similar to those of miR-18a, miR-19a, and miR-92a.
The higher levels of expression were found with miR-19b, miR-20a, and miR-92, followed by those of miR-17–5p, miR-18a, and miR-19a, while that of miR-17–3p was again barely detectable.
Changes in levels of expression of miRNAs encoded by the miR-17–92 miRNA cluster in developing murine first molar mandibular tooth germ
Figure 4 shows the relative levels of expression of miRNAs coded by miR-17–92 cluster in tooth germ at E15.5, P0, and P5. Data from tooth germ at P25 is not presented as isolation of RNA from heavily mineralized teeth gave poor yields. The results for miR-20a and miR-92a demonstrate that the levels of these miRNAs decline progressively from E15.5 as the tooth germ matures, approaching their lower levels of expression at a late developmental stage (P5). For miR-17–5p, miR-18a, miR-19a, and miR-19b no significant decrease were observed between E15.5 and P0. At P5, however, significantly decreased levels of expression are apparent.
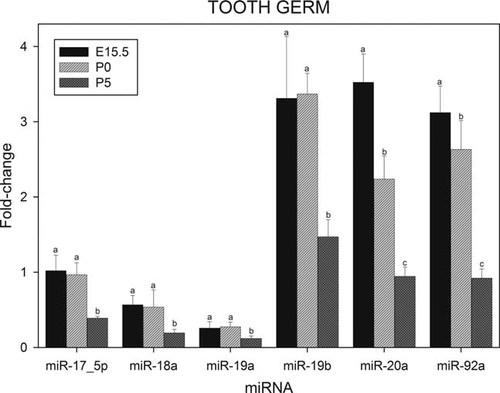
Relative levels of expression of the microRNAs of the miR-17–92 cluster in murine tooth germ at various stages during development. Fractions of RNA enriched with respect to microRNA were isolated from murine tooth germ at the various stages of development shown in Figure. Experimental details are otherwise as given in legend to Figure 1.
Also in tooth germ, miR-19b, miR-20a, and miR-92a exhibited significantly higher levels of expression compared to miR-17–5p, miR-18a, and miR-19a (Fig. 4), the level of expression of miR-17–3p being barely detectable (results not shown).
Changes in expression of miRNAs of the miR-17–92 miRNA cluster in skin, cerebral hemisphere, small intestine, kidney, and heart at E13.5, E15.5, and P25
The levels of expression of constituent miRNAs of the miR-17–92 miRNA cluster were also assayed in a range of tissues to investigate whether diminished expression with progressive development represents a common trait.
Figure 5 show results obtained with skin, cerebral hemisphere, small intestine, kidney, and heart. In all these tissues level of expression at E13.5 is about 10-fold than that at P25 for miR-17–5p. In kidney and cerebral hemisphere the level of miR-18a is significantly decreased from E13.5 to E15.5, decreasing is further at P25. However, in skin and heart a significant decrease was only observed between E15.5 and P25. In small intestine the level of expression of miR-18a is significantly increased from E13.5 to E15.5, although decreasing significantly between E15.5 to P25. For miR-19a distinct differences between tissues were also apparent as in skin, cerebral hemisphere, and small intestine no significant change was detected between E13.5 and E15.5, while a significant decrease was observed in kidney and a significant increase was found in heart. At P25 the level of expression of miR-19a was diminished in all tissues.
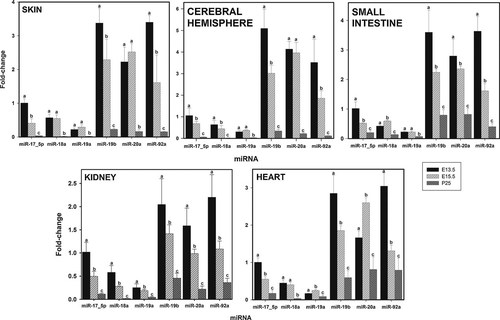
Relative levels of expression of the microRNAs of the miR-17–92 cluster in murine skin, cerebral hemisphere, small intestine, kidney, and heart at E13.5, E15.5, and P25. Fractions of RNA enriched with respect to microRNA were isolated from murine skin, cerebral hemisphere, small intestine, kidney, and heart at the various stages of development shown in Figure. Experimental details are otherwise as given in legend to Figure 1.
For miR-19b the level of expression was significantly decreased from E13.5 to E15.5 in all tissues. Further, at P25 the levels of expression of miR-19b was 30% or less of that measured at E15.5.
The time-course of expression of miR-92a was in all tissues similar to that of miR-19b, but the level of expression at E15.5 was about 50% of that observed at E13.5.
In small intestine and kidney miR-20a also exhibited a time-course of expression similar to that of miR-19b. In skin and brain no significant difference in expression was observed between E13.5 and E15.5, although between E15.5 and P25 its level of expression decreased some 20–30 fold (Fig. 5). In heart the level of expression of miR-20a is significantly increased from E13.5 to E15.5, subsequently decreasing significantly between E15.5 and P25 (Fig. 5).
In all tissues the lower level of expression was found at the later developmental stage (P25), and expression of mir-17–3p was barely detectable and did not exhibit significant changes (data not shown).
Expression of miRNAs of the miR-17–92 cluster in murine embryos at E8.5 and E11.5.
Levels of expression of miRNAs of the miR-17–92 cluster were also measured in mouse embryos at E8.5 and E11.5 (Fig. 6). The results illustrate that five miRNAs of the miR-17–92 cluster (miR-17–5p, miR-18a, miR-19a, miR-19b, and miR-20a) show increased levels of expression at E11.5 compared to E8.5, while that of miR-92a was significantly decreased at E11.5. MiR-19b and mir-92a are highly expressed at an early embryonic stage (E8.5), while miR-20a show a more than 30-fold increase in expression between E8.5 and E11.5. At E11.5, miR-19b, miR-20a, and miR-92a exhibit high, almost identical levels of expression, while miR-17–5p, miR-18a, and miR-19a are expressed at significantly lower levels. Expression of mir-17–3p was barely detectable (data not shown).
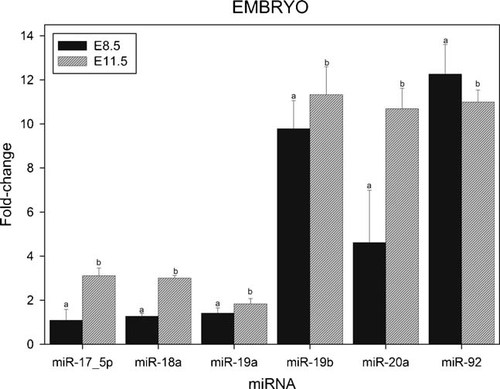
Relative levels of expression of the microRNAs of the miR-17–92 cluster in murine embryos at E8.5 and E11.5. Fractions of RNA enriched with respect to microRNA were isolated from murine embryos at the various stages of development shown in Figure. Experimental details are otherwise as given in legend to Figure 1.
Expression of mmu-pri-miR-17–92 in murine liver, lung, and salivary gland
The level of expression of pri-miR-17 and pri-miR-92a-1 were measured in mouse liver, lung, and salivary gland. Figure 7 shows the level of expression of mmu-pri-miR-92a-1 in liver at E11.5, E13.5, E15.5, E17.5, P0, P5, and P25. The time-course of expression correlates with the expression of miR-17–5p, miR-18a, miR-19a, miR-19b, miR-20a, and miR-92a in liver (Fig. 1). In general, the levels of expression of pri-miR-92a-1 decreased during development. The results show a significant decrease from E11.5 to E15.5. There is also a significant, although smaller, decrease in level of expression from E15.5 to P0. The lower level of expression is observed at P25. Similar results were found with pri-miR-17 (not shown).
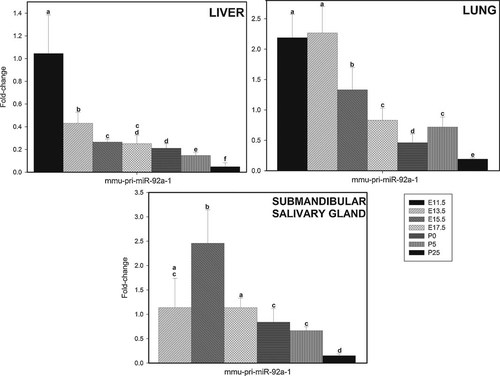
Relative levels of expression of mmu-pri-miR-17–92 transcript in murine liver, lung, and submandibular gland at various stages during development. Total RNA were isolated from murine liver, lung, and submandibular gland at the various stages of development shown in Figure. The mmu-pri-miR-92a-1 was assayed by real-time RT-PCR as described in Experimental Section. Fold changes in expression and statistical parameters were calculated using the GenEx program. The plotted data represents mean values ± SD derived from at least three separate samples of tissue (derived from three separate embryos/pups/young adults). The mean values with the same letter are not significantly different at P < 0.05. All other values are significantly different. Very similar results were obtained with mmu-pri-miR-17 (results not shown).
Figure 7 also show that relative levels of expression of mmu-pri-miR-92a-1 in murine lung at E11.5, 13.5, E15.5, E17.5, P0, P5, and P25 followed a time-course of expression similar to that found in liver. However, levels of expression at E11.5 and E13.5 are relatively high, no significant decrease being detected between these stages. From E13.5 to P0 the level of expression decreases, although subsequently increasing at P5. The lower level of expression is, nevertheless, observed at P25. The increased level of expression at P5 correlates with the observed increased level of miR-17–5p, miR-19a, miR-19b, miR-20a, and miR-92a in lung at P5 (Fig. 2). The significant increase in level of expression of miR-19a, miR-19b, miR-20a, and miR-92a between E11.5 and E15.5 are not, however, reflected in the level of expression of pri-miR-17–92a-1 (Fig. 7).
At E17.5, P0, P5, and P25 the level of expression of pri-miR-92a-1 in murine submandibular salivary gland are similar to that found in liver, decreasing between E15.5 and P25 (Fig. 7). However, a significant increase in level of expression is observed between E13.5 and E15.5, the level of expression at E15.5 being higher compared to all other time-points investigated. This higher level of expression correlates with the higher levels of expression observed with miR-17–5p, miR-19b, and miR-20a in the gland atE15.5 (Fig. 3).
Expression of mature constituents of the miR-17–92 cluster and hsa-pri-miR-17–92 in cultured human squamous carcinoma cells.
Expression of the seven constituent microRNAs of the miR-17–92 cluster was measured at early and late passages during cell cultivation. Results presented in Figure 8a show that with cultured carcinoma cells (E10) no decrease in levels of expression of these microRNAs was observed at the later passages. Rather, the levels of miR-19b, miR-20a, and miR-92a were significantly increased between 6, 15, and 25 passages. However, no increased proliferation of cells was observed at the later passages (results not shown). Levels of expression of miR-17–5p, miR-18a, and miR-19a are significantly increased between 6 and 15 passages, but no significant difference in their levels of expression were observed between 15 and 25 passages. Similar results were also obtained using two additional carcinoma cultures (clones C12 and D2, results not shown).
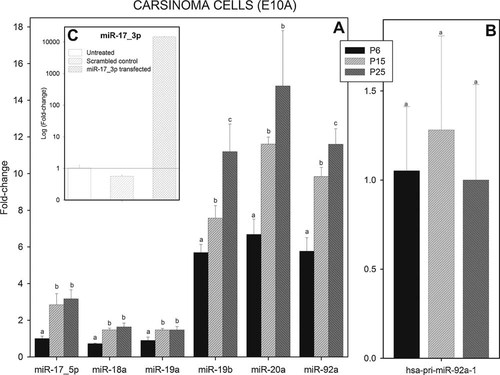
Relative levels of expression members of the miR-17–92 cluster and hsa-pri-miR-92a-1 in cultured human squamous carcinoma cells at various numbers of passages. Total RNA and fractions of RNA enriched with respect to microRNA were isolated from cultured human squamous carcinoma cells and isolated at the various cell passages shown in A and B. Fractions of RNA enriched with respect to microRNA were also isolated from cells transfected with scrambled control and mature miR-17–3p (C). Matured miRNAs (A and C) and hsa-pri-miR-92a-1 (B) were assayed by real-time RT-PCR as described in Experimental Section. Fold changes in expression and statistical parameters were calculated using the GenEx program. The plotted data represents mean values ± SD derived from at least three separate samples of tissue (derived from three separate embryos/pups/young adults). The mean values with the same letter are not significantly different at P < 0.05. All other values are significantly different. Very similar results were obtained with mmu-pri-miR-17 (results not shown).
Also, with cultured carcinoma cells the level of expression of miR-17–3p was barely detectable (results not shown). A positive control experiment was, therefore, carried to confirm that the assay was working also for this miR. The level of expression of miR-17–3p was assay in untreated cells, in cells transfected with scrambled control and in cells transfected with mature miR-17–3. The results showed a massive increase in expression in miR-17–3p-transfected cells, suggesting the assay to be working well.
Results presented in Figure 8b show the level of expression of hsa-pri-miR-92a-1 obtained in cultured carcinoma cell (E10) at P6, P15, and P25. In contrast to the results in Figure 8a, no significant increase between 6, 15, and 25 passages were observed studying hsa-pri-miR-92a-1 expression. Levels of expression of the primary transcript and those of resulting corresponding miRNAs do, therefore, not correlate in carcinoma cells.
Expression of mature constituent of the miR-17–92 cluster and hsa-pri-miR-92a-1 in cultured primary human oral keratinocytes
In cultured primary KCs a consistent decrease in levels of expression of all microRNAs were observed at the higher cell passage number for KC17A and KC22A (Fig. 9a).
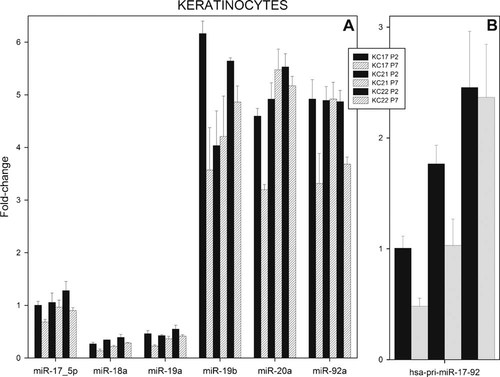
Relative levels of expression of members of the miR-17–92 cluster and hsa-pri-miR-92a-1 in cultured, primary, human KCs at various numbers of passages. Total RNA and fractions of RNA enriched with respect to microRNA were isolated from cultured, primary, human KCs at the various cell passages shown in the Figure. Results obtained with KCs derived from three separate biopsies (KC17A, KC21A, and KC22A) are presented. Mature miRNAs (A) and hsa-pri-miR-92a-1 (B) were assayed by real-time RT-PCR as described in Experimental Section. Very similar results were obtained with hsa-pri-miR-17 (results not shown).
KC21A show a different pattern (Fig. 9a). The levels of expression of miR-19a, miR-19b, miR-20a, and miR-92a were unchanged between passage two and seven, and levels of miR-17–5p and miR-18a were consistently decreased between these two cell passages. MiR-19b, miR-20a, and miR-92a all exhibited significantly higher levels of expression compared to miR-17–5p, miR-18a, and miR-19a, while that of miR-17–3p was barely detectable (results not shown). In conclusion, the levels of expression of individual miRNAs in cultured cells were more like those found in intact tissues.
In some cultures primary KCs (KC17 and KC21) a decrease in levels of expression of pri-miR-92a-1 were observed at the higher cell passage number, while no change was observed with culture KC22 (Fig. 9b). Hence, the level of expression of the primary transcript appeared to correlate with those of the mature miRNAs in KC17, and with levels of miR-17–5p and miR-18a in KC21 (Fig. 9a). No correlation between hsa-miR-17–92a-1 and the corresponding miRNAs were found with KC22.
Discussion
General trends as regards changes in levels of expression of members of the miR-17–92 cluster.
The relative levels of expression of the members of the cluster were surprisingly consistent across all tissues examined, irrespectively of the extent of decrease in expression observed with increasing time of development (Figs. 1-5). In all tissues, apart from lung, the lower level of expression of all members of the cluster was observed at P25. Results presented in Figures 1, 3, and 4 (miR-17–5p, miR-18a, miR-19b, miR-20a, and miR-92) correlates with our previous study using microarrays (Jevnaker and Osmundsen, 2008), while results shown in Figure 2 are in agreement with findings showing these miRNAs to be highly expressed in developing lung (Carraro et al., 2009; Lu et al., 2007).
In all tissues, apart from lung, miR-19b, miR-20a, and miR-92a invariably exhibited the higher relative levels of expression. The level of expression of mir-17–5p, miR-18a, and miR-19a were, in general, relatively low. In lung, however, the levels of expression of miR-19b and miR-92 were higher than those of miR-17–5p and miR-20a, while miR-18a and miR-19a exhibited the lower level of expression. This indicates that their pattern of expression is, to some extent, tissue dependent.
With cultured cells the relative levels of expression of individual miRNAs were similar to those found in tissues. In cultured cells miR-19b, miR-20a, and miR-92a were consistently found to have a several-fold higher level of expression compared to those of the remaining three microRNAs of the cluster (Figs. 8a and 9a).
The level of expression of miR-17–3p was barely detectable in all tissues and cells examined. This suggest that the miR-17–3p strand is degraded in the RISC, miR-17–5p, remaining as the functional strand of this miRNA duplex (Olena and Patton, 2010).
Differences in levels of expression of the various members of the miR-17–92 cluster during development
Individual members of the mir-17–92 cluster exhibited, however, tissue and cell-dependent differences. In liver, skin, cerebral hemisphere, small intestine, kidney, and heart the level of expression of most members of the cluster are either decreased or unchanged at the earlier developmental stages (E11.5, E13.5, and E15.5) (Figs. 1 and 5). In lung, however, the level of expression of miR-19a, miR-19b, miR-20a, and miR-92a increases from E11.5 to E15.5 (Fig. 2), as was observed for miR-17, miR-19b, and miR-20a in the submandibular gland between E13.5 and E15.5 (Fig. 3). In liver and submandibular gland the levels of expression of most members of the miR-17–92 cluster decrease from E15.5 to P25 (Figs. 1 and 3). In lung, however, the lower level of expression is observed at P0 and P25 (Fig. 2).
Also, levels of expression of all members of the cluster, apart from for mir-92a, increase significantly in mouse embryos from E8.5 to E11.5, the increase being most marked for miR-20a (Fig. 6). MiR-17 and miR-20 have been identified in embryonic stem cells and embryos (Foshay and Gallicano, 2009).
The results obtained with the carcinoma cell lines show that the relative levels of the microRNAs increase, or remain unchanged, with an increasing number of cell passages; an increase being most marked for miR-19b, miR-20a, and miR-92a (Fig. 8a). The significant increase in expression of miR-17–5p, miR-18a, and miR-19a observed in carcinoma cells is in line with results suggesting that decreased expression of miR-19a leads to diminished proliferation (Cheng et al., 2005). Further, miR-17–5p has been implicated in regulation of the cell cycle at the G1/S-phase boundary (Cloonan et al., 2008), and it has been proposed that miR-17–5p, miR-20a, and miR-19b enhance proliferation of progenitor cells and inhibit/delay their differentiation (Lu et al., 2007; Lu et al., 2008). This is in agreement with our finding of high levels of expression in cultured carcinoma cells (Fig. 8a). This may also explain why levels of expression of most members of the cluster are decreasing during development of murine tissue (Figs. 1-5).
Results from carcinoma cells contrast the changes observed with cultured, primary, KCs, where miR-18a exhibited consistently decreased expression at the later cell passage (Fig. 9a). A consistent decrease in levels of expression of all microRNAs was observed at the higher cell passage number for cultures KC17A and KC22, while the level of expression of miR-17–5p, miR-19a, miR-19b, miR-20a, and miR-92a are unchanged at the higher cell passage number for KC21A. This likely reflects biological variation as the various KC cultures are derived from separate donors.
Differences in levels of expression of miRNAs belonging to the same miRNA-family
Our results show that members of a single miRNA family are expressed at markedly different levels. In the literature miR-19a and miR-19b often are referred to as miR-19, and are considered to have identical mRNA targets. The observed markedly different level of expression suggests differential regulation of their expression, also hinting of functional differences.
MiR-17–5p and miR-20a are also highly homologues. They target a large number of common mRNAs encoding proteins involved in regulation of cell cycle (Bonauer and Dimmeler, 2009). Our results show that miR-17–5p and mir-20a are consistently expressed at different levels, the level of expression of miR-20a in general being higher than that of miR-17–5p. This indicates that miR-17–5p and miR-20a have different functions, even though they have an identical “seed sequence.” This conclusion is in line with proposal that “seed sequence” recognition alone is insufficient for prediction miRNA binding (Foshay and Gallicano, 2009).
Post-transcriptional regulation of levels of the various members of the miR-17–92 cluster
The observed differences in levels of expression of members of the miR-17–92 cluster indicate that their expression is regulated at a post-transcriptional level, in agreement with Thomson et al. (2006). This implies that the various miRNAs derived from a single transcript are differentially processed. Post-transcriptional processing mediated by Drosha, Exportin 5, or Dicer may be involved in this respect (Miyoshi et al., 2010). Guil and Caceres (2007) reported that hnRNP A1 is required for processing of miR-18a by binding to the primary RNA sequence surrounding the miRNA, suggesting that specific cofactors may regulate processing of individual miRNAs.
Levels of expression of the primary transcript of the miR-17–92 cluster
MiR-17–5p, miR-18a, and miR-19a are located toward the 5′-flank of the gene (Mendell, 2008), and consistently exhibited the lower levels of expression among cluster members (Figs. 1, 3–6). Our finding of no difference in levels of expression between the 5′- and 3′-end of the primary transcript in liver, lung, and submandibular gland (results not shown) suggest that this differences is not caused by selective transcription of the 5′ or 3′ end of the primary transcript. The markedly lower levels of expression of the three miRs located at the 5′-flank must, therefore, be due to subsequent, selective, post-transcriptional processing of the primary transcript.
The level of the primary transcript of the mir-17–92 cluster was consistently markedly higher at the earlier developmental stages (Fig. 7), in line with higher levels of individual miRNAs at these stages (Figs. 1-3). Hence, the rate of transcription is one likely mechanism regulating levels of mature miRNAs.
In cultured carcinoma cells the level of expression of the primary transcript did not alter significantly with increasing number of cell passages (Fig. 8b), and exhibited no apparent correlation with observed increase in levels of expression of miRNAs derived from the cluster (Fig. 8a). The increased level of expression of the mature miRNAs observed in later passages may, consequently, be due to their diminished rates of degradation. This may suggest that mechanisms which ensure correlation between rate of transcription of the cluster (i.e., the level of the primary transcript) and levels of mature miRNAs is not functioning in cultured carcinoma cells.
Cellular functions of the miR-17–92 cluster
Here we show that, irrespectively of the tissue investigated, the level of expression of the miR-17–92 cluster is consistently higher at the earlier stages of development. This was observed with distinctly different tissues. Hence, it is likely that the functions regulated by these microRNAs are shared by phenotypically very different tissues. The functions which are regulated by these miRNAs can therefore be assumed to be common to all tissues investigated, and are therefore likely fundamental to tissue development. This conclusion is in agreement with the finding that a knock-out in the miR-17–92 cluster is incompatible with normal embryonic development (Ventura et al., 2008).
Cells at an early stage of development are known to share several characteristics with carcinoma cells. The higher tissue levels of miR-17–92 miRNAs at early developmental stages and in carcinoma cells may, therefore, be regarded as one such shared characteristic. The changes in levels of expression of the various miRs observed with differentiating primary KCs were more similar to those observed in developing tissues rather than to those observed with cultured carcinoma cells. Cultured primary oral KCs has been shown to differentiate (Locke et al., 2008). Our results therefore suggest that declining levels of expression of the members of the miR-17–92 cluster reflect ongoing KC differentiation.
Acknowledgements
The expert technical assistance of Mrs. Bente Gehrken, Mrs. Toril Woldene, and Mr. Benedicto Geronimo is gratefully acknowledged. We thank Bryn Tannhelsesenter and Mr. Eivind Strøm DDS for donation biopsies to the project. This study was supported by a grant from The Norwegian Cancer Society.