Quiescent epithelial cell rests of Malassez can differentiate into ameloblast-like cells
Abstract
Epithelial cell rests of Malassez (ERM) are quiescent epithelial remnants of Hertwig's epithelial root sheath (HERS) that are involved in the formation of tooth roots. After completion of crown formation, HERS are converted from cervical loop cells, which have the potential to generate enamel for tooth crown formation. Cervical loop cells have the potential to differentiate into ameloblasts. Generally, no new ameloblasts can be generated from HERS, however this study demonstrated that subcultured ERM can differentiate into ameloblast-like cells and generate enamel-like tissues in combination with dental pulp cells at the crown formation stage. Porcine ERM were obtained from periodontal ligament tissue by explant culture and were subcultured with non-serum medium. Thereafter, subcultured ERM were expanded on 3T3-J2 feeder cell layers until the tenth passage. The in vitro mRNA expression pattern of the subcultured ERM after four passages was found to be different from that of enamel organ epithelial cells and oral gingival epithelial cells after the fourth passage using the same expansion technique. When subcultured ERM were combined with subcultured dental pulp cells, ERM expressed cytokeratin14 and amelogenin proteins in vitro. In addition, subcultured ERM combined with primary dental pulp cells seeded onto scaffolds showed enamel-like tissues at 8 weeks post-transplantation. Moreover, positive staining for amelogenin was observed in the enamel-like tissues, indicating the presence of well-developed ameloblasts in the implants. These results suggest that ERM can differentiate into ameloblast-like cells. J. Cell. Physiol. 217: 728–738, 2008. © 2008 Wiley-Liss, Inc.
Epithelial cell rests of Malassez (ERM) are located in the periodontal ligament tissue (PDL) near the developed tooth-root and are derived from the Hertwig's epithelial root sheath (HERS) fragments during advancing root development. ERM form rests in the PDL around all teeth (Lester, 1969; Thomas, 1995; Beertsen et al., 1997). HERS play a role in the induction and differentiation of dental pulp cells into odontoblasts. In addition, HERS continue to proliferate for a limited time and control tooth-root formation (Thomas, 1995). HERS are developed when the cervical loop changes its developmental fate into tooth root. At that time, the stellate reticulum is degenerated and subsequently the inner and outer enamel epithelium are fused into a double layered epithelium known as Hertwig's epithelial root sheath (HERS) (Thomas, 1995; Tummers and Thesleff, 2003). During tooth-crown formation, the cervical loop consists of a basal layer of epithelium. The outside layer is known as the outer enamel epithelium and the inside layer as the inner enamel epithelium. The dental epithelial cells in the cervical loop differentiate into ameloblasts through the inner enamel epithelium (Harada et al., 2006) and subsequently generate enamel tissue by interaction with dental papilla cells derived from neural crest cells (Chai et al., 2000). After formation of the tooth crown, which is composed of an enamel-dentin complex, is completed, tooth-root formation begins.
Thus, one of the key features of tooth-root initiation is a structural change from the cervical loop to the HERS. Generally, no new ameloblasts or enamel are generated from the dental epithelium after the HERS is formed; however, after the root dentin forms, the HERS starts to disintegrate and secrete enamel-related proteins onto the newly formed root dentin surface. At that time, dental follicle cells are able to migrate on the dentin surface through the disintegrated regions of the HERS, and then differentiate into cementoblasts (Slavkin et al., 1989). It is known that ERM can be induced to differentiate into cementoblasts (Thomas, 1995; Bosshardt and Schroeder, 1996) because the disruption of Patched, the hedgehog receptor, leads to shorter roots (Nakatomi et al., 2006).
It has previously been concluded that ERM in PDL must be completely quiescent (Grupe et al., 1967). This conclusion is based on the morphology of their mitochondria and the scarcity of Golgi complexes by TEM analysis (Valderhaug and Nylen, 1966). It has been suggested that ERM might prevent resorption of the root surface (Loe and Waerhaug, 1961). Although these results indicate that in a healthy tooth, ERM are in the G0 phase of the cell cycle, ERM can be stimulated to proliferate in response to injury in rats (Talic et al., 2003). It has been suggested that ERM have the potential to differentiate in two different directions: squamous metaplasia and formation of cystic lesions, and ameloblastic differentiation during the formation of odontogenic tumors (Buchner and Sciubba, 1987; Hamamoto et al., 1998). These results suggest that some ERM are mitotically active (Trowbridge and Shibata, 1967; McCulloch and Melcher, 1983).
In addition, when the ERM are explanted in vitro, they actively proliferate and grow readily upon structures (Brunette, 1984b). Cell culture studies have shown that mechanical stretching, as well as elevation of the intracellular levels of cyclic AMP, increase the growth of ERM (Brunette, 1984a). These results indicate that ERM have the potential to proliferate if there are appropriate extracellular signals, such as mitogens, and are able to enter into the cell cycle. However, the mechanism by which ERM are stimulated to proliferate is not fully understood.
To date there are several reports which show that ERM have the potential to grow in vitro (Mizuno et al., 2005; Shimonishi et al., 2005), however there have been no reports on the in vivo behavior of subcultured ERM. For example, no studies have examined whether ERM are capable of forming normal tooth-tissue. Previously, it has been reported that removal of stem cells from their natural milieu may change their differentiation properties (Bissell and Labarge, 2005). Since the ERM are a direct lineage from the HERS derived from the enamel organ through the cervical loop structures, we hypothesize that the ERM retain their original ability to secrete a matrix conducive to generating enamel in a fashion similar to the enamel organ in the crown formation stage. To investigate this theory we established a cell culture system for ERM in order to preserve as many as possible of the original cellular characteristics. We then designed our study to examine the tissue-forming capability of ERM.
Recently, we developed a cell culture system using a 3T3-J2 feeder cell layer for enamel organ epithelial cells (EOE) derived from porcine tooth buds at the crown formation stage (Honda et al., 2006). 3T3-J2 cells are able to maintain the activity of primary EOE cells. After subculture of the EOE cells, it was determined that these cells expressed ameloblast-related genes by RT-PCR and Western blot analysis. Furthermore, when the subcultured EOE cells are combined with the primary dental pulp cells, enamel-dentin complex structures are produced at 4 weeks post-transplantation (Honda et al., 2007a). The feeder cell layer technique has been widely applied to the skin (Rheinwald and Green, 1975) and oral gingival epithelial cells (OGE) (Hata et al., 1995). We compared the cell characteristics of ERM, EOE, and OGE subcultured cells using the feeder cell layer technique. The subcultured ERM demonstrated the potential to differentiate into ameloblast-like cells and generate enamel-like tissues in combination with primary dental pulp cells in vivo.
Materials and Methods
Localization of epithelial cell rests of Malassez in porcine deciduous incisor teeth
A segment of porcine mandible containing one deciduous incisor tooth was obtained from a 6-month-old pig. The specimens were fixed in 4% PFA, decalcified for 2 months, and embedded in paraffin. They were then examined histologically or by immunohistochemistry to detect the presence of epithelial cell rests of Malassez.
Isolation and subculture of porcine epithelial cell rests of Malassez (ERM)
The three types of epithelial cells [epithelial cell rests of Malassez (ERM), enamel organ epithelial cells (EOE), oral gingival epithelial cells (OGE)] and periodontal ligament cells (PDLC) were prepared from the same porcine mandible in approximately 6-month-old pigs. These cells were used to examine the characteristics of ERM.
The methods for culturing ERM and PDLC from porcine PDL were as described previously (Brunette et al., 1976). Briefly, the deciduous incisor teeth were removed from the mandibles and then the PDL were removed from the middle one-third of the incisor tooth roots. The PDL cells were placed in culture dishes as explant cultures with Dulbecco's modified Eagle's medium (DMEM; Kohjn bio, Saitama, Japan) supplemented with 10% fetal bovine serum (FBS, Nichirei, Tokyo, Japan) and 100 U/ml penicillin/streptomycin. The medium was changed every 3 days and the explants were cultured at 37°C under 10% CO2 in air. ERM and PDLC were grown from the explants. Before the mixed cells reached confluence, the medium was replaced with non-serum epithelial cells selective medium, LHC-9 (Biofluids, Bethesda, MD) including antibiotics. After substitution of non-serum medium, the PDLC were detached from the culture dish and the ERM subsequently survived. However, the growth of the ERM in non-serum medium was quite slow. ERM were then subcultured on 3T3-J2 feeder cell layers with complete-MEM medium as previously described (Hata and Ueda, 1996; Honda et al., 2007a). Subcultured ERM were passaged and this was repeated ten times with feeder cell layers. The experiments were repeated three times with the cells derived from deciduous incisor teeth from three different donors.
In order to obtain the PDLC for use as controls, a mixture of PDLC and ERM were cultured from PDL explants in DMEM plus 10% FBS with antibiotics. After mixed cultures were passaged, PDLC alone were subcultured by altering the culture conditions to select against the ERM. The other controls, EOE and OGE, were obtained as described previously. Briefly, EOE were obtained from porcine third molar tooth buds at an early stage of crown formation and an enamel organ was separated from the dental mesenchyme. Enamel organ tissues were minced and the dissociated enamel organ epithelial cells were seeded in DMEM medium supplemented with 10% FBS (Nichirei) and 100 U/ml penicillin/streptomycin (Den Besten et al., 1998; Honda et al., 2007a). Porcine oral gingival epithelium was cut from the gingiva of the mandible and put on the culture dish. The OGE were grown from the explant tissue (Hata and Ueda, 1996). Both epithelial cell types were trypsinized and inoculated (1 × 105 cells/cm2; as first passage cells) over a feeder cell layer using complete-MEM medium until the fourth passage.
Preparation of 3T3-J2 feeder cell layer
The 3T3-J2 cells (a gift from Dr. H. Green, Harvard Medical School) were prepared as previously described (Hata and Ueda, 1996; Honda et al., 2007a). Briefly, 3T3-J2 cells were treated with 4 µg/ml mitomycin C (WAKO, Osaka, Japan) in DMEM (Khojin bio, Saitama, Japan) without serum to suppress self-proliferation for four hours, then washed with Dulbecco's phosphate buffered saline (PBS (-), Invitrogen) to exclude the mitomycin C before seeding the epithelial cells. The treated 3T3-J2 cells play the role of the feeder layer and inhibit the growth of any other contaminating fibroblasts.
Reverse transcription-polymerase chain reaction (RT-PCR) analysis
TRIZOL reagent (Invitrogen) was utilized to isolate total cellular RNA from the enamel organ, dental pulp, PDL, subcultured ERM (passage1, 4, and 10), EOE (passage 4), OGE (passage 4), and PDLC (passage 4). Five micrograms of RNA was used per reaction and reverse transcribed with specific primers (SuperscriptTM First-Strand Synthesis System for RT-PCR; Invitrogen). For PCR amplification of cytokeratin2 and desmoglein1 as epithelial cell markers, periostin and alkaline phosphatase (ALP) as dental mesenchymal cell markers, amelogenin, ameloblastin enamelin, matrix metalloproteinase 20 (MMP-20), tuftelin, kallikrein 4 (Nagano et al., 2003) (KLK4) as ameloblast-related markers, EGFR, FGFR as dental epithelial marker and GAPDH for an internal control, we used primer sequences which are listed in Table 1. Amplification products were run on a 1.5% agarose gel, and the gels were visualized by ethidium bromide staining.
| Primer | Sequence (5′–3′) | Product size (bp) | Annealing temp. (°C) | Cycles | Accession#/reference |
|---|---|---|---|---|---|
| Cytokeratin 2 | S-AAGAGCACATGGCAGGACTT | 154 | 59 | 25 | DN837335 |
| A-CTTGGAGGTGGCTTCAGTTC | |||||
| desmoglein1 | S-AAGGTTCTGGCTGGGAAGAT | 443 | 60 | 35 | NM001035535 |
| A-AACCCCTGAGTTGTCAATGC | |||||
| Periostin | S-CTGCACATGCAAGGATGAG | 589 | 54 | 25 | AY880669 |
| A-ACATGGAGTTTCCCAGGCTA | |||||
| ALP | S-TCGACCACAGGGTAGGTTTC | 221 | 55 | 25 | AY145131 |
| A-CCCTGCAGTTAGGACTGGC | |||||
| Amelogenin | S-CCTGCCTTTTGGGAGCA | 328 | 50 | 30 | NM213800 |
| A-TGGTGGTGTTGGGTTGGA | |||||
| Ameloblastin | S-ATTCCCAACCTGGCAAGAGG | 380 | 55 | 30 | NM214037 |
| A-AGCGCTTTTAATGCCTTTGC | |||||
| Enamelin | S-TGAGGAGATGATGCGCTATG | 315 | 45 | 30 | NM214241 |
| A-TGAGGTGTCTGGGTTTCCTC | |||||
| MMP20 | S-ATGACTCCTGCAGAAGTGGACA | 289 | 48 | 30 | NM213905 |
| A-AGGTCCGACAGTGCCTGGA | |||||
| Tuftelin | S-AAGTCAGGAGCTGCAGAAG | 392 | 57 | 30 | AF030318 |
| A-GCTGAAGTTGCCATGACTGA | |||||
| KLK 4 | S-ACCATGGGCGAGGACTGCAA | 429 | 57 | 30 | Nagano et al. (2003) |
| A-GTTAACTGGCCTGGATGGTCG | |||||
| GAPDH | S-GGGCATGAACCATGAGAAGT | 497 | 56 | 25 | AF17079 |
| A-CCCCAGCATCAAAGGTAGAA |
- S, sense; A, antisense; ALP, alkaline phosphatase; MMP 20, matrix metalloproteinase 20; KLK 4, kallikrein 4; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Cell growth analysis
The growth of subcultured ERM was compared with that of the subcultured EOE. Subcultured ERM and EOE (1 × 104 cell/cm2) were seeded onto the feeder cell layer (80% confluency) in 12-well culture dishes independently. To count cell numbers, the cultures were first treated with PBS containing 0.2% versene (GIBCO, Grand Island, NY) in order to eliminate the feeder layer cells. The epithelial cells were trypsinized with 0.25% trypsin/0.04% EDTA and the detached cell numbers were counted using a hemocytometer at days 0, 7, and 14 independently. As another control to evaluate the growth of ERM on feeder cell layers, the ERM were subcultured on collagen type I-coated dishes (Asahi Techno Glass, Chiba, Japan) without the feeder cell layer.
Statistical analysis was performed using the Student's t-test. Error bars represent the mean ± standard deviation for three separate experiments. Asterisks indicate a significant difference (P < 0.05) between paired conditions.
Co-culture with ERM and primary dental pulp cells in vitro
To determine whether ERM were able to differentiate into ameloblasts, the subcultured ERM were co-cultured with the secondary subcultured dental pulp cells in vitro. Primary dental pulp cells were obtained from porcine third molar tooth buds as described previously (Honda et al., 2007a). To prevent contamination with enamel organ epithelial cells in the dental pulp, we carefully peeled off the enamel organ epithelium and dental follicle. We then we cut off the edge of the dental pulp and removed the small mass of dental pulp in the center area of the dental pulp tissue. The small mass of dental pulp was then dissociated into a single cell suspension using collagenase (WAKO). Thereafter, the subcultured ERM (passage 4) and subcultured dental pulp cells (passage 2) were seeded onto a cover glass (5 × 103 cells/cm2) and co-cultured for 4 weeks. For controls, subcultured ERM alone with or without feeder layer cells were prepared. Complete MEM medium containing 10% FBS was used in the co-culture experiments and changed every 2 days.
To test for contamination with EOE cells, we observed the dental pulp cells after isolation by phase-contrast microscopy and performed RT-PCR analysis of the isolated dental pulp cells. The experiments were performed in triplicate.
Immunocytochemistry
Subcultured cells were fixed with 4% paraformaldehyde and were then permeabilized with 0.1% TritonX-100 (Sigma–Aldrich, St. Louis, MO). Cells were then incubated in blocking solution (PBS containing 3% BSA) and the immunocytochemical reaction was performed using anti-cytokeration14 (CK14) mouse monoclonal antibody (1:50 dilution; Chemicon International, Temecula, CA), anti-amelogenin rabbit polyclonal antibody (1:1,000 dilution; gift from Dr. J. P. Simmer and Dr. Yamakoshi) and anti-vimentin mouse monoclonal antibody (1:500 dilution; NeoMarkers, Westinghouse, CA) as primary antibodies for mesenchymal cell markers. They were incubated for 20 min at room temperature. The cells were then incubated in Rhodamine-conjugated secondary antibody (Chemicon International) for CK14 and vimentin, and FITC-conjugated secondary antibody (ICN Pharmaceuticals, Inc., Costa Mesa, CA) for amelogenin and vimentin, both diluted 1:200 in PBS containing 1% BSA, for 20 min at room temperature. All samples were sealed with VectaShield containing DAPI diluted 1:2,000 for nuclear staining. Non-immune 1% BSA was used instead of primary antibody as a control.
Preparation of collagen sponge scaffolds
The collagen sponge scaffolds were prepared for the in vivo transplantation study, as we had previously demonstrated that collagen sponge scaffolds (product number, CL025-PH56/FD90H48-02F26; gift from NIPRO Corporation, Osaka, Japan) promote odontogenesis in tooth bud cells (Sumita et al., 2006; Honda et al., 2007b). Briefly, the scaffolds, approximately 10 mm in diameter and 2 mm in thickness, were prepared from a 2.5% aqueous solution of collagen extracted from porcine skin. The scaffolds were composed of 75% (dry weight) type I atelocollagen and 25% type III atelocollagen and were frozen at −40°C and dried in a vacuum to produce a porous matrix (pore volume fraction, 97.5%). Before use the scaffolds were sterilized with γ-radiation at 25 kGy.
Combination study with ERM and dental pulp cells in vivo
All experiments involving the use of animals were approved by the Institutional Animal Care and Use Committees of the Institute of Medical Science at the University of Tokyo, Japan.
We examined whether subcultured ERM had the potential to generate enamel tissue in vivo (Fig. 1). Primary dental pulp cells were taken from porcine third molar tooth buds using the same methods as described above for in vitro co-culture analysis. The cell-seeding technique was described previously (Honda et al., 2007a). Briefly, the primary dental pulp cells were plated (5 × 106cells/scaffold) on the collagen sponge for 30 min on ice and then the subcultured ERM cells (passage 4: 5 × 106 cells/scaffold) were seeded on the top of dental pulp cells. The seeded scaffolds were transplanted into athymic rat omentum at 5–7 weeks of age (F344/n Jcl-rnu, Nihoncrea, Tokyo, Japan) (n = 7). The subcultured OGE cells (passage 4: 5 × 106 cells/scaffold) were combined with fresh dental pulp cells (5 × 106cells/scaffold) in collagen sponge (n = 3) and transplanted into the omentum as a control.
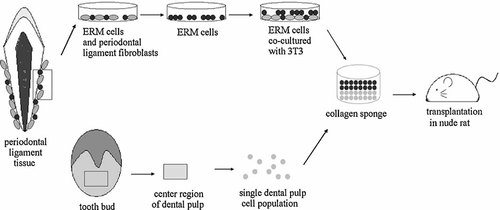
A flow diagram represents the in vivo analysis of enamel growth. The periodontal ligament tissue (PDL) on the root surface was obtained and cultured by explant culture. After the epithelial cell rest of Malassez (ERM) and the periodontal ligament cells (PDLC) were expanded in culture, the ERM were selected using non-serum medium. The subcultured ERM and primary dental pulp cells were seeded onto collagen sponge and transplanted into the rat omentum.
The implants in the experimental group were obtained at 2 weeks (n = 2), 4 weeks (n = 3) and 8 weeks (n = 2) after transplantation. In the control group, the implants were removed after 4 weeks. Both implants were fixed in Bouin's solution and were decalcified and then embedded in paraffin. Five-micrometer sections were cut and stained with haematoxylin and eosin for histological analysis.
Immunohistochemical analysis
Immunohistochemical analyses were performed on paraffin-embedded tissue sections by means of a Vectastain ABC kit (Vector Laboratories, Inc., Burlingame, CA). The following primary antibodies were used: anti-cytokeration14 (CK14) mouse monoclonal antibody (dilution of 1:50; Chemicon International), anti-vimentin mouse monoclonal antibody (dilution of 1:500; NeoMarkers), anti-amelogenin rabbit polyclonal antibody (dilution of 1:1,000), and anti-DSP chick polyclonal antibody (dilution of 1:1,000, gift from Dr Simmer and Yamakoshi, University of Michigan, USA). Standard procedures (Hsu et al., 1981) were modified as described in detail by Chen et al. (1991).
Results
Localization of ERM in porcine deciduous incisor teeth
Firstly, we examined the presence of the ERM in deciduous incisor teeth. In histological sections, clusters of aggregated cells were easily recognized in the periodontal ligament tissues (PDL) of porcine deciduous incisor teeth (Fig. 2A,B) (Lesot et al., 1982; Sculean et al., 1998). The clustered cells revealed the expression of CK14 (Fig. 2C), an epithelial cell marker, but not vimentin (Fig. 2D) a mesenchymal marker. Furthermore, immunolabeling for amelogenin was not observed in any of the samples (Fig. 2E).
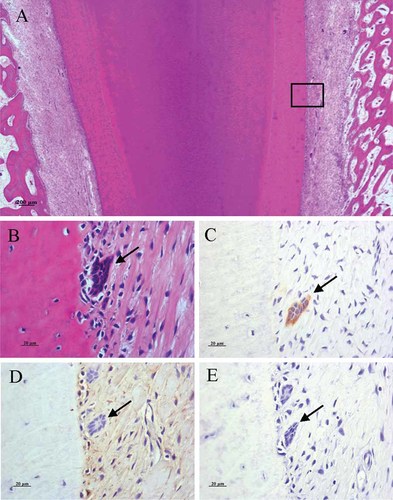
Localization of ERM in porcine deciduous incisor teeth. A: Photomicrograph of a deciduous incisor tooth root in pig at 6-month of age. Dentin (d), cementum (c), PDL (p), and alveolar bone (b) were recognized. B–E: High-magnification views of boxed area in (A). B: Hematoxilin-eosin (H-E) staining shows that roundish ERM (black arrow) located in the PDL are situated along the cementum (c). C: ERM with a typical histological structure expressed CK14 (black arrow). D: Immunohistochemistry showed no expression of vimentin in the ERM (black arrow), but PDL were positive for vimentin. E: No expression of amelogenin was observed in the ERM (black arrow). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Establishment of ERM subculture technique
PDLC and the ERM from explant cultures were established by primary culture (Fig. 3A). After replacing the serum-containing medium with non-serum medium, the PDLC disappeared and the only the ERM were left (Fig. 3B). However the growth of the ERM was quite slow. The selected ERM were then plated on the feeder cell layer (Fig. 3C) and after 7 days of subculture, several small colonies of ERM appeared (Fig. 3D). The colonies increased with time and became confluent after 2 weeks and were then subcultured until the tenth passage (passage 1: Fig. 3E, passage 4: Fig. 3F, passage 10: Fig. 3G). All passaged cells showed the typical polygonal-shaped epithelial morphology. However, when ERM (passage 1) were subcultured on the collagen Type I coated dish without a feeder cell layer, the ERM displayed an extended morphology (Fig. 3H). In addition, when comparing fourth passage ERM (Fig. 3F), EOE (Fig. 3I), and OGE (Fig. 3J), no appreciable differences were found in cell morphology.
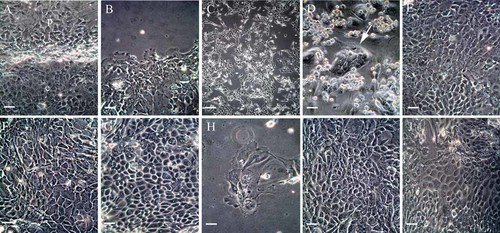
Phase-contrast micrographs. A: Outgrowth of ERM (e) and PDLC (p) from explant culture of PDL; scale bar = 20 µm. B: ERM (e) alone existed in the non-serum medium; scale bar = 20 µm. C: Subcultured ERM (passage 1) on the feeder cell layer after 1 day of cultivation; scale bar = 80 µm. D: After 7 days of cultivation, small colonies of ERM (white arrow) appeared; scale bar = 20 µm. E: After 14 days of cultivation, larger colonies of ERM showed a cobblestone-like appearance; scale bar = 20 µm. F: After four passages, subcultured ERM formed colonies; scale bar = 20 µm. G: After 10 passages, subcultured ERM cells still showed the cobblestone-like appearance; scale bar = 20 µm. H: ERM (passage 1) were cultured on collagen type I coated dishes; scale bar = 20 µm. I: The enamel organ epithelial cells (EOE; passage 4) demonstrated a cobblestone-like appearance; scale bar = 20 µm. J: The appearance of subcultured oral gingival epithelial cells (OGE; passage 4) showed typical epithelial morphology; scale bar = 20 µm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Cell growth analysis
We examined the cell growth of ERM by comparing the growth rate of these cells with that of EOE. All cell populations were able to grow on the feeder cell layer and rates of growth were similar (Fig. 4). However, ERM exhibited a much lower proliferation rate when cultured on the collagen type I coated dishes. There was a significant difference (P < 0.05) between the growth rates in these different culture conditions.
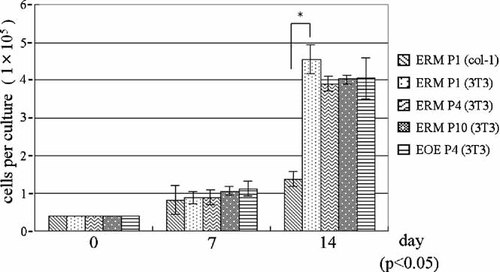
Comparison of the growth of subcultured ERM on collagen coated dishes (passage 1) and on feeder cell layers (passage 1, 4, and 10), and subcultured EOE on feeder cell layers (passage 4). The numbers of cells were counted on days 0, 7, and 14. The growth of ERM with 3T3 feeder cells was faster than that of ERM without 3T3 cells. Asterisks indicate a significant difference (P < 0.05) between paired conditions.
Gene expression pattern of ERM
We used RT-PCR to examine the expression pattern in the subcultured cells. The PDL expressed cytokeratin2 (CK2) which indicated that ERM exist in the PDL (Fig. 5A). Over 10 passages, the ERM expressed CK2 and desmoglein1, but not ALP or periostin, while the PDL and PDLC (passage 4) expressed ALP and periostin (Fig. 5A–D). CK2 and desmoglein1 were selected as the epithelial cell markers in this study. CK2 is expressed in the upper spinous and granular cells of the epidermis and is a marker of late epidermal differentiation. In addition, CK2 supports the nucleus and provides tensile strength for the cell. Desmoglein1 is one of a family of cadherins and plays a role in the formation of desmosomes that join epithelial cells to basal cells (Daugaard et al., 2007)
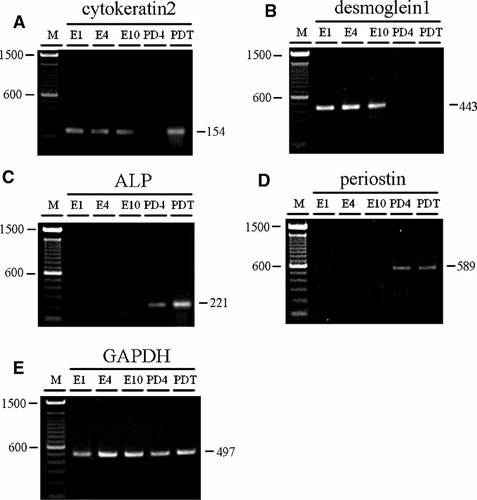
Semi-quantitative RT-PCR analysis with cytokeratin2 (A), desmoglein1 (B), alkaline phosphatase (ALP; C), periostin (D), and GAPDH (E) in subcultured ERM and PDLC (PD4), and PDL (PDT): Subcultured ERM (passage 1, 4, and 10) expressed only epithelial markers, cytokeratin2 and desmoglein1. PDLC (passage 4) expressed only mesenchymal markers, periostin and ALP.
The next step was to examine the characteristics of the subcultured ERM in comparison to the EOE using ameloblast-related markers. Both the ERM and EOE expressed ameloblastin and tuftelin (Fig. 6D,G), while EOE alone expressed amelogenin, MMP 20, and KLK 4 (Fig. 6C, F, and H). None of the cell populations examined expressed enamelin (Fig. 6E). The expression patterns of ameloblast-related genes in ERM were not inconsistent with those in EOE.
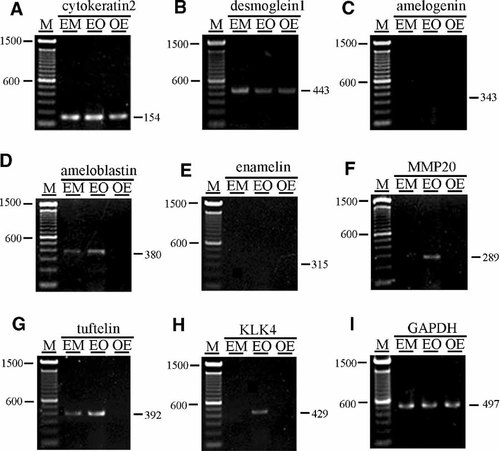
Semi-quantitative RT-PCR analysis of ERM (passage 4) in comparison with EOE (passage 4) and OGE (passage 4) using epithelial cell markers, cytokeratin2 (A), desmoglein1 (B), and ameloblast-related markers, amelogenin (C), ameloblastin (D), enamelin (E), MMP20 (F), tuftelin (G), KLK4 (H), and GAPDH (I). Subcultured ERM (EM), EOE (EO) and OGE (OE) expressed epithelial markers, cytokeratin2 and desmoglein1. All populations were negative for amelogenin and enamelin. Both ERM and EOE expressed ameloblastin and tuftelin (D,G). EOE alone expressed MMP 20 and KLK 4. F,H: OGE did not express any of the ameloblast-related maker genes.
ERM can differentiate into ameloblast-like cells
The possibility that ERM cells can differentiate into ameloblast-like cells was examined in vitro. Phase-contrast micrographs showed the appearance of typical epithelial cells in ERM (Fig. 7A,D) and dental pulp cells (Fig. 7F,I) growing on feeder cell layers. CK14 was detected by immunofluorescence in ERM (Fig. 7B), but no immunoreactivity for amelogenin (Fig. 7C) or vimentin antibodies (Fig. 7E) was observed in the ERM over a 4-week cultivation period.
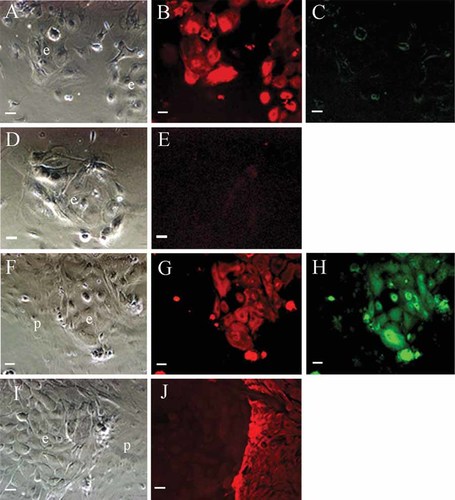
The expression of cytokeratin14 (CK14) and amelogenin. A–E: The ERM were subcultured on feeder cell layers. A,D: Phase-contrast micrographs showed typical epithelial cells(e); scale bar = 20 µm. B: Immunofluorescence showed that ERM were positive for cytokeratin 14; scale bar = 20 µm. C: ERM were negative for amelogenin by immunofluorescence; scale bar = 20 µm. E: ERM were negative for vimentin by immunofluorescence; scale bar = 20 µm. F–J: The ERM were subcultured with second passage dental pulp cells. F,I: Phase-contrast micrographs showed the ERM (e) and dental pulp cells (p); scale bar = 20 µm. G: CK14 expression was detected in the ERM colonies; scale bar = 20 µm. H: Amelogenin expression was detected in the ERM; scale bar = 20 µm. J: Vimentin expression were strongly detected in dental pulp cells, but not the ERM; scale bar = 20 µm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
ERM in the epithelial island, co-cultured with the dental pulp cells, were clearly positive for both CK14 (Fig. 7G) and amelogenin (Fig. 7H) by immunofluorescence. Second passage dental pulp cells were clearly positive for vimentin but it was not detected in ERM (Fig. 7J).
No contamination of dental epithelial cells in isolated dental pulp cells
Before we could perform the differentiation analysis in vitro and the tissue-forming capability analysis in vivo, it was important to check for contamination of the primary dental pulp cells with dental epithelial cells. This was achieved using RT-PCR. Dental pulp cells expressed ALP, however, cytokeratin2 and desmoglein1 were not detected (Fig. 8A). This indicated that there was no contamination with dental epithelial cells. This was confirmed by observation with phase contrast microscopy (Fig. 8B).
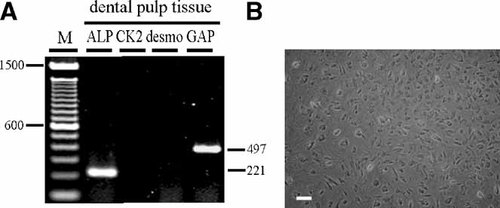
The isolated dental pulp cells were free from dental epithelial cell contamination (A) RT-PCR analysis: primary dental pulp expressed ALP and did not express epithelial cell markers CK2 or desmoglein1 (desmo). B: Secondary cultured dental pulp cells showed no contamination with any of epithelial cells; scale bar = 20 µm.
Tissue-forming capability of subcultured ERM
The capacity of subcultured ERM to produce enamel was examined by transplanting subcultured ERM seeded onto scaffolds into the omentum of athymic rats. The success of all transplanted implants was confirmed following transplantation by assessing the hardness of the implants at 2 and 4 weeks post-transplantation; a solid implant indicated a successful transplantation. Furthermore, histological analysis showed that all implants displayed the appropriate stages of amelogenesis from initiation to maturation. Hematoxylin-eosin staining showed that cylindrical cells were aggregated in the implant at 2 weeks (Fig. 9A) and the rounded shape of a tooth bud-like structure was formed at 4 weeks (Fig. 9B). Specific staining with CK14 was observed in all columnar cells and stellate reticulum-like cells (Fig. 9C). Amelogenin expression was detected in the regions of the tall columnar cells which were in direct contact with hard tissues and stellate reticulum-like cells (Fig. 9D). Columnar shaped odontoblast-like cells demonstrated expression of dentin sialo-protein (Fig. 9E). The odontoblast-like cells, dentin-like tissues, ameloblast-like cells, and stellate reticulum-like cells were distinguished by high magnification of Figure 9B (Fig. 9F). Amelogenin positive staining in ameloblast-like cells and regions of the stellate reticulum-like cells was clearly demonstrated (Fig. 9G). Tooth bud-like structures were not seen in any of the control implants; however bone-like structures were identified (Fig. 9H).
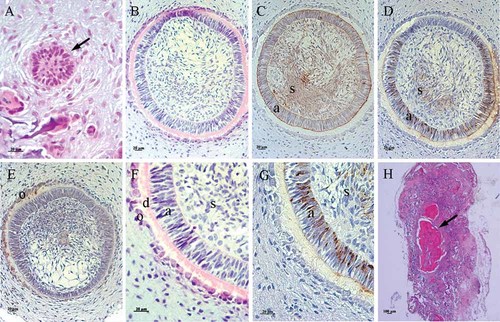
Histology at 2 (A) or 4 weeks after transplantation (B-H) (A) an epithelial cell cluster was revealed within a collagen sponge (black arrow); scale bar = 20 µm. B: The onset of dentin-like tissue formation was clearly shown with haematoxylin-eosin staining; scale bar = 20 µm. C: Immunohistochemistry showed a circular shaped-epithelium expressing the epithelial marker CK14. (a) ameloblast-like cells, (s) stellate reticulum-like cells; scale bar = 20 µm. D: Amelogenin expression was identified in regions of the ameloblast-like cells (a) and stellate reticulum-like cells (s); scale bar = 20 µm. E: DSP expressions were observed in odontoblast-like cells (o); scale bar = 20 µm. F: High magnification of (B) showed columnar odontoblast-like cells (o), dentin-like tissue (d), tall-columnar ameloblast-like cells (a) and stellate reticulum-like cells (s); scale bar = 100 µm. G: High magnification of (B) showed positive staining for amelogenin was seen adjacent to the dentin-like tissues. (a) ameloblast-like cells, (s) stellate reticulum-like cells; scale bar = 100 µm. H: Bone-like structures were observed (black arrow) but not tooth germ-like structures were observed in the control implants; scale bar = 20 µm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
At 8 weeks post-transplantation, enamel-dentin complex-like structures were recognized in the implants (Fig. 10A). At higher magnification, the width of the enamel-like tissue was approximately 100 µm and the stellate reticulum-like structures were observed to some degree (Fig. 10B). The tall columnar ameloblast-like cells were aligned with the surface of the enamel-like tissues. Amelogenin expression revealed enamel-like tissue, ameloblast-like cells, and stellate reticulum-like cells (Fig. 10C). Control sections treated with pre-immune serum exhibited no reactivity, indicating that the staining was specific (Fig. 10D).
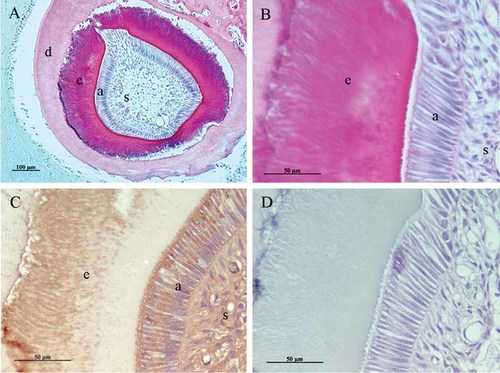
The implants at 8 weeks after transplantation. A: Enamel-like tissue formation (e) on the surface of dentin (d), stained with haematoxylin-eosin. (a) ameloblast-like cells, (s) stellate reticulum-like cells; scale bar = 100 µm. B: High magnification of (A), showing tall columnar ameloblast-like cells (a) are aligned perpendicular to the enamel-like tissues (e). (s) stellate reticulum-like cells; scale bar = 50 µm. C: Amelogenin expression was strongly observed in the enamel-like tissue (e),ameloblast-like cells (a) and stellate reticulum-like cells (s); scale bar = 50 µm. D: Control staining with amelogenin was negative; scale bar = 50 µm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Discussion
The present study provided a new insight into the characteristics of epithelial cell rests of Malassez (ERM), in that they are able to generate enamel-like tissues in a similar fashion to the cervical loop epithelial cells which perform this function at the tooth-crown formation stage. Our hypothesis is that the ERM, which can generate enamel, come from the same cell lineage as cervical loop epithelial cells. It has been reported that the epithelium of Hertwig's root sheath can differentiate into ameloblast-like cells and produce amelogenin (Thomas and Kollar, 1989; Hamamoto et al., 1996), however there has been no report to date on the production of enamel in HERS or ERM.
To demonstrate our hypothesis, firstly we developed a novel culture technique for ERM to expand the cell numbers while retaining their original characteristics. There are many reports that ERM can grow in vitro, however there has been no data on the tissue-forming activity of ERM grown in these conditions. Our previous study showed that subcultured enamel organ epithelial cells (EOE) at the crown formation stage can generate enamel when grown on a feeder cell layer. These results indicated that the technique is effective in retaining tissue-forming potential. We demonstrated that this technique was appropriate for the expansion of ERM by RT-PCR and cell growth analyses. We showed that the subcultured ERM after ten passages have similar characteristics to those at their first passage. ERM are in a quiescent state in periodontal ligament tissues (PDL) after completion of tooth-root formation, therefore it is interesting to find that ERM can expand under in vitro conditions.
A well studied example of this phenomenon involves hematopoietic stem cells. They are modulated by BMP-related and Notch signaling pathways due to their association with osteoblasts in bone (Calvi et al., 2003; Zhang et al., 2003). Generally, the tissue-specific stem cells are thought to mediate repair and regeneration of their respective tissues and they are maintained in a quiescent state until they are required to act on behalf of the tissue. The normal behavior is strictly governed by the stem cell microenvironment or niche, which is composed of other cells, the ECM, and other secreted factors (Ohlstein et al., 2004). ERM which are set free from the PDL are able to grow in vitro and it may be that ERM may have the characteristics of stem/progenitor cells.
The next step was to show that the subcultured ERM are able to secrete amelogenin when co-cultured with primary dental pulp cells. Although RT-PCR analysis demonstrated the expression of amelogenin (data not shown), amelogenin expression has also been reported in odontoblasts (Oida et al., 2002; Papagerakis et al., 2003). We then verified the localization of the amelogenin expressing cells by immunocytochemistry. ERM co-cultured with dental pulp cells expressed amelogenin, however, expression was not detected in subcultured ERM on feeder cell layers. Amelogenin is the predominant matrix protein secreted by ameloblasts, and plays an important role in enamel formation (Gibson et al., 2001). The ERM on the root surface of porcine incisors did not express amelogenin in this study. These results when taken together may indicate that ERM may differentiate into ameloblast-like cells when co-cultured with dental pulp cells. However, the presence or absence of amelogenin is still controversial in ERM. Some articles describe the presence of amelogenin (Slavkin et al., 1989; Diekwisch, 2001; Zeichner-David, 2001), whereas other studies were not able to detect it in ERM (Luo et al., 1991; Fong et al., 1996; Mizuno et al., 2005).
Finally, in the transplantation analysis, enamel-like tissue was produced in the implant when the subcultured ERM were combined with primary dental pulp cells. The previous study showed that subcultured EOE can also generate enamel in combination with primary dental pulp cells at 4 weeks post-transplantation. In the present study, some of the epithelial cells that were in direct contact with the odontogenic calcified tissues showed columnar ameloblast-like cell appearances. These findings are consistent with the theory of epithelial-mesenchymal interaction in tooth development (Jernvall and Thesleff, 2000). At 8 weeks post-transplantation, thick enamel-dentin complex structures were clearly recognized and were stained with CK14 and amelogenin in ameloblast-like cells and enamel. CK14 is a known marker for ameloblasts in a developing tooth prior to the synthesis of amelogenin (Tabata et al., 1996) and it is believed that interactions between CK14 and amelogenin may play an important role in amelogenesis and enamel development (Ravindranath et al., 2001). It cannot be excluded that the primary dental pulp can receive the ERM signal and subsequently differentiate into ameloblasts and produce enamel.
Why can quiescent ERM grow in vivo after transplantation? For quiescent ERM (G0 phase) to divide and proliferate, they have to enter the cell cycle. Extracellular signaling (via mitogens) is required to stimulate cells in the G0 phase to enter the G1 phase of the cell cycle (Arai et al., 2005; Liu et al., 2007). However, since very little is currently known of the roles of the ERM, further study is required to elucidate the mechanism of entry into the cell cycle.
In summary, our study suggests that the ERM have the potential to differentiate into columnar ameloblast-like cells and produce amelogenin. Moreover, when the ERM were directly in contact with dental mesenchymal cells, the ERM have the potential to produce enamel-like tissues. Our results have implications for the understanding of the cellular behavior of ERM. Moreover, ERM may become a candidate cell source for enamel-tissue engineering in adult teeth, because generally the ameloblasts produce enamel and then disappear after the completion of enamel formation.
Acknowledgements
We thank Dr. J. P. Simmer and Dr. Y. Yamakoshi for generously providing the amelogenin and DSP antibodies. The authors thank Dr. H. Green (Harvard Medical School) and Dr. S. Watanabe (IMSUT) for critical reading of the manuscript. This work was supported in part by grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology (Kakenhi Kiban B 19390511 and Houga 20659305 to M. J. H.), and by the DENICS International.




