Cyclin E in centrosome duplication and reduplication in sea urchin zygotes
Abstract
When protein synthesis is completely blocked from before fertilization, the sea urchin zygote arrests in first S phase and the paternal centrosome reduplicates multiple times. However, when protein synthesis is blocked starting in prophase of first mitosis, the zygote divides and the blastomeres arrest in a G1-like state. The centrosome inherited from this mitosis duplicates only once in each blastomere for reasons that are not understood. The late G1 rise in cyclin E/cdk2 kinase activity initiates centrosome duplication in mammalian cells and its activity is needed for centrosome duplication in Xenopus egg extracts. Since the half-time for cyclin E turnover is normally ∼1 h in sea urchin zygotes, the different behaviors of centrosomes during G1 and S phase arrests could be due to differential losses of cyclin E and its associated kinase activities at these two arrest points. To better understand the mechanisms that limit centrosome duplication, we characterize the levels of cyclin E and its associated kinase activity at the S phase and G1 arrest points. We first demonstrate that cyclin E/cdk2 kinase activity is required for centrosome duplication and reduplication in sea urchin zygotes. Next we find that cyclin E levels and cyclin E/cdk2 kinase activities are both constitutively and equivalently elevated during both the S phase and G1 arrests. This indicates that centrosome duplication during the G1 arrest is limited by a block to reduplication under conditions permissive for duplication. The cytoplasmic conditions of S phase, however, abrogate this block to reduplication. J. Cell. Physiol. 217: 626–631, 2008. © 2008 Wiley-Liss, Inc.
The centrosome, the primary microtubule organizing center of the cell, serves to nucleate and organize the interphase microtubule array and the asters at the spindle poles during mitosis (Pickett-Heaps, 1969; Kellogg et al., 1994). Since the purpose of mitosis is the equal segregation of the duplicated genome at mitosis, the cell can contain two and only two centrosomes at the onset of mitosis. If the centrosome fails to duplicate, the next mitosis will either be monopolar or bipolar with one anastral pole. This means that the cell will fail to divide or one daughter will not inherit a centrosome. Over-duplication of the centrosome raises the chances that the cell will assemble a multipolar spindle at mitosis, which inevitably leads to unequal chromosome segregation and aneuploid daughter cells.
To ensure that mitosis will be strictly bipolar, the cell must coordinate centrosome doubling with nuclear DNA synthesis. The link between centrosome duplication and DNA synthesis is ensured by the rise in cyclin E/cdk2 and/or cyclin A/cdk2 kinase activities which participate in determining when both events begin (Hinchcliffe et al., 1999; Lacey et al., 1999; Matsumoto et al., 1999; reviewed in Hinchcliffe and Sluder, 2002). Importantly, the cell must also have a means to ensure that the centrioles do not reduplicate during S phase. A centrosome intrinsic block to reduplication exists, at least in normal human cells, which ensures that once the centrosome has duplicated it will not do so again even though the cellular conditions are permissive for duplication (Wong and Stearns, 2003; Tsou and Stearns, 2006).
To better understand the mechanisms that limit centrosome duplication, we have investigated a phenomenon described by Hinchcliffe et al. (1998). When protein synthesis in sea urchin zygotes is completely blocked from before fertilization, the zygotes arrest in first S phase and the paternal centrosome repeatedly reduplicates (also see Gard et al., 1990; Sluder et al., 1990). This is consistent with observations of centrosome reduplication in zygotes arrested in S phase of the first or second cell cycle by inhibition of DNA synthesis (Hinchcliffe et al., 1998). However, a different pattern of centrosome reproduction was observed when protein synthesis was completely blocked beginning at prophase of first mitosis. The zygotes went through mitosis, because they had completed all preparations for first division before protein synthesis was inhibited. The two blastomeres reformed nuclei and arrested in a G1-like state and each blastomere contained two complete centrosomes for up to 8 h or the equivalent of seven division cycles. The centrosome inherited at the end of mitosis had duplicated just once in a normal fashion, but no more. The difference in centrosome reproduction at the two arrest points is not based in differing levels of cyclin B/cdk1 activity; H1 kinase activity was at the pre-fertilization level for both cell cycle arrest points due to the complete inhibition of protein synthesis (Hinchcliffe et al., 1998). Why centrosomes reduplicate during S phase arrest but are limited to duplicating only once during a G1 arrest has been a mystery.
In the sea urchin zygote, translation of cyclin E mRNA is activated after fertilization and the level of this protein remains constant during early development, suggesting that there is continuous turnover of cyclin E during early development (Sumerel et al., 2001). Perhaps arrest in S and G1 by blocking protein synthesis might bring about a differential decline in cyclin E protein levels under the two arrest conditions and hence a change in its associated kinase activity. To test this possibility, we repeated the experiments of Hinchcliffe et al. (1998) and characterized the levels of cyclin E protein and its associated kinase activity when the zygotes were arrested in first S phase and in the G1-like state under conditions in which protein synthesis was completely blocked.
Materials and Methods
Gamete collection and fertilization
Lytechinus pictus and Strongylocentrotus purpuratus adults, were obtained from Marinus Inc. (Long Beach, CA). Eggs and sperm were collected by intracoelomic injection of 0.5 M KCl and fertilizations were carried out as previously described (Sumerel et al., 2001; Schnackenberg and Marzluff, 2002). Fertilization frequency was judged by monitoring the formation of the fertilization envelopes. The zygotes were cultured at 15°C for S. purpuratus or 18–20°C for L. pictus.
Drug treatments
All drugs were obtained from Sigma (St. Louis, MO). The protein synthesis inhibitors, anisomycin and emetine, were prepared together as a 5× stock in filtered artificial sea water at concentrations of 50 µM and 0.5 mM, respectively (Sluder et al., 1990; Hinchcliffe et al., 1998). The drug cocktail was added to cultures 30 min prior to fertilization or during first prophase (generally 55–60 min post-fertilization). Roscovitine stocks were prepared at a concentration of 10 mM in DMSO.
Immunofluorescence microscopy
Immunofluorescence was performed as previously described (Schnackenberg and Marzluff, 2002; Schnackenberg et al., 2007). Briefly, zygotes were immobilized onto poly-L-lysine coated coverslips, and fixed in −20°C MeOH. Samples were rehydrated in PBS, blocked for 4 h at room temperature, followed by incubation in primary antibodies to α-tubulin (Serotec, Raleigh, NC) and/or cyclin E (Sumerel et al., 2001) overnight at 4°C. The coverslips were then washed, incubated in FITC- and Rho-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) for 1 h at 37°C. The coverslips were washed and mounted in elvanol. Images were collected using a Zeiss LSM-410 confocal microscope.
Extract preparation
Extracts were prepared as previously reported (Schnackenberg and Marzluff, 2002; Schnackenberg et al., 2007). Briefly, eggs or zygotes were collected by centrifugation, washed and suspended in ice-cold lysis buffer (10 mM HEPES, pH 8.0, 250 mM NaCl, 25 mM EGTA, 5 mM MgSO4, 110 mM glycine, 250 mM glycerol, 1 mM DTT, 1 mM PMSF) supplemented with a protease inhibitor cocktail (Sigma) and were lysed by passage through a 27-gauge needle. Whole extract was quick-frozen in liquid N2 and stored at -80°C. Protein concentrations of the extracts were determined using the BCA Protein Assay (Pierce, Rockford, IL).
Western blot
Equal amounts of protein (10 µg) were separated on 12% SDS–polyacrylamide gels and were transferred to nitrocellulose. The nitrocellulose blots were incubated in blocking buffer (5% milk in TBST: 150 mM NaCl, 10 mM Tris, pH 7.5, 0.05% Tween-20) for 1 h at room temperature, followed by incubation in affinity-purified anti-cyclin E antibody (Sumerel et al., 2001) for 1 h at room temperature. The blots were washed in TBST, incubated in HRP-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, Inc.) for 1 h at room temperature, washed, and developed using Super Signal West Pico substrate (Pierce).
Cyclin E dependent kinase assay
The immunoprecipitations and kinase assays were performed as previously described (Sumerel et al., 2001; Schnackenberg and Marzluff, 2002; Schnackenberg et al., 2007). Sea urchin extract (300 µg protein) was diluted in NP-40 Lysis Buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.5% NP-40, 50 mM NaF) to a final volume of 300 µl and was clarified by centrifugation. The supernatant (250 µl) was collected and pre-cleared with 10 µl Protein A Agarose (Invitrogen, Carlsbad, CA). The pre-cleared supernatant was collected (200 µl), 100 ng of affinity-purified cyclin E antibody was added to each sample and was incubated overnight at 4°C. Immune complexes were precipitated with Protein A Agarose beads and were washed four times in NP-40 Lysis Buffer. After the last wash, the supernatant was carefully removed and the beads were suspended in 25 µl kinase buffer (50 mM HEPES, pH 7.0, 10 mM MgCl2, 5 mM MnCl2, 1 mM DTT, 5 µM ATP). The reactions were started by the addition of 5 µCi 32P-γ-ATP and 6 µg recombinant pRb-GST, incubated for 30 min at room temperature, and terminated by the addition of 2× SDS–sample buffer. The samples were separated on a 12% SDS–polyacrylamide gel which was dried and exposed to Kodak X-OMAT AR film.
Densitometry
Films from three separate experiments (for both Western blots and kinase assays) were scanned using a ScanMaker 9800XL flatbed scanner (Microtek Lab, Inc., Carson, CA). Background subtraction and measurement of integrated densities of all bands was performed using Image J (http://rsb.info.nih.gov/ij/). The resulting band densities for all time points were normalized to the unfertilized egg controls. For Western blots, the densities of both 50 and 36 kDa cyclin E bands were measured and summed. For all time points, the Mean Density ± SEM was plotted and the trendline was determined by linear regression.
Results
Sea urchin zygotes are normal, primary cells and a good model system for our work, because centrosome duplication is not dependent upon ongoing protein synthesis. Zygotes contain from the time of fertilization stored pools of all the centrosomal subunits sufficient to make many centrosomes (Gard et al., 1990; Sluder et al., 1990).
Cyclin E/cdk2 activity is necessary for centrosome duplication and reduplication
We first needed to empirically establish if cyclin E/cdk2 is necessary for centrosome duplication and reduplication in the sea urchin zygote. We treated fertilized eggs with roscovitine, a potent inhibitor of mammalian cyclin-dependent kinase activity (Alessi et al., 1998) and monitored the duplication of the sperm centrosome at the time of first DNA synthesis. In sea urchins, the centrosome used in development comes from the sperm and it replicates once 15–20 min post-fertilization (Sluder, 1992; Schatten, 1994). Roscovitine inhibits sea urchin cyclin E/cdk2 activity in a dose-dependent manner, with nearly complete inhibition at 20 µM (Schnackenberg et al., 2007).
Zygotes were treated with 20 µM roscovitine at 1.5 or 15 min post-fertilization and cultured for 1 h before fixation for α-tubulin immunofluorescence. For embryos treated at 1.5 min post-fertilization only one aster was observed next to the zygote nucleus (Fig. 1A), indicating that the paternal centrosome did not duplicate. Since the sperm introduces two spatially separate centrioles at fertilization, the presence of a single aster indicates that the paternal centrosome did not split into sister centrosomes, something it normally does at this time. However if roscovitine is added at 15 min post-fertilization, two asters are observed next to the zygote nucleus indicating that the paternal centrosome replicated and split into two sister centrosomes (Fig. 1B).
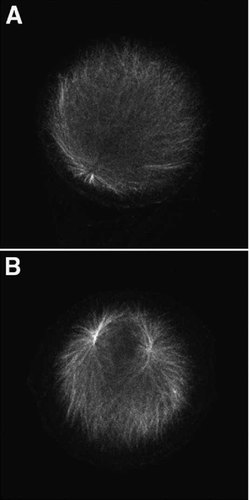
Roscovitine inhibits replication of the paternal centrosome. Sea urchin zygotes were treated with 20 µM roscovitine at 1.5 or 15 min post-fertilization. Replication of the paternal centrosome was assayed for using α-tubulin immunofluorescence. Only one aster was observed in zygotes treated at 1.5 min post-fertilization (A), whereas zygotes treated at 15 min contained two asters (B).
To determine if cyclin E/cdk2 activity is necessary for centrosome re-replication during prolonged S phase, unfertilized eggs were pre-treated with protein synthesis inhibitors for 30 min, fertilized, and cultured at 15°C for 2 h (equivalent to the time of first cleavage for S. purpuratus) to establish the S phase arrest (Hinchcliffe et al., 1998). The culture was then divided into two aliquots. Twenty micro molar roscovitine was added to one culture and the other culture served as a control. Both cultures were incubated for an additional 4 h at 15°C in the continued presence of protein synthesis inhibitors. Samples were fixed and assayed using α-tubulin immunofluorescence to determine the number of asters per cell. As expected, the control culture exhibited multiple rounds of centrosome replication during S phase arrest (Fig. 2A–C), with as many as eight asters in a single zygote (Fig. 2B). However, no centrosome re-replication was evident in the roscovitine treated culture (Fig. 2D–F). Thus, cyclin E/cdk2 activity is necessary for the re-replication of centrosomes in S phase arrested embryos.
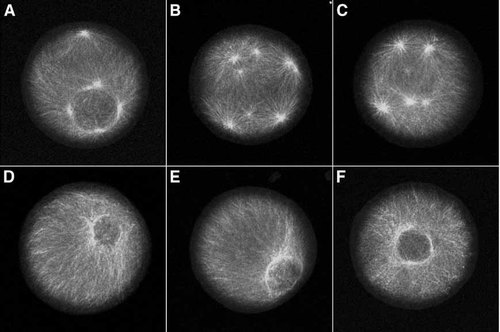
Roscovitine inhibits re-replication of centrosomes during S phase arrest. S phase arrested zygotes were cultured in the absence (A–C) or presence of 20 µM roscovitine (D–F). Immunofluorescence using antibodies to α-tubulin shows that centrosomes re-replicate in S phase arrested zygotes accumulating up to eight centrosomes per cell (B), whereas re-replication was inhibited by roscovitine (D–F).
Cyclin E levels are the same during S and G1 arrests
Unfertilized eggs contain stores of cyclin E/cdk2, and the level of cyclin E expression remains constant for the first 5 h of embryogenesis (Sumerel et al., 2001). Since translation of the cyclin E mRNA is activated after fertilization, this constancy suggests that there is continuous turnover of cyclin E during the early cell cycles (Sumerel et al., 2001). Therefore, blocking protein synthesis might bring about a differential decline in cyclin E protein levels when the zygotes are arrested during S phase arrest and the G1-like state.
Eggs were treated with anisomycin/emetine for 30 min prior to fertilization or 60 min post-fertilization, the time of prophase for first mitosis. The concentrations of translation inhibitors used completely block protein synthesis in these cells (Sluder et al., 1990). Samples of both cultures were collected, fixed, and processed for confocal immunofluorescence microscopy using antibodies to α-tubulin and cyclin E. As previously reported (Hinchcliffe et al., 1998), cultures treated with protein synthesis inhibitors prior to fertilization arrested in first S phase and underwent repeated rounds of centrosome replication; only a few can been seen in any one image plane (Fig. 3A). Embryos treated during first prophase arrested prior to second S phase (in a G1-like state) having undergone only a single round of centrosome replication (Fig. 3C). We found that cyclin E protein is localized to the nuclei of zygotes arrested in first S phase (Fig. 3B) or in a G1-like state (Fig. 3D) and remains nuclear throughout the entire 6 h time course of the experiment. This nuclear localization of cyclin E is consistent with what is seen in untreated zygotes (Schnackenberg and Marzluff, 2002). Our results indicate that centrosome reduplication during S phase arrest and lack of reduplication during arrest in a G1-like state are not a function of different localizations of cyclin E.
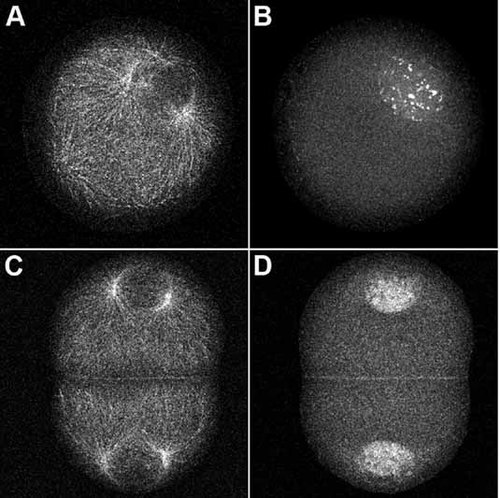
Cyclin E protein remains nuclear during S phase and G1-like arrest. Zygotes arrested in first S phase (A,B) or in a G1-like state (C,D) were immobilized onto coverslips, fixed, and processed for immunofluorescence microscopy using antibodies to α-tubulin (A,C) and cyclin E (B,D). Centrosomes undergo centrosome re-replication during S phase arrest (A), and only one round of replication in a G1-like arrest (C). Cyclin E protein is still present after 6 h of culture in protein synthesis inhibitors and remains localized within the nuclei regardless of the arrest point (B,D).
To quantify cyclin E levels at these two arrest points, Western blot analysis was conducted on extracts prepared at 1 h intervals for 6 h from zygote cultures that were arrested in either first S phase (Fig. 4A) or the G1-like state (Fig. 4B). As previously reported, cyclin E is present in unfertilized eggs and is cleaved to a 36 kDa proteolytic fragment (Sumerel et al., 2001, Fig. 4A,B) due to the release of cytoplasmic stores of proteases during extract preparation. These proteases are normally released into the sea water as part of the fertilization reaction (Vacquier et al., 1972) and consequently are not as much of an issue for post-fertilization samples. However, there are occasionally low levels of proteolysis in embryo extracts (Fig. 4A, lanes 4–6 h). Since the 36 kDa proteolytic fragment retains associated kinase activity, the band densities for both full-length (50 kDa) and truncated (36 kDa) cyclin E were measured and summed for these analyses. We find that treatment with anisomycin/emetine (A/E) does not alter cyclin E protein levels in unfertilized eggs (Fig. 4A, compare lane E–EA/E). Furthermore, cyclin E protein levels remain elevated and equivalent during both S and G1-like arrests with no significant increases or decreases over 6 h (Fig. 4A–C). For S phase arrested zygotes the high zygote-to-zygote variability in the extent of centrosome reduplication under these conditions (Sluder et al., 1990) indicates that there is no correlation between variations in cyclin E levels at each time point and centrosome reduplication. For the zygotes arrested in a G1-like state, there is some rise and fall of cyclin E levels from time point to time point (Fig. 4C), which is likely due to sample variation between experiments since protein synthesis is inhibited throughout the experiment. Since the centrosomes duplicated only once at this arrest point, variations in cyclin E levels have no obvious significance. Since cyclin E turns over with a half-life of about 1 h in normal sea urchin zygotes (Sumerel et al., 2001), blocking protein synthesis must, therefore, stop the turnover of cyclin E in vivo.
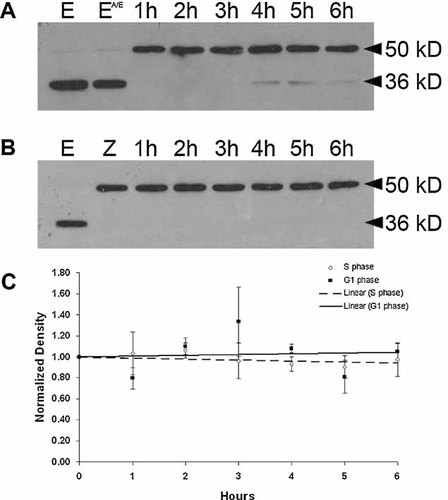
Cyclin E protein levels remain constant during S phase and G1-like arrest. Sea urchin eggs or zygotes were treated with protein synthesis inhibitors and arrested in first S phase (A) or in a G1-like state (B). Extracts were prepared at 1 h intervals. Proteins were separated on a 12% SDS–polyacrylamide gel, transferred to nitrocellulose, and probed with a polyclonal antibody to sea urchin cyclin E. Some proteolysis of cyclin E (full-length 50 kDa) to a 36 kDa fragment occurs during extract preparation. E, unfertilized eggs; EA/E, unfertilized eggs treated with anisomycin/emetine for 30 min; Z, zygotes at 60 min after fertilization when inhibition of protein synthesis begins. The densities of cyclin E bands (both 50 and 36 kDa) from three independent experiments were measured and normalized to the unfertilized egg. For every time point, the mean ± SEM was plotted and the trendline was determined using linear regression (C).
Cyclin E/cdk2 activity in zygotes arrested in S and G1
To quantify cyclin E/cdk2 activity at these two arrest points, cyclin E was immunoprecipitated from the same extracts used for Western blots and these immunoprecipitates were tested for associated kinase activity using recombinant pRb-GST as the substrate. As previously shown, cyclin E is associated with kinase activity in unfertilized eggs (Fig. 5A,B, lane E; Sumerel et al., 2001). Treatment of unfertilized eggs with anisomycin/emetine for 30 min does not affect this activity (Fig. 5A, lane EA/E). Zygotes treated with these translation inhibitors from the time of fertilization arrest in first S phase and the cyclin E associated kinase activity declines only slightly relative to the pre-fertilization level over 6 h (Fig. 5A,C). Given the zygote-to-zygote variability in the extent of centrosome duplication (Sluder et al., 1990) there is no obvious correlation between variations in kinase activity for each time point and centrosome reduplication. For cultures treated with anisomycin/emetine at 60 min post-fertilization to arrest the zygotes prior to second S phase, the cyclin E associated kinase activity also declines only slightly, albeit with some scatter, for the 6 h duration of the experiment (Fig. 5B,C). Since the centrosome in each blastomere duplicated only once, there is no correlation in the fluctuation in kinase activity during G1 arrest and centrosome duplication. The variability between samples taken at different times was not consistent from experiment to experiment and may be attributable to random loss of sample during recovery of beads in the immunoprecipitation steps. Thus cyclin E/cdk2 kinase activity is equivalent and essentially constant when embryos are arrested in the first S phase or in the G1-like state.
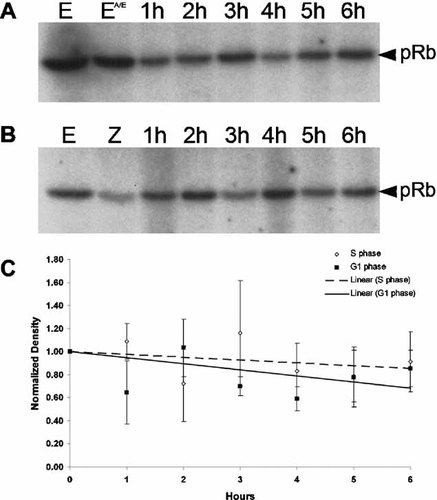
Cyclin E-associated kinase activity remains constant during S phase and G1-like arrest. Cyclin E was immunoprecipitated from extracts prepared from zygotes arrested in first S phase (A) or in a G1-like state (B) and tested for associated kinase activity using recombinant pRb-GST as the substrate. Phosphorylated pRb was detected by autoradiography. E, unfertilized eggs; EA/E, unfertilized eggs treated with anisomycin/emetine for 30 min; Z, zygotes at 60 min after fertilization when inhibition of protein synthesis begins. The densities of the phosphor-pRb bands from three independent experiments were measured and normalized to the unfertilized egg. For every time point, the mean ± SEM was plotted and the trendline was determined using linear regression (C).
Discussion
We investigated whether changes in cyclin E protein levels and/or its associated kinase activity can explain the bimodal regulation of centrosome duplication observed by Hinchcliffe et al. (1998). Several possibilities had already been ruled out. First, both modes of centrosome duplication occurred when the activity of cyclin B/cdk1 activity was at low pre-fertilization values and thus not a factor (Hinchcliffe et al., 1998). Second, this difference in centrosome reproduction cannot be due to limitations in the pools of centrosomal subunits during G1 relative to S phase; the egg before fertilization contains pools of all the subunits needed to make many centrosomes (Gard et al., 1990; Sluder et al., 1990). Third, the difference cannot be due to the differential synthesis of some putative centrosome interacting protein that must be synthesized anew at each cell cycle, because in both cases protein synthesis was completely shut down.
Cyclin E associated kinase activity in centrosome doubling
Our first finding was that the Cdk inhibitor roscovitine blocked the doubling of the paternal centrosome and did not allow centrosome reduplication during S phase arrest. This indicates that cyclin E associated kinase activity is required in the establishment of permissive conditions for centrosome duplication in the sea urchin zygote consistent with previous observations that cyclin E/cdk2 activity is needed for centrosome duplication in mammalian cells and Xenopus egg extracts (Hinchcliffe et al., 1999; Matsumoto et al., 1999; Matsumoto and Maller, 2002; reviewed in Hinchcliffe and Sluder, 2002).
The fact that the paternal centrosome did not split into two asters with a centriole apiece in the presence of roscovitine also indicates that cyclin E/cdk2 activity is needed not only for centriole duplication but also for the splitting of the centrosome into two sister centrosomes. The two centrioles contributed by the sea urchin sperm at fertilization do not appear to be functionally linked. The proximal centriole, which serves as the basal body for the sperm flagellum is separated from the distal centriole located between the sperm nucleus and mitochondrion (Longo and Anderson, 1968), and mild detergent lysis in the presence of protease inhibitors allows one to isolate the distal centrioles of the sperm leaving the proximal centrioles attached to the flagellum and nucleus (Sluder, unpublished work). In sea urchin zygotes and mammalian cells the centrosome can split into two sisters with one centriole apiece even when centriole duplication does not occur (Mazia et al., 1960; Sluder and Begg, 1985; Sluder and Rieder, 1985; Hinchcliffe et al., 1998; Salisbury et al., 2002; Habedanck et al., 2005). Thus, if the paternal centrosome could split without cyclin E/cdk2 activity, it should be able to do so.
Cyclin E levels and associated kinase activity during S and G1 arrests
Since cyclin E has a half-life of about an hour in normally cycling sea urchin zygotes (Sumerel et al., 2001), we tested the possibility that inhibition of protein synthesis might lead to a differential loss of this protein and its associated kinase activity during S and G1 arrests. This might determine whether the centrosome duplicates just once or reduplicates multiple times. In this context, our second primary finding is that the presence of cyclin E and its associated kinase activity are essentially constant and the same during S phase arrest and during the G1-like arrest. Thus, the difference in the reproduction of centrosomes at the two arrest points is not based in differing levels of cyclin E or its associated kinase activity.
The presence of cyclin E associated kinase activity throughout the G1 arrest provides the first indication that the early sea urchin zygote has a block to centrosome reduplication, conceivably similar to that found in mammalian somatic cells (Wong and Stearns, 2003). This block is revealed only when the zygotes are experimentally arrested in a G1-like state, because early sea urchin zygotes normally begin DNA synthesis in telophase and, thus do not exhibit a G1 phase. The nature of this block to reduplication is not clear, but our results indicate that it is functional under conditions that are constitutively permissive for duplication. It is interesting that this block to reduplication is not operational during prolonged S phase; centrosomes reduplicate. This means that the normally cycling early embryo is not rigidly protected from centrosome reduplication during S phase. Since centrosome reduplication during prolonged S phase is slow (Hinchcliffe et al., 1998), the rapid kinetics of the normal cell cycle presumably do not allow enough time for the centrosome to duplicate more than once.
Finally, we found that inhibition of protein synthesis appears to stop the normal turnover of cyclin E. We did not observe a progressive loss of this protein or its associated kinase activity over 6 h of S phase or G1 arrest when new cyclin E could not be synthesized. A possible explanation comes from the finding that cyclin E is degraded by the SCF complex in S phase somatic cells (Singer et al., 1999). One component of the SCF complex, Skp2, accumulates during late G1 and is maximal during S and G2 phases (Zhang et al., 1995; Carrano et al., 1999; Marti et al., 1999). Therefore, blocking protein synthesis might block the accumulation of Skp2 thereby stopping the normal degradation of cyclin E. This notion is consistent with the finding that Skp2 knockout cells accumulate cyclin E (Nakayama et al., 2000).
Acknowledgements
The authors thank Shawn Galdeen and Joshua Nordberg for constructive comments on the manuscript. This work was supported by NIH grant GM59812 to W.F.M. and NIH grant GM30758 to G.S.




