ROCK pathway participates in the processes that 15-hydroxyeicosatetraenoic acid (15-HETE) mediated the pulmonary vascular remodeling induced by hypoxia in rat
Abstract
15-Hydroxyeicosatetraenoic acid (15-HETE), a product of arachidonic acid (AA) catalyzed by 15-lipoxygenase (15-LO), plays an essential role in hypoxic pulmonary arterial hypertension. We have previously shown that 15-HETE inhibits apoptosis in pulmonary artery smooth muscle cells (PASMCs). To test the hypothesis that such an effect is attributable to the hypoxia-induced pulmonary vascular remodeling (PVR), we performed these studies. We found subtle thickening of proximal media/adventitia of the pulmonary arteries (PA) in rats that had been exposed to hypoxia. This was associated with an up-regulation of the anti-apoptotic Bcl-2 expression and down-regulation of pro-apoptotic caspase-3 and Bax expression in PA homogenates. Nordihydroguaiaretic acid (NDGA), which inhibits the generation of endogenous 15-HETE, reversed all the alterations following hypoxia. In situ hybridization histochemistry and immunocytochemistry showed that the 15-LO-1 mRNA and protein were localized in pulmonary artery endothelial cells (PAECs), while the 15-LO-2 mRNA and protein were localized in both PAECs and PASMCs. Furthermore, the Rho-kinase (ROCK) pathway was activated by both endogenous and exogenous 15-HETE, alleviating the serum deprivation (SD)-induced PASMC apoptosis. Thus, these findings indicate that 15-HETE protects PASMC from apoptosis, contributing to pulmonary vascular medial thickening, and the effect is, at least in part, mediated via the ROCK pathway. J. Cell. Physiol. 222:82–94, 2010. © 2009 Wiley-Liss, Inc.
Abbreviations:
15-HETE, 15-hydroxyeicosatetraenoic acid; PVR, pulmonary vascular remodeling; ROCK, Rho-kinase; PAs, pulmonary arteries; PASMCs, pulmonary artery smooth muscle cells; AA, arachidonic acid; 15-LO, 15-lipoxygenase; NDGA, nordihydroguaiaretic acid; SD, serum deprivation.
Pulmonary arterial hypertension (PAH) is a disease of the small pulmonary arteries characterized by sustained vasoconstriction, thickening of pulmonary artery walls, vascular remodeling, progressive increases in pulmonary vascular resistance, leading to right heart failure and death (Humbert et al., 2004; Mandegar et al., 2004; Pidgeon et al., 2004). The enhanced vascular smooth muscle cell (VSMC) proliferation and suppressed normal VSMC apoptosis are likely the major reasons leading to medial hypertrophy, arterial remodeling and vascular lumen narrowing. Indeed, the inadequate apoptosis has been implicated in the development and maintenance of severe pulmonary hypertension, whereas the induction of apoptosis regresses the hypertrophy of pulmonary arterial walls in animal experiments (Rabinovitch, 1998; Zhang et al., 2003). Chronic hypoxia is a major reason of PAH, but the pathogenesis of PAH remains unclear.
The Rho-kinase (ROCK) pathway has been found to regulate a wide range of fundamental cell functions such as contraction, motility, proliferation and apoptosis (Coleman and Olson, 2002; Etienne-Manneville and Hall, 2002; Moore et al., 2004; Kozai et al., 2005). The involvement of the ROCK pathway in the pathogenesis of PAH has been widely studied (Abe et al., 2004). The ROCK pathway acts in both vasoconstriction and vascular remodeling in animal molds of hypoxic PAH (Fagan et al., 2004; Guilluy et al., 2005; Hyvelin et al., 2005; Nagaoka et al., 2005). There are reports showing that RhoA activation and ROCK expression are increased in chronic hypoxic lungs (Jernigan et al., 2004; Guilluy et al., 2005; Nagaoka et al., 2006). ROCK is also involved in endothelial nitric oxide synthase (eNOS) expression and activity. Therefore, it is possible that the ROCK pathway plays a role in VSM cell proliferation and apoptosis, and its abnormality leads to PAH (Takemoto et al., 2002; Furuyama et al., 2006). Supporting this idea are previous studies showing that a long-term inhibition of ROCK by Y-27632 or fasudil, two specific ROCK inhibitors, markedly improves survival when started after development of PAH (Fagan et al., 2004; Fukumoto et al., 2005; Ishikura et al., 2006), inhibits VSM cell proliferation, increases apoptosis, and suppresses the medial thickening of pulmonary arteries (PA) in rats with PAH (Abe et al., 2004; Hattori et al., 2004). The experimental evidence thus indicates that the ROCK pathway plays an important role in the hypoxic PAH development and the associated pulmonary vascular remodeling.
In previous studies, we have found that hypoxia increases the formation of endogenous 15-hydroxyeicosatetraenoic acid (15-HETE) through stimulation of 15-lipoxygenase (15-LO) (Zhu et al., 2003), both endogenous and exogenous 15-HETE increase ROCK II mRNA and protein expression, 15-HETE inhibits apoptosis of pulmonary artery muscle smooth cells (PASMCs) (Li et al., 2009), and the inhibition of ROCK pathway initiates apoptosis in several cell types (Hippenstiel et al., 2002; Li et al., 2002; Ikeda et al., 2003).
These previous findings raise the possibility that 15-HETE inhibits the apoptosis of PASMCs to induce pulmonary artery medial hypertrophy, contributing to pulmonary vascular remodeling through the ROCK pathway. To test this hypothesis, we first examined the effects of endogenous 15-HETE induced by hypoxia on the morphological changes of pulmonary vessels and the expression of apoptotic proteins (caspase-3, Bcl-2, Bax) in PA homogenates and cultured PASMCs of rats. Immunocytochemistry and in situ hybridization were applied to determine the cellular distribution of 15-LO isoenzymes in PAs; Y-27632 (ROCK inhibitor) was employed to elucidate whether ROCK pathway was involved in the inhibitory effect of 15-HETE on PASMC apoptosis. Our results show that endogenous 15-HETE produced by 15-LO-2 in hypoxia inhibits the apoptosis of PASMCs to cause PA medial thickening and lumen obliteration, and attenuates a series of apoptotic events involving mitochondrial dysfunction, caspase-3 activation and DNA fragmentation via ROCK pathway.
Materials and Methods
Materials
15-HETE dissolved in ethanol was obtained from Cayman Chemical Company (Ann Arbor, MI) and was stored at −20° under nitrogen. Cinnamyl 3,4-dihydroxy-[alpha]-cyanocinnamate (CDC), Y-27632, 15-LO-1 and 15-LO-2 polyclonal antibodies were purchased from Cayman Chemical Company. Rabbit anti-MYPT1 and phosphor-specific antibodies against MYPT1 were purchased from Cell Signaling Technology (Danvers, MA). Antibodies against Bcl-2, procaspase-3, Bax and β-actin were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). RT-PCR kit was purchased from Invitrogen Company (Carlsbad, CA). ROCK II and ROCK I polyclonal antibodies, in situ hybridization and SABC immunocytochemistry detection kits were purchased from Boster Biological Technology Co. Ltd. (Wuhan, China). JC-1 probe, the terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) cell apoptosis detection kit and caspase-3 activity kit were obtained from Beyotime Institute of Biotechnology (Haimen, China). Enhanced chemiluminesence (ECL) reagents were from Amersham International (Amersham, UK). All other reagents were from common commercial sources.
Animals and lung tissues preparation
Adult male Wistar rats with a mean weight of 200 g were from the Experimental Animal Center of Harbin Medical University, which is fully accredited by the Institutional Animal Care and Use Committee (IACUC). Twelve-hour light exposure cycles, standard rat chow, and water ad libitum were provided to all rats. Adult male Wistar rats were randomized to 9 days of normal and hypoxic environments with fractional inspired oxygen (FiO2) 0.21 and 0.12, respectively as previously described (Zhu et al., 2003). Normoxic rats were kept in the same room adjacent to the hypoxic chamber. To test the effects of Nordihydroguaiaretic acid (NDGA) on hypoxia, one group of rats had been given NDGA (650 mg/kg b.w. orally, once daily) since 2 days before hypoxia till they were euthanized (the 10th day after hypoxia) (Arteaga et al., 2005). At the end of the 9 days exposure period, we anesthetized each rat with pentobarbital injection (120 mg/kg, i.p.), opened the thorax and removed the heart and lungs to the flat plate. In some experiments, the lungs were quickly removed and further processed for in situ hybridization and immunocytochemistry as described below, and the pulmonary arteries were harvested for Western blot and RT-PCR.
Cell culture and protocols
PASMCs were dispersed according to our previously published protocol (Guo et al., 2008). Cells were cultured in 20% fetal bovine serum (FBS)-DMEM and in a 37°C, 5% CO2 humidified incubator. Cell viability determined by Trypan Blue exclusion was consistently greater than 98%. The purity of PASMCs in the primary cultures was determined by specific monoclonal antibodies raised against smooth muscle α-actin (Boehringer Mannheim, Germany). Passages 2–3 were used for further experimentations. Before each experiment, the apoptosis in PASMC was induced by serum deprivation, and the cells were incubated in DMEM without serum for 24–48 h. Then some cells were treated with Y-27632 (1 µM) or Y-27632 (1 µM) plus 15-HETE (1 µM) in serum deprivation conditions, the others were exposed to hypoxia in absence or presence of CDC (5 µM). The cells cultured in complete medium were used as control. CDC, 15-HETE and Y-27632 at the indicated concentration were added every 24 h with new medium.
Histology
The lung tissues were obtained from anesthetized rats, sliced into tissue blocks, and immersed in 4% paraformaldehyde for overnight fixation. Fixed tissues were then dehydrated, cleared, and embedded in paraffin wax. The tissues were cut into 5 µm thick sections and stained with hematoxylin and eosin (H&E). The areas of the vessel within the external elastic lamina (EEL), the internal elastic lamina (IEL), and the lumen (luminal area) were measured. The intimal area (IEL-luminal area) and the medial area (EEL-IEL) were calculated. Results are expressed as ratios of intima to media. The sections were viewed with an Eclipse 600 Nikon microscope and photographed with a digital camera. Morphometric analysis was analyzed with image software (Image Pro Plus).
Immunocytochemistry
The immunocytochemical methods were carried out by the technique described by Natarajan R and Nadler JL previously (Natarajan and Nadler, 2003). In brief, 5-µm paraffin-embedded tissue sections were deparaffinized and rehydrated in graduated alcohol. The sections were steamed over a 0.1 mol/L citrate buffer solution for 20 min for the heat-induced epitope retrieval and then allowed to cool for 5 min. The endogenous peroxidase activity was blocked. The sections were then treated with normal serum to bind nonspecific sites. The sections were then incubated with primary antibodies (in 1:1,000 dilation). Parallel controls were run without primary antibodies. After an overnight incubation, the sections were washed three times with PBS and then exposed to the secondary antibodies (1:200) for the IgGs of the appropriate species. Subsequently, sections were stained by a modified avidin–biotin complex (ABC) technique. Finally, the staining was visualized with 3,3-diaminobenzidine (brown) and counterstained using hematoxylin. Brown and yellow colors indicated positive stains. The staining was evaluated by Image Pro Plus.
Non-isotopic in situ hybridization
Dig-labeled probes were designed according to the 15-LO-1/2 sequences of rat and synthesized by Invitrogen Inc. (Genbank accession NO. NM_031010, NM_153301). The sequences of Dig-labeled probes against 15-LO-1 mRNA were: 5′-GGCAGG AAGACAAGTAGAGCG-3′. The sequences of Dig-labeled probes against 15-LO-2 mRNA were: 5′-ATTCGCAGCATCAGCACAGT-3′. In situ hybridization was performed using a detection kit on sections of 4% paraformaldehyde-fixed (containing 0.1% diethylpyrocarbonate) of lung tissues according to the manufacturer's instructions. Briefly, the tissues were dehydrated with increasing concentrations of ethanol, embedded in paraffin; 5-µm sections were cut and placed on poly-L-lysine treated slides. The sections were dewaxed, rehydrated in a graded series of ethanol. The endogenous peroxidase was quenched using 3% H2O2 with 0.1% diethylpyrocarbonate water for 10 min in room temperature. The sections were then transferred to RNase-free cold phosphate-buffered saline. The sections were permeabilized with proteinase K and pre-hybridized for 4 h at 33°C in prehybridization mixture. The sections were placed in a humidified chamber and incubated with Dig-labeled single-stranded oligonucleotide probes for hybridization overnight at 37°C. Hybridization with blank probes solution carried out at the same time under identical condition served as a negative control. Post-hybridization washes were done stepwise in room temperature with 2× standard saline citrate (SSC) (1× SSC contains 150 mM NaCl, and 15 mM sodium citrate, pH 7.0) 0.5×, 0.2× and then again with 2× for 15 min. Hybridization signal was visualized through labeled streptavidin biotin method with horseradish peroxidase/3,3-diaminobenzidine (HRP/DAB). The nuclear was counterstained using hematoxylin and the sections were dehydrated and mounted. Brown and yellow colors indicated positive results. The positive staining was evaluated by Image Pro Plus.
MTT
PASMCs were cultured in 60-mm dishes (about 1 × 105), and then subjected to growth arrest for 24 h before being placed in either complete medium (DMEM with 20% FBS) or switched to basal medium for the next 48 h. The concentration of ethanol in the medium was less than 0.1% (v/v). At 48 h of the incubation in 37°C, the cells were incubated for 4 h in a medium containing 0.5% 3-[4,5-dimethylthiazol-2-yl]-2, 5-diphenyl-tetrazolium bromide (MTT). The reaction was terminated by adding DMSO to the medium followed by incubation for 10 min at 37°C. The absorbance was read at 540 nm in a spectrophotometer.
LDH release assay
The activity of lactate dehydrogenase (LDH) released into the culture media was measured using a Cytotoxicity Detection kit to indicate the percentage of injured cells relative to total LDH activity after complete cell lysis. Briefly, a portion of culture medium was reacted with an equal volume of LDH substrate solution for 30 min. The reaction was stopped by adding 5 volume of 0.1 M NaOH, and the absorbance was then measured at 440 nm in a spectrophotometer. Sister cultures were treated with 1/100 volume of 10% Triton X-100 and incubated at 37°C for 30 min. Total LDH activities were determined using medium containing Triton-lysed cellular supernatant.
Western blot analysis
Pulmonary arteries from rats (normoxia, hypoxia and hypoxia with NDGA) were homogenized in a hand-held micro-tissue grinder in ice-cold storage buffer (Tris 50 mM, pH 7.4, NaCl 150 mM, Triton X-100 1%, EDTA 1 mM, and PMSF 2 mM). The homogenates were sonicated on ice and then centrifuged at 16,099g for 10 min at 4°C. The supernatants were colleted and stored at −80°C until use in Western blot analysis.
The cells in 6-well culture clusters were treated according to 2.3. After the treatment for 24 h, the cells were lysed in a lysis buffer (Tris 50 mM, pH 7.4, NaCl 150 mM, Triton X-100 1%, EDTA 1 mM, and PMSF 2 mM) and incubated for 30 min on ice. Phosphatase inhibitor was added to the lysis buffer containing. The lysates were then sonicated and centrifuged at 16,099g for 10 min, and the insoluble fraction was discarded. The supernatants were collected and stored at −80°C until use in Western blot analysis.
Protein concentrations were determined by the Bradford assay using bovine serum albumin (BSA) as standard. Pulmonary artery homogenates containing 50 µg of protein and cells protein samples containing 20 µg of protein were separated by SDS–PAGE and electro blotted onto nitrocellulose sheets, blocked for 1 h in room temperature with a Tris-buffered saline buffer (20 mM Tris, 150 mM NaCl, pH7.6 Tween 20 0.1%) containing 5% nonfat dry milk, and then probed with appropriate antibodies to 15-LO-1 at 1:2,000, 15-LO-2 at 1:1,000, MYPT1 and phospho-MYPT1(Thr853) at 1:1,000, β-actin, procaspase-3, Bcl-2, Bax, ROCK II and ROCK I at 1:500 dilution overnight at 4°C followed by horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5,000 dilution) or rabbit anti-goat IgG (1:5,000 dilution) antibodies for 1 h in room temperature. Immunoreactivity was detected using an enhanced chemiluminescence Western blotting detection kit (Amersham Biosciences, Piscataway, NJ) and exposed to X-ray film. Immunoblots were scanned using a GS-800 densitometer and protein bands were quantified with Quantity One software (Bio-Rad Laboratories, Hercules, CA).
Measurement of caspase-3 activity
Caspase-3 activity was measured by cleavage of chromogenic caspase substrates, Ac-DEVD-pNA (acetyl-Asp-Glu-Val-Asp p-nitroanilide), a caspase-3 substrate. The absorbance of the substrate was measured at 405 nm after cleavage by caspase-3. The optical density value at 405 nm was thus used as indication for the amount of caspase-3. The protein samples were prepared as indicated in Western blot analysis. Then approximate 50 µg of total proteins were added to the reaction buffer containing Ac-DEVD-pNA (2 mM), incubated for 2 h at 37°C, and the absorbance of yellow pNA cleaved from its corresponding precursors was measured using a spectrometer at 405 nm. The specific caspase-3 activity, normalized for total proteins of cell lysates, was then expressed as fold of the baseline caspase activity of control cells cultured in DMEM with 20% FBS.
Mitochondrial depolarization assay
Mitochondrial function was indirectly assessed with the mitochondrial transmembrane potential measured with JC-1 red fluorescence. Relative mitochondrial mass was determined by a fluorescent microscope using 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine iodide (JC-1), analyzed for green fluorescence. The cells were stained with JC-1 probe for the measurement of mitochondrial depolarization. The treated cells were incubated with an equal volume of JC-1 staining solution (5 µg/ml) at 37°C for 20 min and rinsed twice with PBS. Mitochondrial membrane potentials were monitored by determining the relative amounts or dual emissions from both mitochondrial JC-1 monomers and aggregates using an Olympus fluorescent microscope at 488 nm excitation. Mitochondrial depolarization was indicated by an increase in the green/red fluorescence intensity ratio.
TUNEL
TdT-UTP nick end labeling (TUNEL) method was performed to label 3′-end of fragmented DNA of the apoptotic PASMCs. The cells cultured in a 6-well plate were treated as mentioned in mitochondrial depolarization assay, fixed with 4% paraformaldehyde phosphate buffer saline, rinsed with PBS, and then permeabilized by 0.1% TritonX-100 for 2 min on ice followed by TUNEL for 1 h at 37°C. The FITC-labeled TUNEL-positive cells were imaged under a fluorescent microscopy at 488-nm excitation and 530-nm emission. The cells with green fluorescence were defined as apoptotic cells.
RT-PCR
The PAs were snap-frozen in liquid nitrogen and then stored at −80°C. Frozen tissue samples were disrupted and homogenized. Total RNA was extracted from the PAs by using Trizol reagent with a Polytron homogenizer (Brinkmann Instruments, Westbury, NY) and determined by ultraviolet spectrophotometry (absorbance at 260 nm/280 nm). Isolated total RNAs were reverse-transcribed with the Superscript first-strand cDNA synthesis kit (Invitrogen). The cDNA samples were amplified in a DNA thermal cycler (PxG20809, Thermo Electron Co., Waltham, MA). Gene-specific primers were designed from their own coding regions as follows: 15-LO-1 (GenBank accession No. NM_031010): forward: 5′-GACTCGGAAGCAGAA-3′; reverse: 5′-CTCCCTGTAGACCAACGA-3′, fragment size: 369 bp. 15-LO-2 (GenBank accession No. NM_153301): forward: 5′-GGTCAGGAGGATGGCTAA-3′, reverse: 5′-CTCAAACCAGCGGCAGTA-3′, fragment size: 292 bp. β-Actin: forward: 5′-CCGTAAAGACCTCTATGCCAACA-3′, reverse: 5′-CGGACTCATCGTACTCCTGCT-3′, fragment size: 500 bp. β-Actin: forward: 5′-GGCTGAGGTCTTTGCTG-3′, reverse: 5′-CTTTGTCGGTCTTGTAGTG-3′, fragment size: 230 bp. β-Actin: forward: 5′-TTGTAACCAACTGGGACGATATGG-3′ reverse: 5′-GATCTTGATCTTCATGGTGCTAGG-3′, fragment size: 764 bp. Caspase-3 (GenBank accession No. NM_012922): forward: 5′-ACGGTACGCGAAGAAA-AGTGAC-3′, reverse: 5′-TCCTGACTTCGTATTTCAGGGC-3′, fragment size: 282 bp. PCR products were run on 1% agarose gels stained with ethidium bromide and visualized by GelStar gel staining (FMC BioProducts, Patchogue, NY). Images were recorded and band intensities were analyzed using gel imaging analysis system (Alpha Innotech, San Leandro, CA). β-Actin mRNA levels served as an internal standard for normalization of 15-LO-1/2 and caspase-3 mRNA levels. The ratio of optical density (OD) values of the target 15-Lipoxygenase isozymes and caspase-3 to β-actin were then determined, respectively.
Measurement of 15-HETE level
To ascertain whether administration of NDGA to rats can inhibit the generation of endogenous 15-HETE in PAs, the 15(S)-HETE EIA Kit (Catalog No.534721, Cayman) was performed for the detection of the amount of 15(S)-HETE. Pulmonary artery vessels were prepared homogenates by sonication, and ground with mortar and pestle and 0.1 M/L Tris–HCl, pH 7.4 and with 1 × 10−3M/L EDTA and 10−5M/L Indomethacin on ice. Then the amount of endogenous 15-HETE was measured by 15(S)-HETE EIA Kit. The protein concentrations were determined by Bradford protein assay. The results were analyzed by Cayman Chemical Company Enzyme Immunoassay (EIA) Tools.
Statistics
The composite data are expressed as means ± SEM. Statistical analysis was performed with one-way ANOVA followed by Dunnett's test or Student's t-test. Differences were considered to be significant at P ≤ 0.05.
Results
Morphometric analysis of medium-size pulmonary arteries and 15-HETE level in pulmonary arteries after hypoxia and hypoxia with NDGA
The morphology of pulmonary vessels was examined with hematoxylin-eosin (H&E) stain to show potential correlations of the morphological changes with remodeling. Medial thickening was found in medium-size PAs obtained from rats exposed to hypoxia for 9 days. There was significant increase in the intima-to-media ratio over normoxic rats (hypoxic vs. normoxic, 1.29 ± 0.15 vs. 0.86 ± 0.08, n = 18, P < 0.05; Fig. 1B). However, the increase was inhibited by administration of NDGA, 15-LO inhibitor (hypoxic vs. hypoxic + NDGA, 1.29 ± 0.15 vs. 0.92 ± 0.12, n = 18, P < 0.05; Fig. 1B). Furthermore, we found the endogenous 15-HETE level was increased under hypoxic condition, an effect that could be reduced by administration of NDGA to the rats (Fig. 1C, n = 3, P < 0.05). Indeed, NDGA prevented the hypoxia-induced medial thickening of pulmonary arteries.
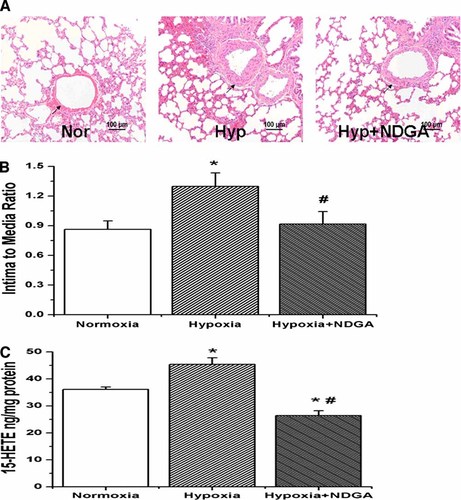
Hematoxylin–eosin staining and the endogenous level of 15-HETE as measured by 15-HETE EIA kit in pulmonary arteries from normoxic, hypoxic and hypoxic with NDGA lung tissues. A: Sections of lung tissues from rats exposed to normoxia, hypoxia and hypoxia with NDGA. B: The ratio of intimal-to-medial areas of the vessel. Hypoxia significantly increased intima to media ratios compared with normoxic rats, which was reversible by administration of NDGA to rats (*P < 0.05 vs. normoxia, #P < 0.05 vs. hypoxia; n = 18). C: Hypoxia induces the generation of endogenous 15-HETE in pulmonary artery vessels, but administration of NDGA to rats decreased the level of endogenous 15-HETE (*P < 0.05 vs. normoxia, #P < 0.05 vs. hypoxia; n = 3). “Nor” means Normoxia, “Hyp” means Hypoxia. Data shown are representative of at least three independent experiments. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Localization and expression of 15-LO isozymes in the lung
The precise locations of 15-LO-1 and 15-LO-2 were examined, both of which metabolize AA to produce 15-HETE, using immunocytochemistry and in situ hybridization histochemistry in the lung tissue sections of normoxic and hypoxic rats. The sections were stained with 15-LO isozyme antibodies and Dig-labeled 15-LO isozyme riboprobes. As shown in Figure 2, 15-LO-1 was majorly localized in PAECs, whereas 15-LO-2 was found in both PAECs and PASMCs. Furthermore, 15-LO isozymes expression was up-regulated by hypoxia, as shown by the intense staining in PAECs and PASMCs from hypoxic rats (Fig. 2A: b,b1, B: f,f1, C: b,b1 D: f,f1). However, when administration of NDGA, the increased expression was down-regulated, as seen by the weaker staining of both PAECs and PASMCs (Fig. 2A: c,c1, B: g,g1, C: c,c1, D: g,g1).
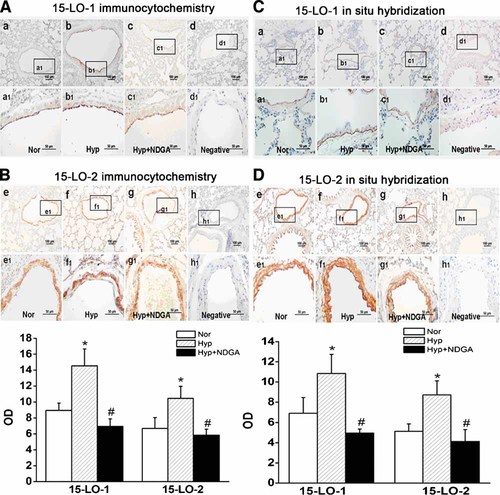
Expression and localization of 15-LO isozymes (15-LO-1 and 15-LO-2) protein and gene in PAs from normoxia, hypoxia and hypoxia with NDGA treated rats. A: 15-LO-1 protein expression and localization through immunocytochemistry. B: 15-LO-2 protein expression and localization through immunocytochemistry. Framed areas in a–d and e–h are shown at high magnification in a1–d1 and e1–h1, respectively. Sections stained without primary antibodies of 15-LO-1 and 15-LO-2 for immnuostaining (d,d1) and (h,h1). 15-LO-1 protein mainly expressed in PAEC and 15-LO-2 protein clearly expressed in PAEC and PASMC. The mean OD values of 15-LO isozymes are mean ± SEM (*P < 0.05 vs. normoxia, #P < 0.05 vs. hypoxia; n = 5). C: 15-LO-1 gene expression and localization through in situ hybridization. D: 15-LO-2 gene expression and localization through in situ hybridization. Framed areas in a–d and e–h are shown at high magnification in a1–d1 and e1–h1, respectively. Sections probed without DIG-labeled 15-LO-1 probes (d,d1) and 15-LO-2 probes (h,h1). 15-LO-1 gene mainly expressed in PAEC and 15-LO-2 gene clearly expressed in PAEC and PASMC. The mean OD values of 15-LO isozymes are mean ± SEM (*P < 0.05 vs. normoxia, #P < 0.05 vs. hypoxia; n = 5). “Nor” means Normoxia, “Hyp” means Hypoxia. Data shown are representative of at least three independent experiments. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
NDGA blocked the effects of hypoxia on mRNA and protein expression of 15-LO-1 and 15-LO-2 in PA homogenates of rats
To determine the gene and protein expression of 15-LO-1/2, RT-PCR and Western blot analysis were performed on total RNAs and proteins extracted from PAs. We found that the hypoxia-induced 15-LO-1/2 mRNA and protein expression were diminished with the administration of NDGA (Fig. 3, n = 3, P < 0.05), suggesting that the hypoxia-induced expression of 15-LO-1 and 15-LO-2 in PA relies on their lipoxygenase activity.
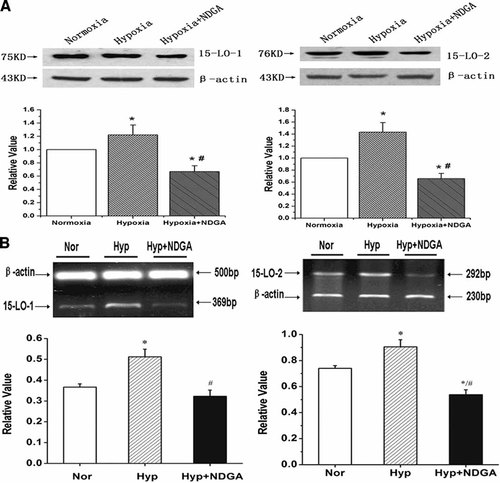
The expression of 15-LO isozymes (15-LO-1 and 15-LO-2) protein and mRNA in rat PA homogenates from normoxia, hypoxia and hypoxia with NDGA treated rats. A: 15-LO-1 (left) and 15-LO-2 (right) protein expression in rat lung homogenates from normoxic, hypoxic and hypoxic with NDGA rats. B: PCR-amplified products were displayed in agarose gels stained with ethidium bromide for 15-LO-1 (left), 15-LO-2 (right) and β-actin. Results showed the effects of hypoxia on expression of 15-LO1/2 protein and mRNA in PA were blocked by NDGA. “Nor” means Normoxia, “Hyp” means Hypoxia. All values are denoted as mean ± SEM from at least three separate experiments (*P < 0.05 vs. normoxia, #P < 0.05 vs. hypoxia; n = 3).
Blockade of 15-HETE production with NDGA inhibited the effects of hypoxia on apoptotic protein expression
To further examine the PA remodeling in hypoxia, we analyzed the expression of apoptotic proteins in PAs. We found that hypoxia induced the expression of Bcl-2 and down-regulated the expression of caspase-3 and Bax in PAs homogenates. These effects were abolished in the presence of NDGA (Fig. 4A–C). We also found that NDGA reduced the expression of ROCK II and ROCK I induced by hypoxia in PA homogenates (Fig. 4D,E). These results suggest that PASMC apoptosis plays an important role in the 15-HETE-induced medial thickening, and ROCK pathway may be involved in the process.
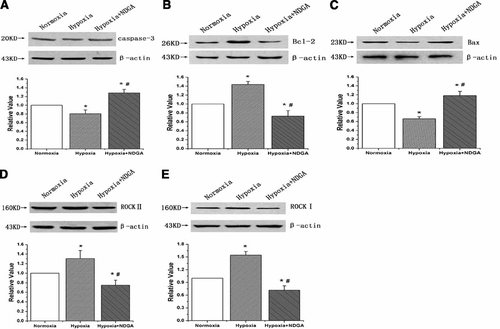
NDGA (15-LO inhibitor) attenuated the effects of hypoxia on apoptotic protein expression in PA homogenates of rats. A: The expression of caspase-3 in PA homogenates from normoxia, hypoxia and hypoxia with NDGA treated rats. B: The expression of Bcl-2 in rat PAs. C: The expression of Bax in rat PAs. D: The expression of ROCK II in rat PAs. E: The expression of ROCK I in rat PAs. Hypoxia significantly induced the expression of Bcl-2, ROCK II and ROCK I while decreased the expression of the pro-apoptotic proteins (caspase-3, Bax), which were reversible by administration of NDGA to rats. All values are denoted as means ± SEM from three or more independent experiments (*P < 0.05 compared with control group; #P < 0.05 compared with hypoxia group; n = 3).
15-HETE improved cell viability and attenuated cell toxicity via Rho-kinase signaling pathway in PASMC
To examine the effects of 15-HETE and Rho-kinase pathway on cell viability and cell toxicity, we applied exogenous 15-HETE and the Rho-kinase inhibitor (Y-27632) to PASMCs. Cell viability was determined by measuring MTT, and cell toxicity was evaluated by the LDH release assay. Our results showed that serum deprivation caused a marked decrease in cell viability. The protective effect of 15-HETE on cell viability was significantly attenuated by incubation with 1 µM/L Y-27632 (Fig. 5A, n = 6, P < 0.05). Hypoxia known to promote the formation of endogenous 15-HETE further enhanced the cell viability after SD. Both CDC (15-LO inhibitor) and Y-27632 inhibited endogenous 15-HETE effects. Exogenous 15-HETE had a protective role in cell viability in the group treated with CDC, but not in the group with Y-27632 (Fig. 5B, n = 6, P < 0.05). To ascertain whether hypoxia had an inhibitory effect on cell apoptosis, we examined the cell viability, and found that hypoxia significantly improved the cell viability and inhibited PASMC apoptosis (Fig. 5D, n = 6, P < 0.05).
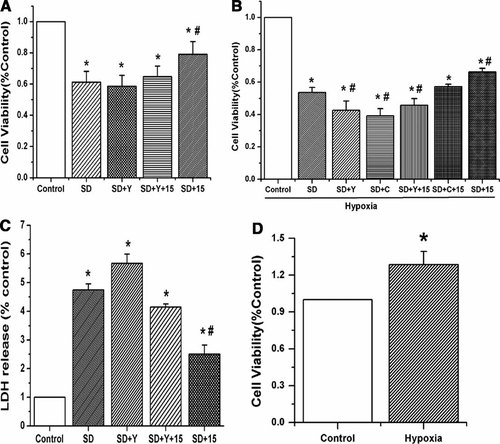
The survival rate of cultured pulmonary artery smooth muscle cells after serum deprivation was studied with MTT and LDH. A: PASMCs were treated as the anticipated groups for 48 h and cell viability was determined by the MTT assay. Y-27632 inhibited the protective effect of 15-HETE on cell viability in serum deprived condition. B: PASMCs exposed to hypoxia were treated in absence or presence with CDC (5 µM) for 48 h and cell viability was determined by the MTT assay. CDC only inhibited the effect of endogenous 15-HETE and the protective effects of exogenous and endogenous 15-HETE were both blocked by ROCK inhibitor. C: PASMCs were treated the same as A, and cell toxicity was determined using LDH release assay. Y-27632 inhibited the protective effect of 15-HETE on cell toxicity in serum deprived condition. D: The effect of hypoxia on cell viability was determined by the MTT assay. Hypoxia alone increased the cell viability and induced little death in PASMCs. “SD” means serum deprivation, “C” means CDC, “Y” means Y-27632, “15” means 15-HETE. All values are denoted as means ± SEM from three or more independent batches of cells (*P < 0.05 compared with the cells cultured in complete medium (Control); #P < 0.05 compared with serum deprived cells in the presence of vehicle (SD)).
Consistently, SD caused more LDH release in comparison with control cells cultured in normal serum medium. Y-27632 itself did not cause LDH release with serum deprivation, which was consistent with our MTT results that Y-27632 did not decrease the cell viability with serum deprivation in PASMC in normoxia. The LDH release was significantly inhibited by 15-HETE, an effect that was attenuated by Y-27632 (Fig. 5C, n = 6, P < 0.05).
Both endogenous and exogenous 15-HETE induced ROCK II and ROCK I expression, and 15-HETE increased ROCK activity in PASMCs
To demonstrate the relationship between 15-HETE and ROCK pathway, the ROCK activity and the expression of ROCK II and ROCK I were studied in PASMCs. We found that 15-HETE increased the ROCK activity after 24 h of stimulation, which was assessed by the amount of phospho-Thr853 in total MYPT1, a downstream target of ROCK (Fig. 6A, n = 3, P < 0.05). Furthermore, the expression was suppressed after serum deprivation, hypoxia and exogenous 15-HETE both induced ROCK II and ROCK I expression. These data suggest that the ROCK pathway is likely involved in the inhibitory effect of 15-HETE on PASMC apoptosis (Fig. 6B, n = 3, P < 0.05).
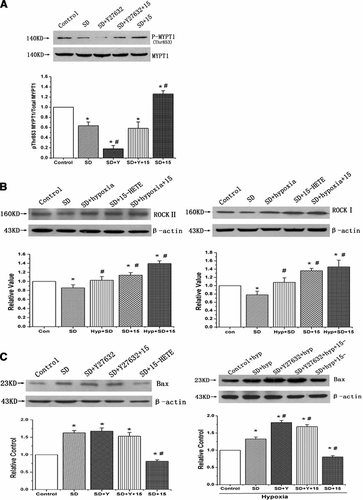
ROCK activity and the expression of ROCK II, ROCK I and Bax in PASMCs. A: MYPT1 phosphatase was examined with Western blot to represent the effects of 15-HETE and Y-27632 on ROCK activity. B: The expression of ROCK II (left) and ROCK I (right) in rat PASMCs. 15-HETE significantly increased the ROCK activity and induced the expression of ROCK II and ROCK I in PASMCs after serum deprivation. C: The expression of Bax under normoxic and hypoxic condition, respectively. Exogenous and endogenous 15-HETE both inhibited the expression of Bax, but the effects were partly inhibited by Y-27632 (ROCK inhibitor). The results mean 15-HETE decreases the expression of Bax in serum deprivation condition through ROCK pathway. “SD” means serum deprivation, “con” means control, “hyp” means hypoxia, “Y” means Y-27632, “15” means 15-HETE. All values are denoted as means ± SEM from three or more independent batches of cells (*P < 0.05 compared with Control; #P < 0.05 compared with SD).
The down-regulation of Bax expression induced by 15-HETE was blocked by ROCK inhibitor
To elucidate whether the ROCK pathway participates in the 15-HETE-induced down-regulation of Bax expression, we blocked ROCK with Y-27632. The inhibitory effect of exogenous 15-HETE on Bax expression was significantly diminished with 1 µM/L Y-27632 (Fig. 6C, left, n = 3, P < 0.05). Endogenous 15-HETE generated in hypoxic condition also suppressed the expression of Bax, while application of exogenous 15-HETE (1 µM/L) had no detectable effect on Bax expression after blocking the ROCK pathway with Y-27632 when compared with SD cells with Y-27632 (Fig. 6C, right, n = 3, P < 0.05).
Inhibition of caspase-3 activation induced by 15-HETE was rescued by ROCK blockade, and the effect of 15-HETE on caspase-3 protein level was likely post-transcriptional
Previous studies have suggested that 15-HETE inhibits the expression and activation of caspase-3 (Li et al., 2009). To understand whether the ROCK pathway plays a role in the inhibitory process, we analyzed the activity of caspase-3 as well as the expression of procaspase-3 and caspase-3. We found that SD promoted the cleavage of procaspase-3 and induced the expression of caspase-3 compared with that in culture medium, and 15-HETE significantly inhibited the cleavage of procaspase-3 and expression of caspase-3 (Fig. 7A,B, n = 3, P < 0.05). Similar results were obtained with the endogenous 15-HETE generated in hypoxic condition (Fig. 7C, n = 3, P < 0.05). The effects of exogenous and endogenous 15-HETE were reversed by ROCK inhibitor. We also determined whether ROCK suppression decreases the inhibitory effect of 15-HETE on the activity of caspase-3, when looking at caspase-3 activity in PASMCs. 15-HETE suppressed the activity of caspase-3 in comparison to SD, and the protective effect of 15-HETE was reversed by ROCK inhibitor (Y-27632) (Fig. 7D, n = 3, P < 0.05). Furthermore, we found there was nearly no change on the mRNA expression of caspase-3 (Fig. 7E, n = 3, P < 0.05). Thus, the effect of 15-HETE on caspase-3 protein level was likely post-transcriptional.
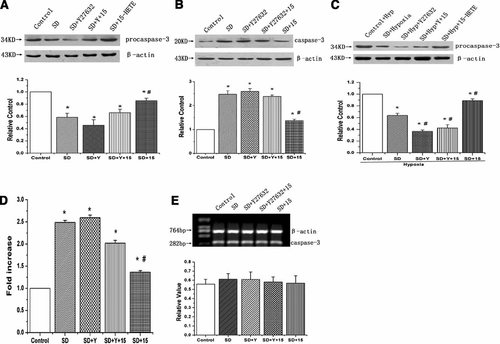
15-HETE suppresses the activity of caspase-3 and the cleavage of procaspase-3 through ROCK pathway. A: The expression of procaspase-3 in rat PASMCs under normoxic condition. B: The expression of caspase-3 under normoxic condition. C: The expression of procaspase-3 under hypoxic condition. 15-HETE inhibited the cleavage of procaspase-3 and the apoptosis induced by serum deprivation through ROCK pathway. D: caspase-3 activity was measured by cleavage of the Ac-DEVD-pNA substrate to pNA. E: The mRNA expression of caspase-3 in PASMCs. 15-HETE decreased the activity and expression of caspase-3 in PASMCs under serum deprivation condition via ROCK pathway, and the effect of 15-HETE on caspase-3 protein level was post-transcriptional. “SD” means serum deprivation, “Hyp” means hypoxia, “Y” means Y-27632, “15” means 15-HETE. All values are denoted as means ± SEM from three or more independent batches of cells (*P < 0.05 compared with Control; #P < 0.05 compared with SD).
15-HETE relieved mitochondrial depolarization and induced Bcl-2 expression in PASMCs after serum deprivation through ROCK pathway
JC-1 was used to assess the changes in mitochondrial membrane potentials. The quantitative analysis of JC-1-stained cells revealed a significant decrease in the red (high ΔΨm) to green (low ΔΨm) ratio in SD-treated cells when compared with control cells, which were cultured in the presence of 20% FBS (n = 10, P < 0.05). A treatment of SD cells with 15-HETE significantly increased the red fluorescence, while an exposure of the Y-27632-treated cells to 15-HETE suppressed the effect of 15-HETE and did not induce marked changes in ΔΨm in comparison to SD cells (Fig. 8A, n = 10, P < 0.05). The result showed that 15-HETE protected against SD-induced loss of ΔΨm and maintained mitochondrial integrity via ROCK pathway.
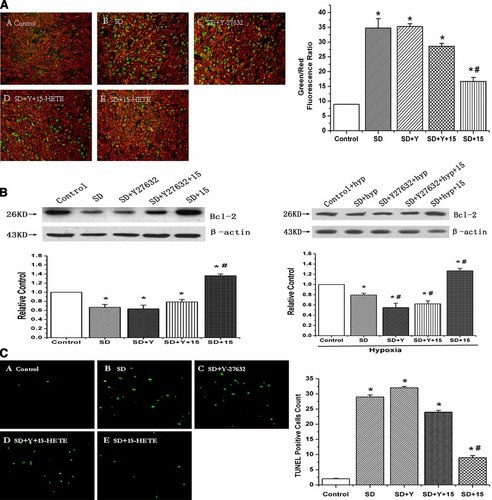
ROCK pathway mediates the inhibitory effect of 15-HETE on mitochondrial potentials reduction and the increase of TUNEL-positive cells in PASMCs. A: The cells were stained with JC-1 probe and imaged by a fluorescent microscope. The individual red and green average fluorescence intensities are expressed as the ratio of green to red fluorescence. The increase of fluorescence ratio, which is represented in the bars, is correlating with an increase in mitochondrial depolarization A-E, representative photographs of JC-1 staining in different groups. Quantitative analysis of the shift of mitochondrial green fluorescence to red fluorescence among groups. B: The expression of Bcl-2 in rat PASMCs under normoxic and hypoxic condition. The expression of Bcl-2 increased by exogenous 15-HETE or hypoxia-induced endogenous 15-HETE was partly inhibited by Y-27632 (ROCK inhibitor). C: Cells undergoing apoptosis were positively stained with TUNEL reagent and are shown in green. A–E: representative photographs of TUNEL staining in different groups. Quantitative analysis of TUNEL positive cells content among groups. “SD” means serum deprivation, “Y” means Y-27632, “hyp” means hypoxia, “15” means 15-HETE. All values are denoted as means ± SEM from ten independent photographs shot in each group. Significant differences are indicated as: *P < 0.05 compared with Control; #P < 0.05 compared with SD. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
We also examined the expression of Bcl-2, which is associated with mitochondrial function. We found that hypoxia and exogenous 15-HETE were able to induce the Bcl-2 expression, and ROCK signal transduction pathway participated in this process (Fig. 8B, n = 3, P < 0.05), consistent with the result of mitochondrial membrane potentials.
Inhibition of DNA fragmentation induced by 15-HETE was blocked by ROCK inhibitor
TUNEL assay was undertaken to determine whether ROCK pathway participated in the exogenous 15-HETE-inhibited DNA fragmentation of PASMCs. As shown in Figure 8C (n = 10, P < 0.05), the number of TUNEL-positive cells was significantly increased after SD for 48 h. In contrast, exogenous 15-HETE significantly decreased the number of TUNEL-positive cells induced by SD. However, the protective effect of 15-HETE was weakened after blocking ROCK pathway with Y-27632.
Discussion
Previous studies have suggested that hypoxia stimulates the production of endogenous 15-HETE in PASMCs (Zhu et al., 2003), and both endogenous and exogenous 15-HETE inhibit PASMC apoptosis (Li et al., 2009). The present study provides a new piece of evidence that 15-HETE, catalyzed by 15-LO-2, inhibits the apoptosis of PASMC to promote PA medial hypertrophy through ROCK pathway.
A major finding of the study is that 15-HETE is involved in PA vessel remodeling in vivo. We have previously demonstrated that 15-HETE causes vasoconstriction and inhibits the apoptosis of PASMCs (Zhu et al., 2003; Han et al., 2007; Li et al., 2009). In the present study, morphological evidence has been found to support the hypothesis that PA vessel remodeling induced by hypoxia in vivo is mediated by 15-LO/15-HETE pathway. We have found that chronic hypoxic exposure induces the mRNA and protein expression of 15-LO-1 and 15-LO-2, which are reversed by NDGA (a 15-LO inhibitor). Although there are data variations in quantitative immunostainings, we judge the approximate tendency of hypoxia and NDGA on the expression of 15-LO-1 and 15-LO-2 through strictly control of the staining time. Furthermore, to ascertain the tendency, we determine the protein and mRNA expression of 15-LO-1 and 15-LO-2 with Western blot and RT-PCR. We also find hypoxia-induced increase of medial wall thickening is reversed by NDGA, suggesting that hypoxia-induced PA remodeling is mediated by 15-HETE. Moreover, Our data prove that administration of NDGA to rats reduces the endogenous 15-HETE level in pulmonary arteries and the inhibition of endogenous 15-HETE decreases PA blood pressure (unpublished data), indicating that the PAH induced by hypoxia is relieved by blocking the generation of endogenous 15-HETE with NDGA in hypoxic rat models. To our knowledge, this result is the first effort to identify that hypoxic PA remodeling is mediated through 15-LO/15-HETE pathway.
As the major components of the pulmonary vascular media, PASMCs are the main contributors to medial wall thickening. It is believed that apoptosis plays a key role in the resolution of vascular remodeling (Gibbons and Dzau, 1994; Uhal, 2002). There are reports that the enhanced vascular smooth muscle cell proliferation or attenuated apoptosis has been attributed to regression in medial hypertrophy (Cowan et al., 1999, 2000). Moreover, we find down-regulation of caspase-3 and Bax expression and up-regulation of Bcl-2 in PA homogenates of rats exposed to chronic hypoxia. And the effects of hypoxia on the expression of apoptotic proteins are abolished after blocking 15-LO with NDGA. Taken together with the results of H&E, these results indicate that 15-HETE induces medial thickening by inhibiting apoptosis of PASMC. It is consistent with our previous report that 15-HETE inhibits the apoptosis in PASMC (Li et al., 2009).
Recent studies have indicated that RhoA/ROCK pathway contributed to the pathogenesis of chronic hypoxia-induced PAH in rodents, and ROCK is regarded as an important therapeutic target in PAH (Fagan et al., 2004; Nagaoka et al., 2005). It is reported that the expression of ROCK II and ROCK I are increased in chronic hypoxic lung (Jernigan et al., 2004; Nagaoka et al., 2005), and ROCK II is the predominant subtype of Rho-kinase expressed in the pulmonary artery and ROCK II expression is increased in chronically hypoxic rats PAs (Khan et al., 2005). In our study, we find that the effects of hypoxia on ROCK II and ROCK I expression are weakened in the presence of NDGA in PA homogenates of rats, and exogenous 15-HETE and endogenous 15-HETE induced by hypoxia significantly promote the expression of ROCK II and ROCK I. Furthermore, it has been proved that inhibition of ROCK pathway attenuated PAH in rats. Fasudil, a ROCK inhibitor, induces a marked improvement of medial wall thickening of pulmonary arteries by enhancing VSMC apoptosis and suppressing VSMC proliferation (Abe et al., 2004). Y-27632, another ROCK inhibitor, relieves the development of hypoxia-induced PAH in rats (Fagan et al., 2004). The evidence is in accordance with our hypothesis that 15-HETE inhibits the apoptosis in PASMC to induce pulmonary vessel remolding through Rho-Kinase pathway.
We further apply SD-induced apoptotic model to examine whether 15-HETE inhibits the apoptosis in cultured PASMCs through ROCK pathway. We have found that the effect of 15-HETE on cell viability is significantly blocked by the ROCK inhibitor (Y-27632). However, the protective effect of hypoxia on cell viability does not seem to be revealed in our study. A possible explanation is that the scan of PASMCs cultured in dishes for MTT experiment is about 5 × 104 to 1 × 105 per dish each time, different number of cells results in obviously different cell viability. We have done the MTT experiment between control and hypoxia and proved the protective effect of hypoxia on cell viability in PASMCs. We also have found that 15-HETE decreases the number of TUNEL-positive cells. And the inhibitory effect of 15-HETE on the apoptotic indication is attenuated by ROCK pathway inhibitor. These findings imply that inhibition of ROCK blocks the protective effect of 15-HETE on SD-induced apoptosis.
Previous reports have indicated that serum deprivation triggers apoptotic responses through mitochondrial pathway leading to the mitochondrial dysfunction. Detection of mitochondrial permeability events provides early indication of the initiation of cellular apoptosis (Salido et al., 2007; Wang et al., 2008). The inhibitory effect of 15-HETE on the SD-induced loss of membrane potential is blocked in the presence of Y-27632. 15-HETE also enhances the expression of Bcl-2, which is localized to the outer mitochondrial membranes and controls the stabilization of mitochondrial membranes. On the other hand, caspase family is central to the proteolytic events of apoptosis, especially caspase-3 (Porter and Janicke, 1999). In our studies, changes in activity of caspase-3, the protein expression of procaspase-3 and cleaved caspase-3 indicate that 15-HETE, through ROCK signal pathway, inhibits the SD-induced apoptosis by acting on a mitochondria-dependent ways in PASMCs.
Interestingly, we have found that hypoxia up-regulates the expression of 15-LO-1 and 15-LO-2 in both mRNA and protein levels in rat PA vessels. However, the cellular distribution of the 15-LO-1 and 15-LO-2 is different under hypoxic condition. 15-LO-1 mainly expressed in PA endothelia under normoxia is up-regulated by hypoxia, while 15-LO-2 is induced by hypoxia in PASMCs. We speculate that the cellular distribution of 15-LO-1 and 15-LO-2 has different pathophysiological effects on the development of hypoxic pulmonary arterial hypertension. Our previous studies have shown that 15-HETE prevents PASMC from apoptosis and the formation of endogenous 15-HETE is blocked by NDGA in PASMCs under hypoxic condition (Zhu et al., 2003; Li et al., 2009). In our present study, we have found that the medial thickening, which results from inhibiting PASMC apoptosis, is reversed by NDGA. These results imply that endogenous 15-HETE in PASMCs is catalyzed by 15-LO-2, which plays an important role in pulmonary vascular remodeling induced by hypoxia.
Taken together, our data indicate that hypoxia-induced PVR, an important change in PAH, is mediated by 15-LO/15-HETE pathway and endogenous 15-HETE, which is catalyzed by 15-LO-2, inhibits the apoptosis of PASMC to promote PA medial hyperplasia and hypertrophy through ROCK pathway. Although we have demonstrated ROCK pathway is involved in the inhibitory role of 15-HETE against PASMC apoptosis, further studies are needed to evaluate whether ROCK is activated by RhoA or others in PASMCs. In addition, PAEC, which is an alternative important component in the pathogenesis of PAR, should be addressed in future studies.
In conclusion, our results imply that ROCK signal pathway is involved in 15-HETE-induced pulmonary vessel remodeling in PAH induced by hypoxia. This study establishes signal transduction pathway and mechanism of 15-HETE-inhibited PASMC apoptosis. Furthermore, the regulation of Rho-kinase pathway by 15-HETE may be an important mechanism underlying the treatment of pulmonary artery hypertension and provide a novel therapeutic in sights for the future.
Acknowledgements
The excellent assistance of Chun Jiang made this work possible.




