Interleukin-1β induces ICAM-1 expression enhancing leukocyte adhesion in human rheumatoid arthritis synovial fibroblasts: Involvement of ERK, JNK, AP-1, and NF-κB
Abstract
Interleukin-1β (IL-1β) has been shown to induce the expression of adhesion molecules on various cell types and contributes to inflammatory responses. However, the molecular mechanisms by which IL-1β induced intercellular adhesion molecule (ICAM)-1 expression remain unclear in human rheumatoid arthritis synovial fibroblasts (RASFs). Here, we demonstrated that IL-1β induces ICAM-1 gene expression via the de novo protein synthesis through transcription and translation, which is attenuated by pretreatment with actinomycin D and cycloheximide, respectively. IL-1β-induced ICAM-1 expression, extracellular signal-regulated kinase (ERK) and c-Jun-N-terminal kinase (JNK) phosphorylation, AP-1 activation, and nuclear factor-κB (NF-κB) p65 translocation were attenuated by the inhibitors of MEK1/2 (U0126), JNK (SP600125), AP-1 (tanshinone IIA), and NF-κB (helenalin) or transfection with respective short hairpin RNA plasmids. Moreover, IL-1β-stimulated NF-κB p65 translocation was blocked by helenalin, but not by U0126 or SP600125, revealing that MAPKs and NF-κB pathways were independent on these responses. IL-1β-stimulated AP-1 activation was blocked by U0126 or SP600125, revealing that ERK and JNK linked to AP-1 on these responses. IL-1β-stimulated ICAM-1 gene expression was attenuated by pretreatment with U0126, SP600125, tanshinone IIA, or helenalin, revealed by ICAM-1 promoter assay and real-time RT-PCR analysis. Finally, up-regulation of ICAM-1 enhanced the adhesion of leukocytes to RASFs exposed to IL-1β. These results suggest that in human RASFs, activation of ERK, JNK, AP-1, and NF-κB are essential for IL-1β-induced ICAM-1 expression and leukocyte adhesion. J. Cell. Physiol. 224: 516–526, 2010. © 2010 Wiley-Liss, Inc.
Rheumatoid arthritis (RA) progression involves the thickening of the synovial lining due to the proliferation of synovial fibroblasts and infiltration by inflammatory cells. The infiltration of inflammatory cells may be due to up-regulation of adhesion molecules including intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 (Szekanecz et al., 1996; Dinarello, 2005; Sidiropoulos et al., 2008). Adhesion molecules expressed on the surface of cells which mediate the adhesion of the cells to other cells or to the extracellular matrix. These proteins play numerous crucial functions between the interface of cells and its environment from a similar or different cell types (Yusuf-Makagiansar et al., 2002; Blankenberg et al., 2003). Both VCAM-1 and ICAM-1 are inducible cell transmembrane glycoproteins of the immunoglobulins which not only express on several cell types but also implicate in the pathogenesis of chronic inflammatory responses such as RA. The up-regulation of VCAM-1 and ICAM-1 has been reported to promote leukocyte adhesion to endothelial cells and their migration in RA (Carter and Wicks, 2001; Cook-Mills, 2002; Gonlugur and Efeoglu, 2004). However, the detail molecular mechanisms by which cytokines induce ICAM-1 expression are not completely understood.
Elevated levels of pro-inflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) are predominate in the pathogenesis of RA (Firestein et al., 1990; Arend, 2001), both are produced by macrophages and leukocytes, which accumulate maximally in the area adjacent to the cartilage pannus junction (Youssef et al., 1998; Choy and Panayi, 2001). The enhanced production of cytokines during the acute phase of RA triggers pathogenic effects such as appearance of anti-neutrophil cytoplasmic antibodies, initiation of vascular thrombosis, and an increase in production of auto-antigens contributing to tissue damage (Matei and Matei, 2002). Moreover, IL-1β exerts as a potent stimulus in inflammatory responses through up-regulation of many genes, including adhesion molecules (Lin et al., 2004; Wang et al., 2005; Lin et al., 2007). Adhesion molecules such as ICAM-1 have been implicated in numerous inflammatory diseases as a key regulator of leukocyte trafficking to the sites of inflammation in RA. Soluble ICAM-1 levels have been correlated with clinical severity and progression of RA (McMurray, 1996; Littler et al., 1997; Furuzawa-Carballeda and Alcocer-Varela, 1999; Klimiuk et al., 2002). Therefore, up-regulation of ICAM-1 on cytokine-triggered RASFs enhances the targeted transmigration of lymphocytes into extravascular space of inflammation (Smith et al., 1989; Gonlugur and Efeoglu, 2004).
Although the roles of cytokines and adhesion molecules in PMN adhesion to endothelial cells have been well described, little is known about the mechanism(s) underlying the interaction between lymphocytes and human RASFs. Several reports have demonstrated the induction of ICAM-1 by cytokines such as IL-1β, TNF-α, and interferon-γ (IFN-γ) in various cell types (Kaiserlian et al., 1991; Mulligan et al., 1993; Ju et al., 2002; Lin et al., 2007). Cytokines such as IL-1β have been reported to activate all of these mitogen-activated protein kinases (MAPKs; McEwan, 2002; Pfeffer, 2003), including p42/p44 MAPK (Boulton et al., 1999), p38 MAPK (Han et al., 1994), and c-Jun-N-terminal kinase (JNK; Kyriakis et al., 1994), activated by a dual specificity of MAPK kinase. Moreover, IL-1β has been shown to induce most of gene expression via MAPKs pathways. However, the relationship between the activation of these pathways and expression of adhesion molecules has been controversial. For example, IL-1β-induced VCAM-1 and ICAM-1 expression in mouse Sertoli cells does not require the activation of p38 kinase, whereas activation of JNK is essential for these responses (Szlosarek and Balkwill, 2003). In human tracheal smooth muscle cells, activation of p42/p44 MAPK, p38, JNK, and nuclear factor-κB (NF-κB) pathways are essential for IL-1β-induced VCAM-1 expression (Wang et al., 2005). Furthermore, IL-1β has been shown to induce ICAM-1 expression mediated through activation of p42/p44 MAPK, p38, JNK, and NF-κB in A549 cells (Lin et al., 2005) and through AP-1 in endothelial cells (Berendji-Grün et al., 2001). Cardiac cells require p38 MAPK activation for VCAM-1 and ICAM-1 expression (Kacimi et al., 1998). These discrepancies imply that there are divergent pathways leading to ICAM-1 expression induced by IL-1β, depending on the nature of stimuli, cell types, and target genes. Moreover, the expression of ICAM-1 and other genes appears to be highly regulated by a number of MAPKs, NF-κB, and AP-1 in various cell types (Ballestas and Benveniste, 1995; Barnes and Karin, 1997; Bian et al., 2001; Lee et al., 2006). In addition, the promoters of both ICAM-1 and VCAM-1 genes contain several binding sites for transcription factors like NF-κB and AP-1 (Ballantyne et al., 1992), regulated by IL-1β through MAPKs activation in several cell types (Barnes and Karin, 1997; Bian et al., 2001). In this regard, several genes regulated by MAPKs are mediated through activation of NF-κB and AP-1 for transcription (Bian et al., 2001). It is, therefore, important to examine whether the activation of these MAPK pathways, NF-κB, and AP-1 by IL-1β linked to ICAM-1 expression in RASFs.
In addressing these questions, the experiments were performed to investigate the roles of MAPKs, AP-1, and NF-κB in IL-1β-induced ICAM-1 expression in RASFs. We found that activation of extracellular signal-regulated kinase (ERK) and JNK was required for ICAM-1 gene expression induced by IL-1β in human RASFs. These findings suggest that the increased expression of ICAM-1 correlates with increased adhesion of leukocytes to IL-1β-challenged RASFs, at least in part, mediated through ERK, JNK, AP-1, and NF-κB.
Materials and Methods
Materials
Dulbecco's Modified Eagle Medium (DMEM)/Ham's nutrient mixture F-12 (F-12), fetal bovine serum (FBS), and TRIZOL were purchased from Invitrogen (Carlsbad, CA). Cell culture dishes were from Costar (Corning, NY). Hybond C membrane and enhanced chemiluminescence (ECL) Western blotting detection system were from GE Healthcare Biosciences (Buckinghamshire, England). Polyclonal antibodies ICAM-1 (sc-7891), IκB-α (sc-847), and NF-κB (p65; sc-7151) were from Santa Cruz (Santa Cruz, CA). Anti-GAPDH antibody (4699–9555) was from Biogenesis (Boumemouth, UK). PhosphoPlus ERK (9101L), p38 MAPK (9211L), SAPK/JNK (9255), and phospho-IκB-α (#2859) antibody kits were from Cell Signaling (Danver, MA). Anti-goat, rat, or mouse horseradish peroxidase secondary antibodies were from Vector Lab. (Burlingame, CA). U0126, SB202190, SP600125, actinomycin D, cycloheximide, helenalin, and tanshinone IIA were from Biomol (Plymouth Meeting, PA). All inhibitors were dissolved in dimethyl sulfoxide (DMSO) and the final level of DMSO was less than 1% in the culture medium. Bicinchoninic acid (BCA) protein assay kit was from Pierce (Rockford, IL). Enzymes and other chemicals were from Sigma (St. Louis, MO).
Cell culture
Human RASFs were isolated from synovium (synovial fibroblast) obtained from patients meeting the criteria for Chang Gung Memorial Hospital RA patients who have undergone knee or hip surgery for synovectomy or total joint replacement surgery, according to the guidance by the Institutional Human and Animal Care and Use Committee of Chang Gung University (Supplementary Table 1). Synovial strips were cut into small pieces and placed in 10 cm dishes. After 2 weeks, the synovial tissues were removed from the cultured medium. The cells were grown in DMEM/F-12 containing 10% FBS and antibiotics (100 U/ml penicillin G, 100 µg/ml streptomycin, and 250 ng/ml fungizone) at 37°C in a humidified 5% CO2 atmosphere. When the cultures reached confluence (about 3 weeks), cells were treated with 0.05% (w/v) trypsin/0.53 mM EDTA for 5 min at 37°C. The cell suspension was diluted with DMEM/F-12 containing 10% FBS to a concentration of 2 × 105 cells/ml. The cell suspension was plated onto 12-well culture plates (1 ml/well) and 10 cm culture dishes (10 ml/dish) for the measurement of protein expression and mRNA accumulation, respectively. Culture medium was changed after 24 h and then every 3 days. Over 95% of the cells were fibroblasts which were characterized by an immunofluorescence staining using an antibody specific for a fibroblast protein vimentin (Supplementary Fig. 1). Experiments were performed with cells from passages 3–8.
Preparation of cell extracts and Western blot analysis
RASFs were plated onto 12-well culture plates and made quiescent at confluence by incubation in serum-free DMEM/F-12 for 24 h. Growth-arrested cells were incubated with different concentrations of IL-1β at 37°C for the indicated time. When inhibitors were used, they were added 1 h prior to the application of IL-1β. The cells were washed with ice-cold phosphate-buffered saline (PBS), scraped, and collected by centrifugation at 1,000g for 10 min. Protein concentration of each sample was determined by the BCA reagents. Samples from these supernatant fractions (30 µg protein) were denatured and subjected to SDS–PAGE using a 10% (w/v) running gel. Proteins were transferred to nitrocellulose membrane and the membrane was incubated successively at room temperature with 1% (w/v) bovine serum albumin (BSA; Calbiochem, San Diego, CA) in TTBS [(50 mM Tris–HCl, 150 mM NaCl, 0.05% (w/v) Tween 20, pH 7.4)] for 1 h as previously described (Lin et al., 2007). Membranes were incubated overnight at 4°C with an anti-phospho-ERK, anti-phospho-JNK, anti-phospho-p38, anti-ICAM-1, or anti-GAPDH antibody used at a dilution of 1:2,000 in TTBS. Membranes were washed with TTBS four times for 5 min each, incubated with a 1:1,500 dilution of anti-goat or anti-mouse horseradish peroxidase antibody for 1 h. Following each incubation, the membrane was washed extensively with TTBS. The immunoreactive bands detected by ECL reagents were developed by Hyperfilm-ECL. Density analysis was performed using an UN-SCAN-IT gel 6.1 program (Silk Scientific, Inc. Orem, UT).
Plasmids and transfection
The plasmids encoding dominant negative mutants of MEK1/2 (MEK K97R), ERK2 (ERK K52R), JNK1(T183A/Y185F), IKK-α(KM), and IKK-β(KM), and short hairpin RNA (shRNA) of ERK2, p38, and JNK2 were used in this study. All plasmids were prepared by using QIAGEN plasmid DNA preparation kits. RASFs were plated at 3 × 105 cells/ml in 12-well culture plates for 24 h. Cells were transfected with 1 µg/well of dominant negative mutants using DNA PLUS-Lipofectamine and incubated at 37°C for 3 h. One milliliter of DMEM/F-12 medium containing 10% FBS was added and incubated for additional 19 h. The cells were washed twice with PBS and maintained in serum-free DMEM/F-12 for 24 h before treatment with IL-1β. The transfection efficiency (approximate 60%) was determined by transfection with EGFP.
Total RNA extraction and real-time RT-PCR analysis
Total RNA was isolated from RASFs treated with IL-1β for the indicated time in 10 cm culture dishes with Trizol according to the protocol of the manufacturer. RNA concentration was spectrophotometrically determined at 260 nm. Real-time PCR was performed with the TaqMan gene expression assay system, using primers (sense: 5′-TGTCCGGGAAACTGGACGT-3′; antisense: 5′-TGCCCTCAAGATCTCGAGTGA-3′) and probe (5′-AATTCCCAGCAGACTCCAATGTGCC-3′) mix for ICAM-1, and primers/probe sets Hs00958116_m1 for vimentin was from Applied Biosystems (Foster City, CA) as a control. PCRs were performed using a 7500 Real-Time PCR System (Applied Biosystems). Relative gene expression was determined by the ΔΔCt method, where Ct = threshold cycle. All experiments were performed in duplicate or triplicate.
NF-κB translocation analyses
RASFs were seeded in a 10 cm dish and starved for 24 h in serum-free DMEM/F-12 medium. The cells were incubated with 15 ng/ml IL-1β for the indicated time intervals, washed once with ice-cold PBS, and 200 µl of homogenization buffer A (20 mM Tris–HCl, pH 8.0, 10 mM EGTA, 2 mM EDTA, 2 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 25 µg/ml aprotinin, 10 µg/ml leupeptin) was added to each dish. The cells were scraped into a 1.5-ml tube using a rubber policeman. The suspension was sonicated for 10 sec at output 4 with a sonicator (Misonix, Farmingdale, NY) and centrifuged at 8,000 rpm for 5 min at 4°C. The supernatant was collected as the cytosol fraction and the pellet as the nuclear fraction. The pellet was resuspended in 300 µl of homogenization buffer B (1% Triton X-100 in buffer A) and sonicated for 10 sec. The suspension was centrifuged at 15,000 rpm for 15 min at 4°C. The supernatant was collected as a nuclear lysate fraction. The translocation of NF-κB was identified and quantified by Western blot analysis using an anti-IκB-α or anti-NF-κB (p65) antibody as previously described.For NF-κB translocation by immunofluorescence staining, RASFs were plated on six-well culture plates with coverslips. Cells were further cultured in serum-free DMEM/F-12 for 24 h and treated with 15 ng/ml of IL-1β for the indicated time. After washing two times with ice-cold PBS, the cells were fixed with 4% (w/v) paraformaldehyde in PBS for 30 min, and then permeabilized with 0.3% Triton X-100 in PBS for 15 min. The staining was performed by incubating with 10% normal goat serum in PBS for 30 min followed by incubating with primary anti-NF-κB (p65) polyclonal antibody (1:100 dilution) for 1 h in PBS with 1% BSA, washing three times with PBS, incubating for 1 h with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit antibody (1:100 dilution) in PBS with 1% BSA, washing three times with PBS, and finally mounting with aqueous mounting medium. The images were observed under a fluorescent microscope (Zeiss, Axiovert 200M, Hall-bergmoos, Germany).
Measurement of AP-1 and ICAM-1 promoter-luciferase activity
For construction of the ICAM-1-Luc plasmid, human ICAM-1 promoter, a region spanning −1,716 to −119 bp (kindly provided by Dr. W.C. Aird, Department of Molecular Medicine, Beth Israel Deaconess Medical Center, Boston, MA) was cloned into pGL3-basic vector (Promega, Madison, WI). ICAM-1-Luc and AP1-Luc (Clontech Laboratories, Inc., Mountain View, CA) activity were determined as previously described (Lin et al., 2007) using a luciferase assay system (Promega) according to the manufacturer's instructions. Firefly luciferase activities were standardized for β-galactosidase activity.
Leukocyte isolation and adhesion assay
Peripheral blood leukocytes were isolated from whole venous blood of healthy individuals by dextran sedimentation followed by density separation over Ficoll–Hypaque and hypotonic lysis. The leukocytes were then resuspended in Tyrode-HEPES buffer (128 mM NaCl, 2.7 mM KCl, 0.5 mM MgCl2, 0.36 mM NaH2PO4, 2 mM CaCl2, 12 mM NaHCO3, 10 mM HEPES; pH 7.4) and adjusted to 1 × 107 cells/ml. Leukocytes were used within 4 h of purification (Wang et al., 2005). Leukocytes–RASFs adhesion was measured by a parallel plate chamber according to the methods as previously described (Wang et al., 2005). RASFs grew on glass coverslips were incubated with 15 ng/ml of IL-1β for 16 h. After the chamber was assembled, it was placed on the stage of an inverted microscope (Nikon, Tokyo, Japan) equipped with a CCD video camera (Mintron Enterprise Co., Taipei, Taiwan). The inlet of the chamber was then connected to a perfusion system (KdScientific Inc. New Hope, PA), and leukocytes were gently infused into the chamber and kept there for 30 min at 37°C. The flow chamber was flushed with Tyrode-HEPES buffer for 5 min at a flow rate of 0.4 ml/min, corresponding to surface shear stress of 2 dyne/cm2. The non-adherent leukocytes were washed away from the slide. Following flow perfusion, the number of leukocytes adhering to RASFs was analyzed with an inverted light microscope (Nikon Co. Tokyo, Japan) connected to an image analysis system (Moti Images plus 2.0; Micro-optic Industrial Group Co., Richmond, Canada).
Analysis of data
Data were expressed as the mean ± SEM using the GraphPad Prism Program (GraphPad, San Diego, CA). Quantitative data were analyzed with a one-way ANOVA to make comparisons with Bonferroni's test at a P < 0.05 level of significance.
Results
IL-1β induces ICAM-1 expression in RASFs
To determine the effect of IL-1β on the expression of ICAM-1 protein and mRNA, RASFs were treated with various concentrations of IL-1β for the indicated time. As shown in Figure 1A (upper part), IL-1β induced ICAM-1 protein expression in a time- and concentration-dependent manner, determined by Western blot. There was a significant increase in this response being observed within 4 h. A maximal response was achieved within 8–16 h and sustained over 24 h. The amount of ICAM-1 protein expression was increased with increasing concentrations of IL-1β (Fig. 1A, lower part). The blots were stripped and re-probed with an anti-GAPDH antibody to demonstrate equivalent amount of GAPDH expression. Furthermore, to examine whether the effect of IL-1β on ICAM-1 expression involved at the level of transcription, ICAM-1 mRNA level was determined by a real-time RT-PCR. As shown in Figure 1B, IL-1β induced ICAM-1 mRNA accumulation in a time-dependent manner. A maximal response was obtained within 4 h and sustained over 6 h during the period of observation.
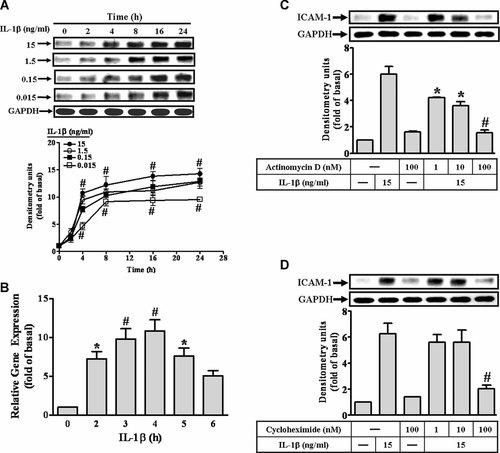
IL-1β induces ICAM-1 expression at transcription and translation levels in RASFs. A: For ICAM-1 protein expression, RASFs were incubated with IL-1β for the indicated times. The cell lysates were subjected to SDS–PAGE and then blotted using an antiserum reactive with ICAM-1 or GAPDH as a control (upper part). Data are summarized from the time course study and expressed as mean ± SEM of three independent experiments (lower part). B: IL-1β-induced ICAM-1 mRNA expression, the cells were incubated with IL-1β (15 ng/ml) for the indicated time. The total RNA samples were prepared and analyzed ICAM-1 and vimentin mRNA by a real-time RT-PCR. Data were analyzed by the comparative cycle threshold method and expressed as the fold relative to those of non-stimulated cells. C,D: Cells were pretreated with actinomycin D (C) or cycloheximide (D) for 1 h and then incubated with IL-1β for 6 h. The ICAM-1 protein level was determined by Western blotting. Data are expressed as mean ± SEM of three independent experiments. *P < 0.05; #P < 0.01, as compared with the cells incubated with vehicle (A,B) or IL-1β (C,D) alone.
To determine whether IL-1β-induced ICAM-1 expression is dependent on de novo protein synthesis, the cells were pretreated with actinomycin D or cycloheximide for 1 h and then exposed to IL-1β for 6 h. As shown in Figure 1C,D, pretreatment with either actinomycin D or cycloheximide significantly attenuated IL-1β-induced ICAM-1 expression in a concentration-dependent manner, suggesting that IL-1β-induced ICAM-1 expression is mediated via the de novo protein synthesis through transcription and translation.
IL-1β induces ICAM-1 expression via ERK1/2 phosphorylation
Pro-inflammatory cytokines have been shown to induce the outgrowth of synovial cells by up-regulation of synoviolin via the ERK1/2-ETS1 pathway (Gao et al., 2006). Next, we investigated whether IL-1β-induced ICAM-1 expression was also mediated via ERK in RASFs. Data in Figure 2A showed that pretreatment with a MEK1/2 inhibitor U0126 (Favata et al., 1998) for 1 h prior to IL-1β exposure for 6 h caused an attenuation of ICAM-1 expression in a concentration-dependent manner.
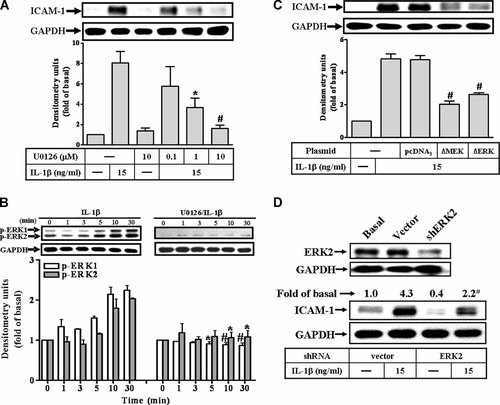
IL-1β-induced ICAM-1 protein expression via ERK1/2 phosphorylation in RASFs. A: Cells were preincubated with U0126 for 1 h and then incubated with IL-1β for 6 h. B: IL-1β-stimulated ERK1/2 phosphorylation, cells were incubated without (control) or with U0126 (1 µM) for 1 h and then stimulated with 15 ng/ml IL-1β for the indicated times. C,D: To ensure the involvement of MEK1/2–ERK1/2 in IL-1β-induced ICAM-1 expression in RASFs, the cells were transfected with a dominant negative mutant of MEK1/2 (BMEK) or ERK2 (BERK2) and shRNA of ERK2 for 16 h, shifted to DMEM/F-12 containing 10% FBS for 24 h, replaced with serum-free DMEM/F-12 medium for 24 h and then incubated with 15 ng/ml IL-1β for 6 h. The cell lysates were subjected to SDS–PAGE and determined the level of ERK2 (D), ICAM-1 protein (A,C,D), and phosphorylation of ERK1/2 (B) as described in Fig. 1. Data are expressed as mean ± SEM of three independent experiments. *P < 0.05; #P < 0.01, as compared with the cells incubated with IL-1β alone.
To determine whether ERK1/2 phosphorylation was necessary for IL-1β-induced ICAM-1 expression, activation of these kinases was assayed using an antibody specific for the phosphorylated, active forms of ERK1/2 determined by Western blotting. As shown in Figure 2B (left part), IL-1β stimulated a transient phosphorylation of ERK1/2 in a time-dependent manner in RASFs. A maximal response was obtained within 10 min. To further examine the involvement of MEK1/2 in ERK phosphorylation, RASFs were pretreated with U0126 and then stimulated with IL-1β. Pretreatment with U0126 (10 µM) almost completely inhibited IL-1β-stimulated ERK1/2 phosphorylation during the period of observation (Fig. 2B, right part). To ascertain that the activation of MEK1/2–ERK1/2 was required for IL-1β-induced ICAM-1 expression, RASFs were transfected with a dominant negative mutant of MEK1/2 or ERK2 and then incubated with IL-1β for 6 h. As shown in Figure 2C, transfection with dominant negative mutants of MEK1/2 and ERK2 significantly reduced ICAM-1 expression induced by IL-1β. Moreover, to determine whether ERK2 was involved in this response, transfection of cells with ERK2 shRNA down-regulated the expression of ERK2 protein (Fig. 2D, upper part) and significantly attenuated IL-1β-induced ICAM-1 expression (Fig. 2D, lower part). These results suggested a link between activation of MEK1/2–ERK1/2 cascade and induction of ICAM-1 expression by IL-1β in RASFs.
IL-1β induces ICAM-1 expression via JNK1/2 phosphorylation
To determine whether JNK1/2 also involved in IL-1β-induced ICAM-1 expression in RASFs, a selective JNK1/2 inhibitor SP600125 (Bennett et al., 2001) was used. As shown in Figure 3A, pretreatment of RASFs with SP600125 blocked ICAM-1 expression in a concentration-dependent manner. In addition, to ensure IL-1β-stimulated JNK1/2 phosphorylation, activation of this kinase was assayed using an antibody specific for the phosphorylated, active form of JNK1/2 by Western blot. IL-1β stimulated a time-dependent phosphorylation of JNK1/2 with a maximal response within 15 min and sustained over 30 min in RASFs (Fig. 3B, left part). Pretreatment with SP600125 attenuated JNK1/2 phosphorylation stimulated by IL-1β during the period of observation (Fig. 3B, right part). To further ascertain that the activation of JNK was required for the IL-1β-induced expression of ICAM-1, RASFs were transfected with a dominant negative JNK mutant (▵JNK) or a selective JNK2 shRNA and then incubated with IL-1β (15 ng/ml) for 6 h. As shown in Figure 3C,D, transfection with ▵JNK or JNK2 shRNA significantly reduced ICAM-1 expression induced by IL-1β. These results suggested that activation of JNK1/2 was also involved in ICAM-1 expression induced by IL-1β.
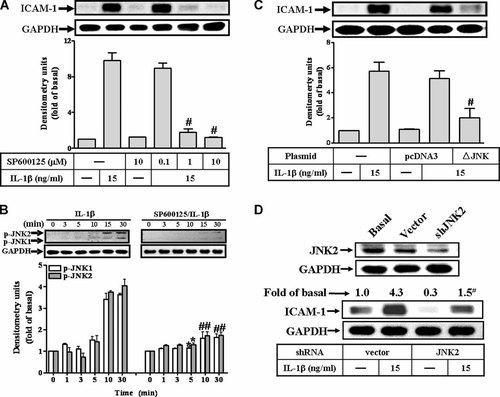
Involvement of JNK1/2 in IL-1β-stimulated ICAM-1 protein expression in RASFs. A: Cells were preincubated with SP600125 for 1 h and then incubated with IL-1β for 6 h. B: IL-1β-stimulated JNK1/2 phosphorylation, cells were pretreated without (control) or with SP600125 (10 µM) for 1 h and then incubated with 15 ng/ml IL-1β for the indicated times. C,D: To ensure the involvement of JNK1/2 in IL-1β-induced ICAM-1 expression in RASFs, the cells were transfected with a dominant negative mutant of JNK (BJNK) or JNK2 shRNA for 16 h, shifted to DMEM/F-12 containing 10% FBS for 24 h, replaced with serum-free DMEM/F-12 for 24 h and then incubated with 15 ng/ml IL-1β for 6 h. The cell lysates were subjected to SDS–PAGE and determined the level of JNK2 (D), ICAM-1 protein (A,C,D), and phosphorylation of JNK1/2 (B) as described in Fig. 1. Data are expressed as mean ± SEM of three independent experiments. *P < 0.05; #P < 0.01, as compared with the cells incubated with IL-1β alone.
NF-κB inhibitors suppress IL-1β-induced ICAM-1 expression
Inflammatory responses following stimulation with cytokines such as IL-1β are highly dependent on various transcription factors, such as NF-κB. Moreover, NF-κB is one of the major mediators of the intracellular functions of IL-1β. In addition, IL-1β has been shown to involve in ICAM-1 gene expression through NF-κB cascade in various cell types (Chen et al., 2000; Lin et al., 2005; Wang et al., 2005). Therefore, the involvement of NF-κB in ICAM-1 expression induced by IL-1β was further confirmed using a pharmacological inhibitor helenalin, a specific sesquiterpene lactone compound which is known to inhibit NF-κB (Lyss et al., 1998). As shown in Figure 4A, pretreatment of RASFs with helenalin for 1 h prior to exposure to IL-1β attenuated ICAM-1 expression in a concentration-dependent manner.
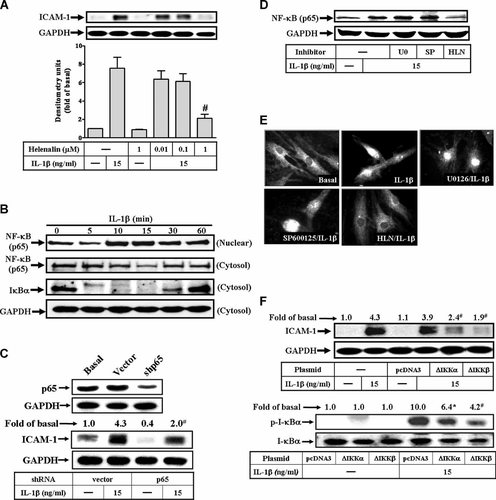
NF-κB is required for IL-1β-stimulated ICAM-1 expression in RASFs. A: Cells were pretreated with helenalin for 1 h and then incubated with IL-1β for 6 h. B: IL-1β-induced IκB-α degradation and NF-κB (p65) translocation, cells were treated with 15 ng/ml IL-1β for the indicated time. C: The involvement of NF-κB (p65) in IL-1β-induced ICAM-1 expression was confirmed by transfection with NF-κB p65 shRNA and then incubated with IL-1β (15 ng/ml) for 6 h. D: Effect of inhibitors of MEK1/2, JNK1/2, and NF-κB in IL-1β-induced NF-κB translocation, cells were pretreated with U0126 (U0, 1 µM), SP600125 (SP, 10 µM) or helenalin (HLN, 10 µM) for 1 h and then incubated with IL-1β (15 ng/ml) for 30 min. Cells were harvested and centrifuged to prepare cytosolic and nuclear fractions (B,D). The resultant fractions were analyzed using an anti-NF-κB (p65), anti-IκB-α, or GAPDH (as a control) antibody. E: Nuclear translocation of NF-κB determined by immunofluorescence staining, RASFs were pretreated with U0126 (1 µM), SP600125 (10 µM), or helenalin (HLN, 10 µM) for 1 h and then stimulated with 15 ng/ml IL-1β for 30 min. Cells were fixed and labeled with anti-NF-κB (p65) antibody and a fluorescein isothiocyanate (FITC)-conjugated secondary antibody. Individual cells were imaged as described in Materials and Methods Section. Figure represents one of three similar experiments. F: The involvement of IKK in IL-1β-induced ICAM-1 expression was ensured by transfection with dominant negative mutant of IKKα (ΔIKKα) or IKKβ (ΔIKKβ) and then incubated with 15 ng/ml IL-1β for 6 h. The levels of NF-κB p65 (C) and ICAM-1 protein expression (A,C,F) were determined as described in Figure 1. Data are expressed as mean±SEM of three independent experiments. #P < 0.01, as compared with the cells exposed to IL-1β alone.
It has been shown that cell activation by cytokines leads to the degradation of IκB-α, accompanied by NF-κB translocation to the nucleus. To determine whether IL-1β stimulated degradation of IκB-α and NF-κB translocation, the cells were incubated with 15 ng/ml of IL-1β for various time periods. The cytosolic and nuclear fractions were prepared to determine the degradation of IκB-α and translocation of NF-κB by Western blotting using an anti-IκB-α or anti-NF-κB (p65), respectively. As shown in Figure 4B, IL-1β rapidly stimulated degradation of IκB-α within 5 min, reached a maximal response within 15 min, and returned to the basal level within 60 min. In addition, IL-1β also stimulated translocation of NF-κB (p65) into nucleus within 10 min and sustained over 60 min. Moreover, to confirm the p65 subunit of NF-κB was required for this response, transfection of cells with p65 shRNA significantly attenuated the p65 protein expression and IL-1β-induced ICAM-1 expression in RASFs (Fig. 4C).
Activation of ERK1/2, JNK1/2, and NF-κB was necessary for the ICAM-1 protein expression induced by IL-1β in RASFs, it is important to differentiate whether these MAPKs phosphorylation was associated with NF-κB activation. To test this possibility, activation of NF-κB was assessed following IL-1β stimulation in the presence of inhibitors for MEK1/2 (U0126), JNK1/2 (SP600125), or NF-κB (helenalin). IL-1β-stimulated NF-κB (p65) translocation was significantly inhibited by pretreatment with helenalin, but not by U0126 and SP600125 (Fig. 4D). Similar results were obtained with the images of immunofluorescence staining (Fig. 4E).
To further characterize whether activation of NF-κB by IL-1β was mediated through the upstream components IκB kinases (IKKs), RASFs were transfected with a dominant negative mutant of IKK-α (▵IKKα) or IKK-β (▵IKKβ), and then incubated with IL-1β (15 ng/ml) for 6 h. As shown in Figure 4F, transfection with ▵IKKα or ▵IKKβ significantly reduced the IKK activity (lower part) and IL-1β-induced ICAM-1 expression (upper part). Taken together, these results indicated that IL-1β-stimulated NF-κB (p65) translocation mediated through IKKα/β and independent on ERK1/2 and JNK1/2 activation, was essential for ICAM-1 up-regulation in RASFs.
MAPKs–AP-1 and NF-κB are required for IL-1β-induced ICAM-1 gene expression
We further determined whether IL-1β-induced AP-1 activation via ERK1/2 and JNK1/2, a promoter-luciferase reporter construct containing AP-1 binding sites was used. As shown in Figure 5A, IL-1β induced AP-1 transcriptional activity in a time-dependent manner. Pretreatment with the inhibitor of MEK1/2 (U0126), JNK1/2 (SP), or AP-1 (tanshinone IIA, TSIIA) significantly attenuated IL-1β-induced AP-1 activation (Fig. 5B), suggesting that IL-1β-induced AP-1 activation is mediated through ERK1/2 and JNK1/2 pathways. Moreover, we examined whether these MAPKs, AP-1, and NF-κB were involved in IL-1β-induced ICAM-1 gene expression at transcriptional level in RASFs, a human ICAM-1 promoter reporter construct was used. As shown in Figure 5C, IL-1β stimulated ICAM-1 promoter luciferase activity in a time-dependent manner, which was attenuated by pretreatment with the inhibitor of MEK1/2 (U0126), JNK1/2 (SP), NF-κB (HLN), or AP-1 (TSIIA; Fig. 5D). Moreover, we also demonstrated that IL-1β-induced ICAM-1 mRNA expression via these pathways assessed by a real-time RT-PCR. As showed in Figure 5E, pretreatment with the inhibitor of MEK1/2 (U0126), JNK1/2 (SP), or NF-κB (HLN) significantly blocked IL-1β-induced ICAM-1 mRNA expression. These results further demonstrated that IL-1β-induced ICAM-1 expression is mediated through ERK1/2, JNK1/2, AP-1, and NF-κB in RASFs.
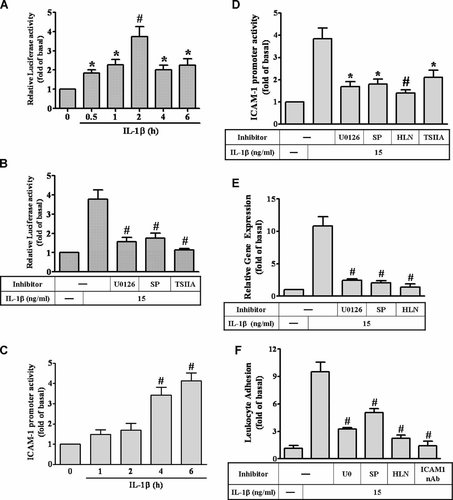
Involvement of MAPKs–AP-1 and NF-κB in ICAM-1 gene expression induced by IL-1β. For AP-1 activity, RASFs were transiently transfected with an AP-1-Luc reporter gene and (A) then incubated with 15 ng/ml IL-1β for the indicated times, or (B) pretreated with U0126 (1 µM), SP600125 (SP, 10 µM), or tanshinone IIA (TSIIA, 10 µM) for 1 h and then indicated with 15 ng/ml IL-1β for 6 h. For ICAM-1 promoter activity, RASFs were transiently transfected with an ICAM-1-Luc reporter gene and (C) then incubated with 15 ng/ml IL-1β for the indicated times, or (D) pretreated with U0126 (1 µM), SP600125 (SP, 10 µM), tanshinone IIA (TSIIA, 10 µM), or helenalin (HLN, 10 µM) for 1 h and then indicated with 15 ng/ml IL-1β for 6 h. Luciferase activity was determined in the cell lysates. E: For ICAM-1 mRNA expression, cells were pretreated with U0126 (1 µM), SP600125 (SP, 10 µM), or helenalin (10 µM) for 1 h and then incubated with 15 ng/ml IL-1β for 4 h. The ICAM-1 mRNA was analyzed by a real-time RT-PCR. F: The functional response of IL-1β-induced ICAM-1 expression, RASFs were pretreated with 1 µM U0126, 10 µM SP600125 (SP), 1 µM helenalin (HLN), or ICAM-1 neutralizing antibody (ICAM1 nAb) for 1 h and then incubated with 15 ng/ml IL-1β for 6 h prior to the addition of leukocytes. The functional activity of ICAM-1 was determined by leukocyte adhesion assay. Data are expressed as mean ± SEM of three independent experiments. *P < 0.05; #P < 0.01, as compared with the cells exposed to vehicle (A) or exposed to IL-1β (B,C) alone.
The functional response of IL-1β-induced ICAM-1 expression in RASFs: Leukocyte adhesion
IL-1β has been recognized as a potent proinflammatory mediator that increases the expression of adhesion molecules and the adhesiveness between leukocytes and several cell types (Kacimi et al., 1998; Szlosarek and Balkwill, 2003; Wang et al., 2005; Lin et al., 2007). To test the functional activity of ICAM-1 expression on RASFs, we assessed the ability of purified leukocytes adhering to these cells exposed to IL-1β. As shown in Figure 5F, the amount of leukocytes adhering to RASF monolayer was significantly increased (approximate 10-fold) by the incubation with 15 ng/ml of IL-1β for 6 h (P < 0.05, n = 3, as compared with the basal level). This enhancing leukocyte adhesion was significantly attenuated by pretreatment of RASFs with U0126 (1 µM), SP600125 (SP, 10 µM), or helenalin (HLN, 10 µM) prior to exposure to IL-1β (Fig. 5F). To further determine the surface molecules responsible for leukocyte adhesion to RASF monolayer, pretreatment of IL-1β-challenged RASFs with a ICAM-1 neutralizing antibody (sc-7891; Santa Cruz) significantly inhibited the amount of leukocyte adhesion (Fig. 5F), suggesting that IL-1β enhanced leukocyte adhesion to RASFs through induction of ICAM-1 in RASFs.
Discussion
Up-regulation of adhesion molecules on the surface of synovial lining may play a key role in recruitment and infiltration of lymphocytes at sites of inflammation in RA (Szekanecz et al., 1996; Li et al., 2000). IL-1β has been demonstrated to induce the expression of ICAM-1 in synovial tissues, but little is known about the intracellular signaling pathways leading to its expression. IL-1β has also been shown to activate all of three MAPK pathways in several cell types (McEwan, 2002; Pfeffer, 2003). However, in RASFs, whether IL-1β-induced ICAM-1 expression mediated through the activation of MAPKs, AP-1 and NF-κB was still unclear. In this study, IL-1β-induced ICAM-1 expression was significantly attenuated by the inhibitors of MEK1/2, JNK, AP-1, and NF-κB, or transfection with respective dominant negative mutants such as MEK1/2, ERK, JNK, IKK-α, and IKK-β and shRNAs of ERK2, JNK2, and p65. Our results established that the mechanisms underlying activation of ERK1/2 and JNK1/2 as well as the translocation of NF-κB by IL-1β are essential for up-regulation of ICAM-1 in RASFs. The implication of NF-κB in the ICAM-1 expression induced by IL-1β results from the degradation of IκB-α by activation of IKKα/β and in turn leads to translocation of NF-κB. Moreover, we also demonstrated that IL-1β induces ICAM-1 expression via ERK1/2- and JNK1/2-dependent activation of AP-1 in RASFs. Finally, up-regulation of ICAM-1 enhances the adhesion of leukocytes to RASFs.
In this study, our results demonstrated that activation of MAPKs was necessary for IL-1β-induced ICAM-1 expression in RASFs. Pretreatment with U0126 attenuated IL-1β-induced ICAM-1 expression and ERK1/2 phosphorylation in these cells (Fig. 2A,B). Moreover, transfection with dominant negative mutants of MEK1/2 or ERK2 significantly reduced ICAM-1 expression induced by IL-1β (Fig. 2C,D), confirming that the MEK1/2–ERK1/2 cascade is required for ICAM-1 expression. These results were consistent with those of IL-1β-induced expression of ICAM-1 through MEK1/2–ERK1/2 in various cell types (Wang et al., 2005; Lin et al., 2007). Next, the involvement of JNK in IL-1β-induced ICAM-1 expression has also been demonstrated in several cell types (Wang et al., 2005; Lin et al., 2007). Thus, we investigated the role of JNK in IL-1β-induced ICAM-1 expression using a JNK1/2 inhibitor SP600125, transfection with dominant negative mutant JNK or JNK2 shRNA attenuated IL-1β-induced ICAM-1 expression and JNK phosphorylation (Fig. 3), consistent with reports indicating that activation of JNK is essential for the up-regulation of ICAM-1 and VCAM-1 in various cell types (Wesche et al., 1997; De Cesaris et al., 1999; Lin et al., 2007).
In addition to ERK and JNK, the involvement of p38 MAPK in IL-1β-induced ICAM-1 expression was also investigated using a p38 MAPK inhibitor SB202190 (Singh et al., 1999). In RASFs, pretreatment with SB202190 significantly blocked p38 MAPK phosphorylation stimulated by IL-1β, but had no effect on ICAM-1 expression, implying that p38 MAPK is not involved in IL-1β-induced ICAM-1 expression in RASFs (data not shown). These results are consistent with the expression of VCAM-1 and ICAM-1 in Sertoli cells (Szlosarek and Balkwill, 2003) and A549 cells (Chen et al., 2000). Taken together, IL-1β-induced ICAM-1 expression is mediated through the activation of ERK and JNK in RASFs.
It has been well established that inflammatory responses following exposure to cytokines are highly dependent on the transcription factor NF-κB which plays an important role in the expression of several genes (Ghosh et al., 1998; Li et al., 2000; Bian et al., 2001). In this study, ICAM-1 expression induced by IL-1β was completely abolished by a NF-κB inhibitor helenalin (Fig. 4A) and transfection with p65 shRNA (Fig. 4C), indicating that NF-κB is essential for IL-1β-induced ICAM-1 expression. The increasing in NF-κB translocation correlated with the rapid and transient degradation of IκB-α in the cytosol of RASFs stimulated by IL-1β (Fig. 4B), suggesting that degradation of IκB-α may initiate translocation of active NF-κB into the nucleus. These results are consistent with those of reports by Lin et al. (2005), indicating that IL-1β-induced expression of ICAM-1 is mediated through NF-κB activation. Interestingly, activation of ERK and JNK as well as NF-κB appeared to be involved in IL-1β-induced ICAM-1 expression. However, it remains unclear how the activation of ERK and JNK is associated with ICAM-1 gene expression. Previously, it has been established that MEKK1 induces activation of both IKKα and IKKβ leading to NF-κB activation (Barnes and Karin, 1997; Ghosh et al., 1998). Thus, ERK may be required for NF-κB activation following exposure to IL-1β. In this study, our results demonstrated that IL-1β-stimulated NF-κB activation was not significantly inhibited by U0126 and SP600125 (Fig. 4D,E), indicating that both MAPKs cascade and NF-κB independently regulate gene expression of ICAM-1 in RASFs. These results suggest that activation of NF-κB and ERK and JNK are mediated by distinct pathways leading to ICAM-1 expression (Wesselborg et al., 1997; Lee et al., 2006) or these MAPKs pathways may converge at a step downstream of NF-κB transactivation (Wesselborg et al., 1997; Vanden Berghe et al., 1998). However, we further confirmed the upstream components of NF-κB in this response by transfection with dominant negative mutants of IKKα and IKKβ (Fig. 4F), indicating that IL-1β induces ICAM-1 expression via IKKα/β activating NF-κB cascade. Furthermore, we demonstrate that IL-1β stimulates activation of ERK and JNK linking to another transcription factor AP-1 in ICAM-1 induction event (Fig. 5).
Moreover, our results showed that pretreatment with U0126, SP600125, helenalin, and tanshinone IIA significantly attenuated ICAM-1 promoter luciferase activity stimulated by IL-1β (Fig. 5D), suggesting that activation of MAPKs–AP-1 and NF-κB pathways regulates ICAM-1 expression at a transcriptional level. In addition, NF-κB and AP-1 binding sites have been previously identified in the ICAM-1 gene promoter (Ballantyne et al., 1992; Chen et al., 2000), which might explain the modulation exerted by IL-1β through NF-κB and AP-1 activation.
This study is the first showing the involvement of ERK and JNK linking to AP-1 as well as NF-κB in the IL-1β-induced ICAM-1 expression in RASFs. The present study provides the evidence that activation of MEK1/2–ERK1/2 by IL-1β is necessary for ICAM-1 expression in RASFs. In addition, JNK1/2 has been shown to be an additional MAPK required for IL-1β-induced ICAM-1 expression in these cells. We also found that IL-1β-induced ICAM-1 expression is mediated through activation of NF-κB based on the early NF-κB nuclear translocation. Based on the observations from the literatures and our findings, a schematic pathway depicts a model for the roles of ERK, JNK, AP-1, and NF-κB activation associated with IL-1β-induced ICAM-1 expression and leukocyte adhesion to RASFs (Fig. 6). The mechanisms by which IL-1β induces ICAM-1 expression and leukocyte adhesion in RASFs may be an important linkage in the pathogenesis of inflammatory diseases in RA. Therefore, understanding the mechanisms underlying IL-1β-induced ICAM-1 expression in RASFs is important to develop new therapeutic strategies.
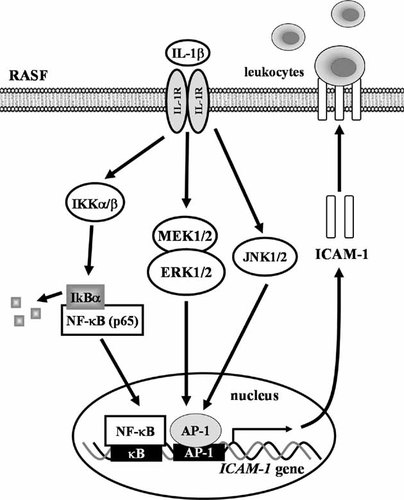
Schematic representation of signaling pathways involved in IL-1β-induced ICAM-1 expression in RASFs. Binding of IL-1β to its receptors results in activation of MEK1/2-ERK, JNK linking to AP-1, and IKKα/β–NF-κB pathways. ICAM-1 transcription is independently regulated by these MAPKs–AP-1 and NF-κB pathways. These signaling pathways might enforce each other and contribute to sustained activation of transcription factors required for ICAM-1 expression and leukocyte adhesion in RASFs.
Acknowledgements
This study was financially supported by National Science Council, Taiwan (No. NSC95-2320-182-047-MY3) to C.M.Y. and Chang Gung Medical Research Foundation (No. CMRPG350643) to S.F.L. (No. CMRPG350653) to C.C.L., and (CMRPD180061) to C.M.Y. The authors appreciate Dr. K.L. Guan (Department of Biological Chemistry, University of Michigan, MI, USA), M.H. Cobb (Department of Pharmacology, University of Texas Southwestern Medical Center, TX, USA), C.C. Chen (Department of Pharmacology, National Taiwan University, Taipei, Taiwan), M. Karin (Department of Pharmacology, University of California, San Diego, CA), and C.P. Tseng (Department of Medical Biotechnology and Laboratory Science, University of Chang Gung, Tao-Yuan, Taiwan) for providing dominant negative mutants MEK1/2 (MEK K97R), ERK2 (ERK2 K52R), JNK, IKK-α(KM), and IKK-β(KM), ICAM-1-Luc, and shRNA (ERK2, p38, and JNK2) and EGFP constructs, respectively.




