NESCA: A new NEMO/IKKgamma and TRAF6 interacting protein†
Antonio Leonardi and Matilde Valeria Ursini contributed equally to this work.
Abstract
NEMO/IKKγ is the essential regulatory subunit of the IkB Kinase (IKK) complex, required for the activation of Nuclear Factor kB (NF-kB) in many physiological processes such as inflammation, immunity, apoptosis, or development. NEMO works at a converging point of the NF-kB pathway as it interacts with upstream signaling molecules to orchestrate its activation. Here we report on the identification of a novel NEMO-interacting protein, NESCA, an adapter molecule previously shown to be involved in the NGF-pathway via the TrkA receptor. We demonstrated that NESCA and NEMO interact by their N-terminal region. Beside to NEMO, we revealed that NESCA directly associates to the E3 ubiquitin ligase TRAF6, which in turn catalyzes NESCA polyubiquitination. Finally, we demonstrated that NESCA overexpression strongly inhibits TRAF6-mediated polyubiquitination of NEMO. In summary, our results highlight that NESCA represents a novel missing link in the NEMO-mediated NF-kB activation pathway. J. Cell. Physiol. 220: 410–417, 2009. © 2009 Wiley-Liss, Inc.
Abbreviations:
NEMO, NF-kB Essential MOdulator; NESCA, new molecule containing SH3 at the carboxy-terminus; IKK, IkB kinase; TNF, tumor necrosis factor; LPS, lypo-polisaccharide; TNF-R, TNF-receptor; TCR, T cell receptor; IL1R, interleukin-1 receptor; TLR, toll-like receptor; IRAK1, interleukin-1 receptor-associated kinase 1; MALT1, mucosa associated lymphoid tissue lymphoma translocation gene 1; Bcl10, B-cell leukemia/lymphoma 10; NGF, nerve growth factor; TrkA, tropomyosin-related kinase A; TRAF6, TNF receptor-associated factor 6; PKC, protein kinase C; HEK, human embryonic kidney; PCR, polymerase chain reaction.
NF-kB is a family of transcription factors that regulates the expression of a broad number of genes involved in immune response, cell survival, differentiation and proliferation (Hayden and Ghosh, 2008). The canonical pathway of NF-kB activation takes place through the activation of the IkB kinase (IKK) complex, that is composed by two catalytic subunits, IKKα and IKKβ, and the regulatory subunit NEMO/IKKγ which represents the point of convergence of most stimuli activating NF-kB (Yamaoka et al., 1998). NEMO contains two coiled-coil motifs, a leucine zipper, a C-terminal Zinc Finger domain and a recently characterized Ubiquitin-binding domain (Ea et al., 2006; Wu et al., 2006). These domains are required for the correct assembly of the IKK complex (Yamaoka et al., 1998) and for the recruitment of upstream signaling molecules, whose interaction with NEMO is essential for the IKK-mediated NF-kB activation. Indeed, numerous proteins producing either NF-kB activation (Leonardi et al., 2000; Poyet et al., 2000; Chariot et al., 2002) or inhibition (Zhang et al., 2000a; Kovalenko et al., 2003; Mauro et al., 2006) have been shown to interact with NEMO/IKKγ (Sebban et al., 2006). In addition to the physical interaction of NEMO with upstream molecules, a novel mechanism of IKK recruitment and activation is linked to the ability of NEMO to recognize polyubiquitinated signaling intermediates. This activity is required for NF-kB activation in many signaling pathways, such as TNF-R, TCR, or IL1R/TLR. Upon stimulation, K63-lynked polyubiquitination of downstream molecules such as RIP (Li et al., 2006; Wu et al., 2006), IRAK1 (Conze et al., 2008), MALT1 (Oeckinghaus et al., 2007), and Bcl10 (Wu and Ashwell, 2008), promotes the binding of NEMO to these molecules, thus determining the recruitment and activation of the IKK complex. Moreover, NEMO itself can undergo K63-lynked polyubiquitination (Tang et al., 2003; Zhou et al., 2004; Sebban-Benin et al., 2007), even if the functional significance of this latter modification has not been fully understood.
In the nervous system, the features of the canonical NF-kB activation cascade are conserved. Many of the stimuli activating NF-kB in the immune system, do so also in the nervous system (Mattson and Meffert, 2006; Memet, 2006). However, other stimuli specific of neuronal cells, such as NGF (Wood, 1995; Carter et al., 1996), glutamate (Guerrini et al., 1995; Kaltschmidt et al., 1995), amiloid β peptide (Behl et al., 1994), membrane polarization and sleep deprivation (Chen et al., 1999; Brandt et al., 2004), can activate NF-kB. NF-kB activation is involved in neuron survival, cognitive functions and behavior (Meffert and Baltimore, 2005) and also in regulating growth of neural processing in developing nervous system. Indeed, inhibition of NF-kB activity with super-repressor IkBα resulted to substantially reduce the complexity of neurite arbors of sensory neurons (Gutierrez et al., 2005). Moreover, neurite outgrowth during NGF-induced differentiation of PC12 cells requires several components of the NGF-induced NF-kB activating pathway, such as TRAF6 (Geetha et al., 2005), p62 (Wooten et al., 2005), or IKKβ (Azoitei et al., 2005).
Among the specific stimuli activating NF-kB in the nervous system, those emanating from the TrkA and p75NTR receptors in the NGF pathway are well characterized. Binding of NGF to TrkA induces dimerization (Khursigara et al., 1999), autophosphorylation (Friedman and Greene, 1999) and internalization to signaling vesicles (Riccio et al., 1997), which mediates NGF-induced differentiation (Zhang et al., 2000b). An important role in this process is played by the E3 ubiquitin ligase TRAF6. The interaction of TRAF6 with the adapter molecule p62 (Sanz et al., 2000) allows both the dimerization of the TrkA and p75NTR receptors (Wooten et al., 2001) and the TRAF6-mediated polyubiquitination of TrkA (Geetha et al., 2005). This latter event seems to be necessary for the receptor internalization and signaling to NF-kB (Geetha et al., 2005), but it is not clearly understood how the IKK complex can be recruited and activated.
To date, the molecular link(s) between NEMO activity and central nervous system function is still unclear. Mutations in the NEMO gene are the most common cause of Incontinentia Pigmenti, an X-linked pathology often associated with severe defects such as mental retardation, microcephaly, or seizueres (Smahi et al., 2000; Fusco et al., 2004, 2008) suggesting that NEMO has an important role in the nervous system development.
To get insight into the molecular mechanisms driving NF-kB activation through NEMO in the central nervous system, we searched for NEMO-interacting proteins in this tissue, via yeast two-hybrid. Here we show the identification of a new NEMO interacting protein, New molecule containing SH3 at carboxy-terminus (NESCA), which has been previously shown to bind to the TrkA receptor and to be necessary in the NGF-induced neurite growth of PC12 cells (MacDonald et al., 2004). We demonstrated that NESCA interacts with NEMO by its N-terminal region and that the NESCA-binding region on NEMO overlaps with the IKK-binding region, even if the interaction does not affect the IKK complex stability.
We also found that NESCA directly interacts by its N-terminal region with TRAF6 that in turn can catalyze its polyubiquitination. Finally, we demonstrated that NESCA overexpression completely abolishes the TRAF6-mediated polyubiquitination of NEMO.
Taken together, our results show that NESCA is a novel NEMO and TRAF6 binding protein that could represent a novel molecular link between NGF signaling and the IKK complex activity.
Materials and Methods
Cell culture and biological reagents
HEK293 cells were maintained in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 mg/ml streptomycin, and 1% glutamine. PC12 cells were grown in RPMI (Invitrogen) containing 10% horse serum, 5% fetal bovine serum, 1% glutamine, and antibiotics (100 U/ml penicillin, 100 mg/ml streptomycin).
Monoclonal and polyclonal antibodies against HA epitope and polyclonal antibodies against NEMO/IKKγ were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA). Monoclonal and polyclonal anti-FLAG antibodies coupled to agarose or not were purchased from Sigma (St. Louis, MO).
Plasmids
NESCA was amplified by PCR from a Human Fetal Brain cDNA library (Clontech, San Jose, CA) and cloned into pcDNA3.1-HA and -FLAG (Invitrogen) for expression in mammalian cells. NEMO/IKKγ, IKKβ, TRAF6, and ubiquitin expression vectors were previously described (Fusco et al., 2004; Mauro et al., 2006). NESCA, NEMO, and TRAF6 deletion mutants were prepared by conventional PCR and cloned into pcDNA3.1-HA or -FLAG vectors. Sequence of primers used in the preparation of all expression vectors, is available upon request ([email protected]).
Yeast two-hybrid screening
The cDNA encoding the N-terminal part of Human NEMO/IKKγ (amino acids 1–399) was cloned in-frame into the GAL-4 DNA-binding domain vector pGBKT7 (Clontech). The resulting plasmid pGBKT7-NEMO/IKKγ was used as a bait in a yeast two-hybrid screen of a Human Fetal Brain cDNA library (Clontech) in Saccharomyces cerevisiae strain AH109.
Transfection and immunoprecipitation assay
Lipofectamine or Lipofectamine 2000 mediated transfections were performed according to the manufacturer's instructions (Invitrogen). All transfections included supplemental empty vector to ensure that the total amount of transfected DNA was kept constant in each dish culture.
For immunoprecipitation of transfected proteins, HEK293 cells (4 × 106) were transiently transfected and 24 h after transfection cells were lysed in Triton X-100 lysis buffer (20 mM Hepes, pH 7.4, 150 mM NaCl, 10% glycerol, 1% Triton X-100, and Complete Protease Inhibitor mixture). After an additional 10 min on ice, cell extracts were centrifuged for 10 min at 14,000g at 4°C and supernatants were incubated for 4 h at 4°C with anti-FLAG antibodies bound to agarose beads (M2, Sigma). The immunoprecipitates were washed five times with Triton X-100 lysis buffer and subjected to SDS–PAGE.
In vivo ubiquitination assays
HEK293 cells (1 × 105) were co-transfected with expression vectors containing Epitope-tagged Ubiquitin, FLAG-NEMO/IKKγ, HA-NESCA and HA-TRAF6, in different combinations. Twenty-four hours after transfection, cell lysates were prepared as above, proteins were dissociated by heating for 5 min at 95°C in 1% SDS, samples were diluted 1:10 and immunoprecipitated with anti FLAG antibodies as described above. Immunoprecipitated extracts were analyzed for polyubiquitination of NEMO/IKKγ by Western blot with anti-HA-ubiquitin or anti-NEMO antibodies.
In vitro translation
In vitro transcription and translation were carried out with 1 µg of plasmid constructs according to the TNT Quick Coupled Transcription/Translation System protocol (Promega, Madison, WI). Proteins were immunoprecipitated as described above.
Results
NESCA interacts with NEMO by its N-terminal region
The molecular mechanism driving NF-kB activation through NEMO in the Central Nervous System is to date unclear. To get insight into how NEMO modulates the activation of NF-kB in this tissue, we used NEMO as a bait to screen a Human Fetal Brain library for new NEMO-interacting proteins, by the yeast two-hybrid system. Three out of fifty clones isolated encoded for overlapping fragments of NESCA, a protein recently shown to be involved in the NGF-induced differentiation of PC12 cells (MacDonald et al., 2004). Co-immunoprecipitation experiments in HEK293 cells confirmed the interaction between HA-NESCA and FLAG-NEMO (Fig. 1A). Moreover, confocal microscopy revealed that these two proteins co-localize in the cytoplasm of PC12 cells, even if NESCA seems to have also a nuclear localization (data not shown).
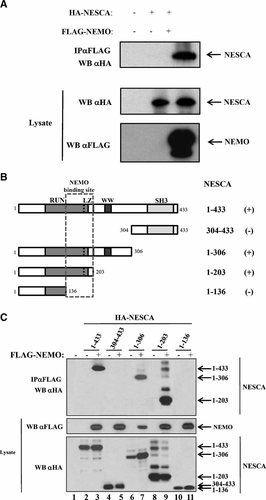
NESCA interacts with NEMO by its N-terminal region. A: HA-NESCA and FLAG-NEMO were co-transfected in HEK293 cells; cell lysates were immunoprecipitated with anti-FLAG antibodies. Immunoprecipitates were subjected to SDS–PAGE and subsequently to Western immunoblotting. B: Schematic representation of the NESCA deletion mutants used to map the NEMO-binding site (indicated in the dashed part). The interaction of each construct with NEMO is indicated with a plus. RUN, Leucine Zipper (LZ), WW and Src Homology 3 (SH3) domains are indicated. C: Mapping of the NEMO-interaction site; HEK293 cells were transfected with the indicated combinations of expression constructs encoding for FLAG-NEMO and HA-NESCA deletion mutants. Cells extracts were analyzed by immunoblotting either directly or after immunoprecipitation with anti-FLAG antibodies.
To define the region of NESCA that interacts with NEMO we tested various N- and C-terminal deletion mutants of NESCA in co-immunoprecipitation assays. FLAG-NEMO was transfected in HEK293 cells together with HA-NESCA or HA-NESCA-mutants lacking different regions of the protein (Fig. 1B). Data shown in Figure 1C indicate that the region necessary for the interaction with NEMO is located between amino acids 137 and 203 of NESCA; this region contains a Leucine-Zipper domain.
Mapping of the NESCA binding site on NEMO
In order to define the region of NEMO that is critical for the interaction with NESCA, several FLAG-tagged deletion mutants of NEMO (Fig. 2A) and HA-NESCA were co-transfected in HEK293 and their interaction was examined by co-immunoprecipitation assays. In this screening we found that only the constructs containing the region between amino acids 61–91, that is a part of the first coiled-coil motif of NEMO, were able to co-immunoprecipitate with NESCA (Fig. 2B), demonstrating that this region is essential for the association between the two proteins.
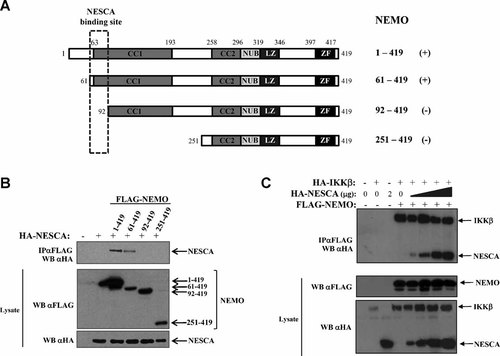
NESCA interacts with the IKK complex by the N-terminal region of NEMO. A: Schematic representation of the NEMO-deletion mutants tested for their ability to interact with NESCA. The interaction of each construct with NESCA is indicated with a plus. The NESCA-interaction site is indicated in the dashed square. Coiled-coil 1 (CC1), Coiled-coil 2 (CC2), NEMO-Ubiquitin Binding (NUB), Leucine Zipper (LZ), and Zinc Finger (ZF) domains are shown. B: HEK293 cells were transiently transfected with the indicated FLAG-tagged NEMO deletion mutants and HA-NESCA; cell extracts were immunoprecipitated with anti-FLAG antibodies and immunoblotted to assess the ability of NESCA to co-immunoprecipitate with each deletion mutant. C: Increasing amounts of HA-NESCA (0.1–2 µg) were transfected in HEK293 cells together with FLAG-NEMO and HA-IKKβ. Cell lysates were generated after 24 h and subjected to direct Western blotting or immunoprecipitated with anti-FLAG antibody. Immunoprecipitates were immunoblotted with anti-HA antibodies.
As the NESCA-binding site overlaps with the IKK-binding region of NEMO (Leonardi et al., 2000), we tested if the interaction between NEMO and NESCA can interfere with the stability of the IKK complex. We co-transfected increasing amounts of HA-NESCA together with FLAG-NEMO and HA-IKKβ and we examined their interaction. NEMO was able to interact with both IKKβ and NESCA in all experimental points (Fig. 2C), demonstrating that the binding to NESCA does not disrupt the IKK complex.
NESCA directly associates with TRAF6 by its N-terminal region
Adapter molecules are generally involved in regulating the dynamics of molecular interactions. Since NESCA represents a new adapter in the NGF-pathway (MacDonald et al., 2004), we sought to verify the presence of other NESCA-interacting proteins belonging to the NGF-mediated NF-kB pathway. We found that NESCA is able to co-immunoprecipitate with TRAF6 when both proteins were overexpressed in HEK293 cells (Fig. 3A). This result was further confirmed by using in vitro translated proteins, demonstrating the direct interaction between NESCA and TRAF6 (Fig. 3B).
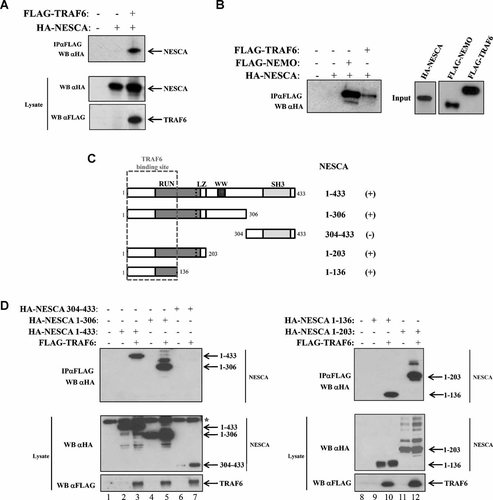
NESCA directly binds to TRAF6 by its N-terminal region. A: HEK293 were transfected with the indicated constructs encoding for HA-NESCA and FLAG-TRAF6. Cells were lysed and immunoprecipitated with anti-FLAG antibodies. Bound proteins were subjected to SDS–PAGE and immunoblotted with anti-HA antibodies. B: In vitro translated HA-NESCA and FLAG-NEMO or FLAG-TRAF6 were incubated 4 h with anti-FLAG antibodies. Immunoprecipitates were subjected to anti-HA Western blotting. C: Schematic representation of the NESCA-deletion mutants tested for their binding to TRAF6. Mutants able to interact with TRAF6 are indicated with a plus. Dashed square indicates the TRAF6-interaction site. RUN, Leucine Zipper (LZ), WW, and Src Homology 3 (SH3) domains are indicated. D: HEK293 cells were transfected with the indicated combination of HA tagged NESCA-deletion mutants and FLAG-TRAF6. FLAG-TRAF6 was immunoprecipitated with anti-FLAG antibodies and bound proteins were revealed by anti-HA Western blotting. *Non-specific bands.
To map the region of NESCA responsible for the interaction with TRAF6, we co-transfected different deletion mutants of NESCA (Fig. 3C) together with FLAG-TRAF6 in HEK293 cells. Co-immunoprecipitation assays revealed that the N-terminal region of NESCA is required for the binding to TRAF6 and in particular the first 136 amino acids of NESCA are sufficient to produce this interaction (Fig. 3D).
NESCA is polyubiquitinated by TRAF6
Polyubiquitination of signaling intermediates of the NF-kB pathway has been shown to be an essential modification occurring upon stimulation of many external triggers (Chen, 2005; Perkins, 2006), including the NGF. Because NESCA is able to interact with TRAF6, we sought to verify whether it undergoes polyubiquitination. To test this hypothesis we transfected HEK293 cells with NESCA in the presence of exogenous ubiquitin, revealing that NESCA undergoes polyubiquitination (Fig. 4, lane 4) and that this modification was strongly enhanced in the presence of TRAF6 (Fig. 4, lane 5). Under stringent SDS-denaturing conditions, TRAF6 did not co-immunoprecipitate with NESCA, excluding the possibility that the polyubiquitination observed was the result of auto-ubiquitinated TRAF6 (not shown). In addition, a dominant negative mutant of TRAF6 abolished the polyubiquitination of NESCA (Fig. 4, lane 6), indicating that TRAF6 acts as an E3 ubiquitin ligase for NESCA.
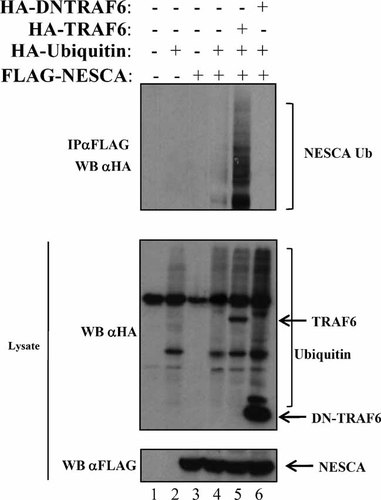
NESCA undergoes TRAF6-mediated polyubiquitination. A: HEK293 were transfected with the indicated plasmids encoding FLAG-NESCA with or without HA-Ubiquitin, HA-TRAF6 and HA-Dominant Negative TRAF6 (DNTRAF6). Cells extracts were boiled in 1% SDS for 15 min, immunoprecipitated with anti-FLAG antibodies and subjected to SDS–PAGE. Ubiquitin-bound NESCA was revealed by anti-HA Western blotting.
NEMO ubiquitination is affected by NESCA overexpression
NEMO is a well characterized target for TRAF6-mediated polyubiquitination (Sebban-Benin et al., 2007). Because NESCA interacts with both NEMO and TRAF6, we wondered whether NESCA has a role in the process of polyubiquitination of NEMO. To test this hypothesis we transfected FLAG-NEMO in the presence of HA-Ubiquitin and HA-TRAF6 without (Fig. 5A, lane 4) or with HA-NESCA (Fig. 5A, lane 5). This experiment revealed that the overexpression of NESCA completely abolished the TRAF6-mediated polyubiquitination of NEMO, as it appears by using either anti-HA (Fig. 5A) or anti-NEMO (Fig. 5B) antibodies on immunoprecipitated extracts.
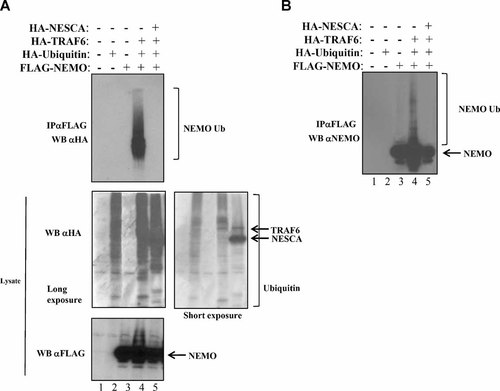
NEMO polyubiquitination is abolished in the presence of NESCA. A: HEK293 cells were transfected with the indicated combination of constructs encoding FLAG-NEMO, HA-Ubiquitin and HA-TRAF6 with or without HA-NESCA. After boiling in 1% SDS, cell extracts were immunoprecipitated with anti-FLAG antibodies. Ubiquitin-bound NEMO was revealed by Western blotting using both anti-HA or (B) anti-NEMO antibodies.
TRAF6 is a well known activator of NF-kB and its overexpression results in both polyubiquitination of signaling proteins and NF-kB activation (Sun et al., 2004; Sebban-Benin et al., 2007; Conze et al., 2008). In our experiment, although the overexpression of NESCA is able to abolish the polyubiquitination of NEMO, this event does not seem to correlate with an impaired NF-kB activation, measured by an NF-kB driven luciferase plasmid transfected in each experimental point (data not shown).
Discussion
In the present study we report on a novel NEMO and TRAF6 interacting protein, NESCA, a molecule previously shown to bind to the TrkA receptor and to be important in the NGF-mediated neurite growth of PC12 cells (MacDonald et al., 2004). NF-kB is one of the transcription factors activated upon NGF stimulation, whose activity is necessary for neuronal differentiation (Carter et al., 1996; Foehr et al., 2000a). Several molecules have been shown to belong to the NGF-induced NF-kB pathway, such as the E3 ubiquitin ligase TRAF6 (Khursigara et al., 1999), the adapter molecule p62 (Wooten et al., 2005), and the PKC atypical kinases (Wooten, 1999; Wooten et al., 2001). Albeit the effects of overexpression or downregulation of these molecules impact on neuronal survival or differentiation and on NF-kB activation (Foehr et al., 2000b; Yeiser et al., 2004; Joung et al., 2005), it is not known how these molecules can recruit and regulate the IKK complex. NESCA represents (one of) the molecular link(s) connecting the IKK complex to upstream molecules. Because it is able to bind to the TrkA receptor (MacDonald et al., 2004) and also to TRAF6 and to NEMO, NESCA could play a role as a novel central adapter in the NGF-induced NF-kB pathway. This raises the question of which is the functional role of NESCA in such pathway. In general, NF-kB signaling requires adapter molecules, such as RIP1, which binds to NEMO to recruit the IKK complex to the receptor and therefore to induce its activation (Poyet et al., 2000). A central event in this process is the polyubiquitination of RIP upon TNFα stimulation. Therefore, polyubiquitinated RIP provides a platform for the recruitment and modulation of the IKK complex through the Ubiquitin-binding domain of NEMO (Ea et al., 2006; Li et al., 2006; Wu et al., 2006). One can imagine that NESCA may operate in a way quite similar to RIP. This hypothesis is supported by some experimental findings: first, despite its binding to the region between amino acids 61–91 of NEMO, that overlaps with the IKK-binding region, NESCA does not disrupt the IKK complex, considering that NEMO can interact with both NESCA and IKKβ simultaneously (Fig. 2C) and that IKKβ can co-immunoprecipitate with NESCA only in the presence of NEMO (not shown). Second, NESCA can be polyubiquitinated by TRAF6, because we observed that TRAF6 overexpression strongly enhances the polyubiquitination of NESCA (Fig. 4). Third, polyubiquitinated forms of NESCA are able to co-immunoprecipitate with NEMO, as it appears either by transfecting HA-NESCA and exogenous ubiquitin (not shown), or by transfecting a deletion mutant of NESCA (HA-NESCA 1-203) that seems to be constitutively ubiquitinated (Fig. 1C, lane 9). These data suggest that NESCA could be poly-ubiquitinated by TRAF6 and that this event could stimulate the IKK complex recruitment.
Which is the functional significance of the NESCA–IKK complex binding? Because NESCA can bind to both NEMO and TRAF6, we wondered whether polyubiquitination of NEMO could be altered by the overexpression of NESCA. Surprisingly, NESCA overexpression completely abolishes the TRAF6-dependent NEMO polyubiquitination, without producing any change of the NF-kB activity, measured by luciferase transcription assay. The polyubiquitination of NEMO has been generally accepted to have a positive effect on the activation of the IKK complex (Tang et al., 2003; Zhou et al., 2004; Yamamoto et al., 2006), although some recent papers bring this thesis into question. Indeed, it has been shown that a point mutation in the C-terminal region of NEMO (K392R) results in a defective LPS-induced NEMO polyubiquitination, without affecting the activation of NF-kB (Ni et al., 2008). Moreover, another C-terminal NEMO mutant (K399R) shows a defective CARMA1-Bcl10-Malt1 induced polyubiquitination, even if this mutation has only a slight effect on inducible NF-kB activation in T cells (Oeckinghaus et al., 2007). These last data are in agreement with our findings suggesting that additional and not yet clearly understood mechanisms of IKK complex regulation through the polyubiquitination of NEMO may occur.
In summary, we have identified NESCA as a novel adapter involved in the NF-kB pathway. In addition to its ability to bind to the TrkA receptor (MacDonald et al., 2004), NESCA can also bind to NEMO and to TRAF6, which in turn catalyzes the polyubiquitination of NESCA. The functional consequence of these interactions is that NESCA could recruit the IKK complex and regulate the levels of NEMO polyubiquitination, even if the functional significance of this latter event in the NGF or other signaling pathways remains to be estabilished.
Acknowledgements
We would like to give our thanks to Dr. M. G. Miano, Dr. A. Pescatore and Dr. C. Mauro for helpful discussion.




