The calcium binding protein S100A9 is essential for pancreatic leukocyte infiltration and induces disruption of cell–cell contacts†
J. Schnekenburger and V. Schick equally contributed to this work.
Abstract
Leukocyte infiltration is an early and critical event in the development of acute pancreatitis. However, the mechanism of leukocyte transmigration into the pancreas and the function of leukocytes in initiating acute pancreatitis are still poorly understood. Here, we studied the role of S100A9 (MRP14), a calcium binding protein specifically released by polymorph nuclear leukocytes (PMN), in the course of acute experimental pancreatitis. Acute pancreatitis was induced by repeated supramaximal caerulein injections in S100A9 deficient or S100A9 wild-type mice. We then determined S100A9 expression, trypsinogen activation peptide (TAP) levels, serum amylase and lipase activities, and tissue myeloperoxidase (MPO) activity. Cell–cell contact dissociation was analyzed in vitro with biovolume measurements of isolated acini after incubation with purified S100A8/A9 heterodimers, and in vivo as measurement of Evans Blue extravasation after intravenous application of S100A8/A9. Pancreatitis induced increased levels of S100A9 in the pancreas. However, infiltration of leukocytes and MPO activity in the lungs and pancreas during acute pancreatitis was decreased in S100A9-deficient mice and associated with significantly lower serum amylase and lipase activities as well as reduced intrapancreatic TAP-levels. Incubation of isolated pancreatic acini with purified S100A8/A9-heterodimers resulted in a rapid dissociation of acinar cell–cell contacts which was highly calcium-dependent. Consistent with these findings, in vivo application of S100A8/A9 in mice was in itself sufficient to induce pancreatic cell–cell contract dissociation as indicated by Evans Blue extravasation. These data show that the degree of intrapancreatic trypsinogen activation is influenced by the extent of leukocyte infiltration into the pancreas which, in turn, depends on the presence of S100A9 that is secreted from PMN. S100A9 directly affects leukocyte tissue invasion and mediates cell contact dissociation via its calcium binding properties. J. Cell. Physiol. 216: 558–567, 2008. © 2008 Wiley-Liss, Inc.
Recent investigations suggest that inflammatory cell infiltration into pancreatic tissue occurs much earlier and plays a much greater role in the course of pancreatitis than previously thought (Bhatia et al., 2005; Mayerle et al., 2005). Surprisingly, not only initial events in acinar cells attract inflammatory cells, but the infiltrating inflammatory cells also trigger intraacinar cell events during the early phase of pancreatitis (Sandoval et al., 1996; Gukovskaya et al., 2002). We recently found that leukocyte infiltration into the pancreas begins within the first 60 min after the onset of acute pancreatitis and also involves an intricate set of mechanisms for the dissociation of acinar cell–cell contacts (Mayerle et al., 2005). This cell–cell contact dissociation particularly concerns adherens junctions of acinar cells with their tightly regulated cadherin/cadherin complexes (Schnekenburger et al., 2005). While much is known about the mechanisms through which polymorph nuclear leukocytes (PMN) adhere to and migrate across the endothelial barrier, the protein repertoire involved in the invasion of epithelial tissue is less well understood.
The two calcium binding proteins S100A8 and S100A9 have been suggested to exert regulatory activities towards migration or adherence of inflammatory cells (Newton and Hogg, 1998; Kerkhoff et al., 1999a; Srikrishna et al., 2001; Ehlermann et al., 2006; Anceriz et al., 2007). They are abundant in phagocytes, form stable heterodimers, and are secreted upon cellular activation (Kerkhoff et al., 1998). S100A8/A9-positive leukocytes belong to the first cells invading sites of inflammation, and both proteins are upregulated during various inflammatory processes resulting in increased circulating serum levels (Nacken et al., 2003). A recent report found S100A8/A9 to downregulate endothelial adherens junction proteins in cultured cells via an unknown mechanism (Viemann et al., 2005). These data prompted us to study the role of S100A8/A9 in inflammatory cell infiltration and in cell–cell contact regulation during pancreatitis.
When we employed S100A9 knockout mice (Hobbs et al., 2003; Manitz et al., 2003) in an experimental pancreatitis model the complete absence of S100A9 in PMN leukocytes—the only cell type where S100A9 was found to be expressed—resulted in a failure of leukocytes to infiltrate the pancreatic tissue and a greatly reduced severity of pancreatitis. In vitro recombinant S100A8/A9 rapidly dissociated cell–cell contacts between pancreatic acinar cells, a process that could be reversed by adding calcium to the buffer, and in vivo S100A8/A9 application dissociated pancreatic adherens junctions as indicated by an induction of Evans Blue extravasation. Taken together these results indicate an essential function of secreted S100A8/S100A9 for leukocyte infiltration into the pancreas and as underlying mechanism for the dissociation of calcium-dependent cell–cell contacts. Since this process appears to determine the severity of pancreatitis S100A8/S100A9 also appears to be an ideal target for the development of treatment strategies against pancreatitis.
Materials and Methods
Materials
Caerulein was obtained from Pharmacia (Freiburg, Germany). Collagenase from Clostridium histolyticum (EC.3.4.24.3) was from SERVA (lot # 14007, Heidelberg, Germany; collagenase activity 1.50 PZ-U/mg). Human myeloperoxidase (MPO) was from Calbiochem (San Diego, CA) and was purified from blood by HPLC (catalog # 475911, 1 mg/10 ml protein concentration, 150–200 U mg protein specific protein activity, in 100 mM NaCl, 50 mM Na-acetate, purity >95%). The biologically active phosphorylated CCK octapeptide [Tyr(SO3H)27]-cholecystokinin fragment was obtained from Sigma (Taufkirchen, Germany, catalog # 2175). TAP-EIA was purchased from Biotrin International (Dublin, Ireland). Antibodies specific for CD11B were obtained from Stressgen (San Diego, CA), secondary HRP coupled antibodies from GE Healthcare Life Sciences (Little Chalfont, UK) and Cy3 conjugated antibodies from Dianova (Hamburg, Germany). All other chemicals were of highest purity and were obtained either from Sigma–Aldrich (Eppelheim, Germany) or Merck (Darmstadt, Germany), Amersham Pharmacia Biotech (Buckinghamshire, UK), or Bio-Rad (Hercules, CA).
S100A8/A9 was purified from human neutrophils as previously described (Kerkhoff et al., 1999b). Prior to use, the proteins were re-chromatographed by anion exchange using a UnoQ column (BioRad, Munich, Germany). SDS–PAGE revealed a purity of >95%.
Methods
Supramaximal secretagogue stimulation in vivo
Male S100A9 wild-type and S100A9 deficient mice (Manitz et al., 2003) (40–50 g) received 7 hourly intraperitoneal injections of supramaximal caerulein concentrations (50 µg/kg/h) (Niederau et al., 1985). Saline injected animals served as controls. All animal experiments were conducted according to the guidelines of the local Animal Use and Care Committee.
Preparation of serum and tissue samples
Mice were sacrificed at intervals between 0 and 48 h after the first intraperitoneal injection of caerulein. Whole blood samples were centrifuged at 4°C, and serum was stored at −80°C for further studies. Tissue from pancreas and lung was removed on ice, weighed, trimmed of fat, immediately frozen in liquid nitrogen, and stored at −80°C. Tissue blocks were embedded in Tissue-Tek (Sakura Finetek, Zoeterwoude, The Netherlands) for cryosectioning. Tissue for the measurement of pancreatic enzyme activities was thawed and homogenized in iced medium containing 5 mM MOPS, 1 mM MgSO4, 250 mM sucrose at pH 6.5. Samples were sonicated and centrifuged for 5 min at 16,000g. Sample preparation for TAP immunoassay was performed as previously described (Hurley et al., 1988). Tissue homogenates were supplemented with 1 mM EDTA and 0.1% Triton X-100 and boiled for 10 min. Supernatants generated at 16,000g for 5 min were used in the subsequent immunoassays. The total protein concentration of the supernatants was determined as previously described (Halangk et al., 2002).
Enzyme activity and activation measurements
Amylase and lipase activities were determined by commercially available assays (Roche, Mannheim, Germany). For MPO measurements pancreatic or lung tissue was homogenized in 20 mM potassium phosphate buffer at pH 7.4 and centrifuged for 10 min at 10,000g. The pellet was resuspended in 50 mM potassium phosphate buffer, pH 6.0, containing 0.5% cetyltrimethylammonium bromide. The suspension was freeze-thawed four times, sonicated twice for 10 sec each at 30% power setting, and centrifuged at 20,000g for 10 min at 4°C. MPO activity was assayed after mixing 50 µl supernatant in 200 µl of 50 mM potassium phosphate buffer (pH 6) containing 0.53 mM O-dianisidine and 0.15 mM H2O2. The initial increase in absorbance at 450 nm was measured at room temperature with a Dynatech MR 5000 Elisa reader. The results are expressed in units of MPO on the basis of 1 U to oxidize 1 µmol H2O2 per min per mg pancreatic protein (Halangk et al., 2000).
TAP was assayed using an enzyme-immunoassay (Biotrin). Each sample was measured, in duplicate a standard curve was generated from free TAP protein. TAP levels were expressed as ng/ml total protein.
For measurement of intracellular trypsin activation in living pancreas acinar cells, acini were freshly prepared from the pancreas of S100A9 wild-type and S100A9 deficient mice by collagenase digestion, suspended in HEPES (24.5 mM) buffered medium (pH 7.5) containing NaCl (96 mM), KCl (6 mM), MgCl2 (1 mM), NaH2PO4 (2.5 mM), CaCl2 (0.5 mM), glucose (11.5 mM), Na-pyruvate (5 mM), Na-glutamate (5 mM), Na-fumarate (5 mM), minimum essential medium (1%, v/v), and bovine serum albumin, fraction V (1%, w/v). After an equilibration period of 30 min the cholecystokinin analogue caerulein (Bachem, Heidelberg, Germany) was added at supramaximal (10 nM) concentrations for 30 min and at a temperature of 37°C. Acini were then washed and resuspended in medium without secretagogue but in the presence of the synthetic trypsin substrate (CBZ-Ile-Pro-Arg)2-rhodamine-110 (10 µM, Molecular Probes, Carlsbad, CA). To quantify substrate cleavage acini, together with the substrate, were transferred to 96-well microtiter plates and the ΔF/Δt ratio was determined by cytofluorometry (Fluostar Optima fluorometer, BMG, Offenbach, Germany) over 60 min as previously reported (Kruger et al., 2000).
Assessment of acinar cell-contact dissociation
Acinar cell–cell contact dissociation assays were performed as recently standardized and reported in detail (Mayerle et al., 2005; Schnekenburger et al., 2005) In brief, pancreatic acini were prepared by collagenase digestion (Collagenase NB8, Serva) as previously described (Lerch et al., 1993; Williams et al., 1978). Acini were washed and centrifuged at 50g for 1 min in DMEM medium containing 0.2% BSA. Living acini were incubated for up to 70 min with buffer alone (DMEM with 0.2% BSA and 0.02% soybean-trypsin-inhibitor) or with buffer containing either, purified S100A8/A9 protein (0.46 or 4.6 µM) in the presence of 0.5 mM calcium or S100A8/A9 (4.6 µM) with 2 mM calcium or no calcium. The biovolume of living acini was determined to quantitate cell contact dissociation. For this assay suspensions of freshly prepared acini were diluted (1:200) in filtered (particle free) physiological NaCl-buffer and their biovolume was determined with a cell analyzing system (CASY I, Schärfe Systems, Reutlingen, Germany) which is based on resistance measurements with pulse-surface analysis (Schnekenburger et al., 2005). Measurements during incubation with the above reagents were made for single cells (11–23 µm) and intact acini (24–80 µm) and the results expressed as percent of control incubations with buffer alone. Graphs indicate the means ± SEM of three measurements representative of five experiments.
In vivo cell contact dissociation
We used Evans blue extravasation to quantify the in vivo effects of purified S100A8/A9 on the integrity of pancreatic cell–cell contacts (Saria and Lundberg, 1983). We have injected 100 µg purified S100A8/A9 in 200 µl buffer resulting in a final serum concentration of approx. 50 µg/ml (the murine blood volume being approx. 2 ml). We have calculated the amount of S100A8/A9 for injection from our in vitro cell dissociation experiments. A concentration of 10 µg/ml was sufficient to dissociate cell–cell contacts in the presence of 2 µM calcium. The murine in vivo blood calcium concentration is in the range from 1.7 to 2 mM. We have therefore increased the concentration to 50 µg/ml blood to achieve an effect but to avoid systemic toxic effects. Evans blue (30 mg/kg of a 3% solution in 0.9% NaCl) was injected into the tail vein of C57BL/6 mice. Ten minutes later the mice were anesthetized with intraperitoneal ketamine (10 mg/100 g) and pentobarbital (10 mg/100 g) and a cannula was placed into the left ventricle with its tip in the aorta. The right atrium was opened, and mice were perfused with 50 ml phosphate-buffered saline (PBS; 15 mM phosphate buffer, 140 mM NaCl, 27 mM KCl, pH 7.4), followed by 100 ml of 1% paraformaldehyde in 50 mM citrate buffer, pH 3.5. The pancreas was removed and rinsed in saline, gently plotted dry and weighed. One half of the tissue was placed in 1 ml of formamide at room temperature for 48 h. Evans blue was quantified by measuring the optical density of the formamide solution at 620 nm using a spectrophotometer (Fluostar Optima, BMG-Labtech). Absorbance was compared to standard curve of 0.05–25 µg/ml Evans blue in formamide. Dye Extravasation was expressed as micrograms Evans blue per mg tissue. The other part of the tissue was fixed in 4% paraformaldehyde and 2.5% parabenzochinone for 24 h at 4°C, plotted dry and shock frozen in Tissue-Tek O.C.T. compound (Sakura Fintek, Zoeterwoude, The Netherlands) and stored at −80°C for later fluorescence microscopy.
Western blotting
Pancreatic tissue was homogenized with a Dounce S glass homogenizer (Braun, Melsungen, Germany) in iced Triton-X-100 lysis buffer containing protease inhibitors (1 ml/mg tissue, 10 µg/ml aprotinin, 10 µg/ml leupeptin, 0.01 M sodiumpyrophosphate, 0.1 M sodiumfluoride, 1 mM dihydrogenperoxide, 1 mM L-phenyl-methyl-sulfonyl-fluoride [PMSF] and 0.02% soybean-trypsin-inhibitor). Protein concentration was determined by a modified Bradford-assay (BioRad Laboratories) and equal amounts of protein were used in subsequent experiments. SDS polyacrylamide gel electrophoresis was performed in a discontinuous buffer system and gels were blotted on nitrocellulose membranes (Hybond C, Amersham Pharmacia). After overnight blocking in NET-gelatine (10 mM Tris/HCl pH 8.0, 0.15 mM NaCl, 0.05% TWEEN 20, 0.2% gelatine) immunoblot analysis was performed using a polyclonal S100A9 antibody followed by enhanced chemoluminescence detection (Amersham Pharmacia) using horseradish peroxidase coupled goat anti-rabbit IgG (Amersham Pharmacia) (Manitz et al., 2003; Schnekenburger et al., 2005).
Immunofluorescence and immunohistochemical staining
For immunocytochemical analysis, paraffin sections of formaldehyde-fixed mice pancreata were mounted on Polylysine microslides (Menzel Gläser, Braunschweig, Germany). For antigen retrieval, dewaxed and rehydrated sections were immersed in 10 mmol/L citric acid, pH 6.0, and boiled in a pressure cooker at an operating pressure of about 103 kPa/15 psi for 2 min. After cooling until all pressure was released, the slides were removed, quickly washed in running distilled water and transferred to PBS solution. Subsequently, sections were encircled with a water-repellent PAP-pen (Dianova) and rinsed with PBS. PBS was used for all dilutions and washing steps. After blocking non-specific binding sites (Fc-receptors) with BSA-c basic blocking solution (1:10 in PBS, Aurion, Wageningen, The Netherlands), sections were incubated overnight at 4°C with rabbit antibody against the leukocyte marker CD11b (Stressgen). Bound primary antibody was detected with corresponding species-specific secondary antibody conjugated with Cy3 (Dianova). After nuclei counterstaining with DAPI (Sigma) for 15 sec, samples were mounted with Vectashield (Vector Laboratories, Burlingame, CA).
For immunohistochemistry S100A9 was stained with a polyclonal antibody as previously characterized (Manitz et al., 2003) and detected by a donkey anti-rabbit peroxidase linked species specific secondary antibody (GE Healthcare Life Sciences). Bound CD11b antibody was detected with a horseradish-peroxidase conjugated secondary antibody. After washing with PBS sections were incubated with NovaRed (Vector Laboratories), rinsed with destilled water and stained with hematoxylin for 2 min.
Microscopy and image processing
Immunostained preparations were examined on a Leica DM LB light and fluorescence microscope (Leica Microsystems, Heerbrugg, Switzerland) equipped with appropriate filters. Composite images were imported as JPEG files. Microscopy controls were not incubated with primary AB and in those sections no immunolabeling was observed. Hematoxylin and eosin stained sections were examined on a Leica DM LB light microscope and the images were captured digitally as described above.
Analysis of data
The results reported are expressed as means ± SEM values obtained from multiple determinations in five or more separate experiments. In all figures, vertical bars denote SEM values, and the absence of such bars indicates that the SEM was too small to show. The significance of changes was evaluated using the Student t-test when data consisted of only two groups and with Bonferoni correction for multiple comparisons. A P-value of ≤0.05 indicated a significant difference.
Results
S100A9 expression and localization in acute pancreatitis
The two calcium binding proteins S100A8 and S100A9 are predominantly expressed in cells of the myeloid lineage. The detection of S100A9 by a specific antibody in pancreatic tissue homogenates showed no significant expression in control tissue but an up regulation of S100A9 expression in the course of pancreatitis (Fig. 1A).
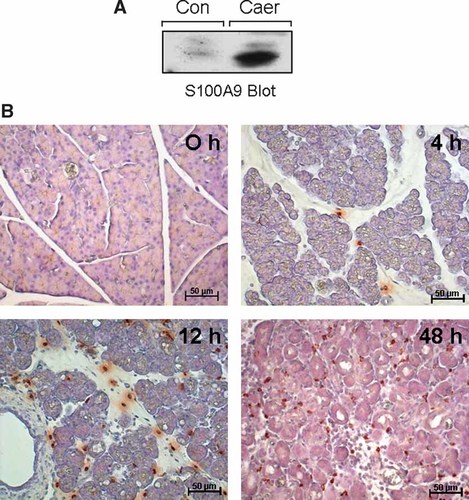
Pancreatic expression and intrapancreatic localization of S100A9 during acute experimental pancreatitis: S100A9 wild-type animals received 7 hourly injections of caerulein (50 µg/kg/h intraperitoneally), animals were then sacrificed at the indicated time points and the pancreas prepared for histology, H and E staining and micrographs were taken (X200) as described under Methods Section. Part of the pancreatic tissue was snap frozen and homogenized for Western blot analysis, in order to ensure antibody specificity of the immunostaining (A). Leukocytes were stained with a polyclonal S100A9 specific antibody on paraffin embedded sections. We observed transmigrated S100A9 positive leukocytes in the pancreatic parenchyma at 4 h after the last injection of Caerulein and the rate of infiltrated neutrophils steadily increased over the next 48 h. Please note the signs of acute pancreatitis such as vacuolization, edema formation and the widening of the interstitial space (B).
Immunostaining of pancreatic tissue revealed S100A9 expression exclusively in infiltrating leukocytes during the course of pancreatitis. S100A9 was not detected in pancreatic parenchymal or stromal cells of control or caerulein-treated tissue (Fig. 1B). As shown in Figure 1B, leukocyte infiltration into pancreatic tissue was evident at 4 h after the last caerulein injection. The number of infiltrating S100A9-positive leukocytes increased over the next 48 h. In agreement with previous reports the first 48 h of experimental pancreatitis were characterized by intracellular vacuolization, loss of cell–cell contacts, pancreatic necrosis and edema formation with a maximum after 12 h (Halangk et al., 2000). In contrast to these microscopic acinar cell changes which were partly restored at 48 h, the infiltration of leukocytes steadily increased up to 48 h.
Acute pancreatitis in S100A9 deficient mice
S100A9 deficient mice lack a functional S100A8/A9 complex since S100A8 was also undetectable in peripheral leukocytes of S100A9 deficient mice (Manitz et al., 2003). The severity of acute experimental pancreatitis was analyzed at 4 h after the last caerulein injection and corresponding to the start of S100A9-positive-neutrophil transmigration.
Serum pancreatic enzyme activities
Treatment of S100A9 wild-type mice with caerulein rapidly increased serum amylase (Fig. 2A) and serum lipase activities (Fig. 2B). Both activities were significantly reduced to less than 50% in S100A9 deficient mice, indicating reduced pancreatic tissue damage.
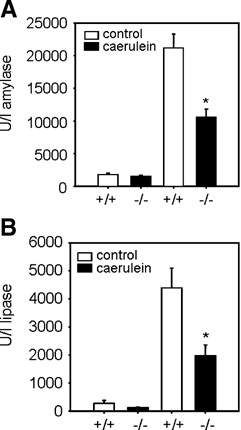
Effect of S100A9 deficiency on caerulein induced pancreatitis (50 µg/kg/h intraperitoneally) after the last injection. Serum activities for lipase and amylase were measured as described in Materials and Methods Section. In S100A9 deficient mice serum amylase activity (A) and serum lipase activity (B) were significantly reduced. Control groups received saline injections instead of caerulein and displayed no increase in serum lipase and amylase activities. The values reported are means ± SEM from five mice at the reported time point. Asterisks (*) indicate 5% significance levels (P < 0.05) if S100A9 wild-type mice were compared to S100A9 knockout animals.
Intrapancreatic trypsinogen activation
The premature activation of trypsinogen is a key event in the initiation of acute pancreatitis. Trypsinogen is converted to active trypsin by cleavage of the N-terminal octapeptide, the Trypsinogen-activation peptide (TAP). TAP is established as a disease specific marker of acute pancreatitis and correlates with the severity of disease (Borgstrom et al., 2002). Intrapancreatic trypsinogen activation was measured using a TAP ELISA (Biotrin). Supramaximal caerulein stimulation induced a prominent increase in pancreatic TAP levels in S100A9 wild-type mice. In contrast, S100A9 deficient mice showed a limited increase of only up to 25% of the concentrations observed in wild-type animals (Fig. 3), indicating an ameliorated course of acute pancreatitis.
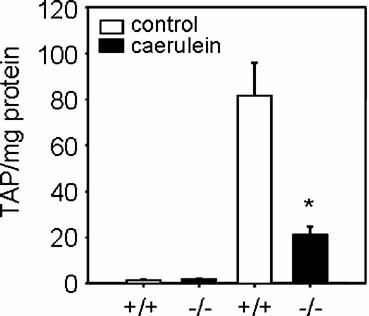
Impact of S100A9 deficiency on intrapancreatic trypsinogen activation, indicated by the concentration of the trypsinogen activated peptide (TAP) in pancreatic tissue (TAP/mg protein), after caerulein stimulation (50 µg/kg/h intraperitoneally). Animals were sacrificed 4 h after the last injection as described in Materials and Methods Section. In S100A9 deficient mice TAP as a marker of trypsin activation was significantly reduced in pancreatic homogenates. Control groups (S100A9 deficient mice and S100A9 wild-type animals) received saline injections and showed no change in TAP levels in the pancreas. The values reported are means ± SEM from five mice at the indicated time point. Asterisks (*) indicate 5% significance level (P < 0.05) if treated wild-type animals were compared to treated S100A9 deficient animals.
Leukocyte infiltration and pancreatic morphology
The S100A8/A9 heterodimer has been suggested to play a role in leukocyte function during acute inflammatory events, but clear evidence for this assumption is sparse and the mechanism how S100A8/A9 acts remains unknown (Vandal et al., 2003). Since transmigration of inflammatory cells is an early and essential event in acute pancreatitis we studied leukocyte infiltration in S100A9 deficient and S100A9 wild-type animals by quantification of MPO activity in pancreas homogenates and immunohistochemical staining for the leukocyte marker CD11b in pancreatic tissues (Mayerle et al., 2005). Supramaximal caerulein stimulation increased the activity of the leukocyte marker enzyme MPO within 4 h after the last injection in S100A9 wild-type mice from 0.4 ± 0.07 mU of baseline to 2.6 ± 0.5 mU in the secretagogue treated group. S100A9 deficient mice showed a significantly reduced pancreatitis associated MPO activity from 0.2 ± 0.1 at baseline to 0.9 ± 0.3 after caerulein stimulation (Fig. 4A). Immunohistochemical staining of the leukocyte marker CD11b revealed similar results: in contrast to wild-type mice we could not detect infiltrating leukocytes in pancreatic tissue sections of caerulein treated S100A9 knockout mice (Fig. 4B). Furthermore, the morphological characteristics of acute pancreatitis including interstitial edema formation and vacuolization in acinar cells were only detected in tissues of secretagogue treated wild-type, but not the knock-out mice (Fig. 4B). These data demonstrate that the reduced severity of acute pancreatitis is associated with a decreased rate of infiltrating leukocytes in S100A9 knock out mice.
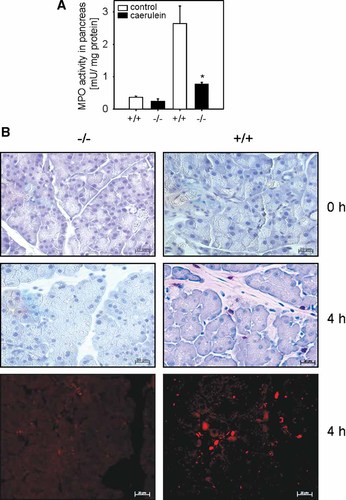
Impact of S100A9 deficiency on the level of myeloperoxidase activity (MPO) in pancreatic homogenate 4 h after the last caerulein injection. MPO was measured as a marker for leukocyte infiltration in the pancreatic parenchyma in caerulein induced pancreatitis. MPO activity was significantly reduced in S100A9 deficient mice compared to S100A9 wild-type animals (A) suggesting that S100A9 plays a critical role for the transmigration of neutrophils to the site of acute inflammation. To give a second line of evidence confirming the MPO data, we performed immunostaining for the leukocyte marker CD11b (X300). B: Representative micrographs with no leukocyte infiltration in untreated wild-type and knockout animals and reduced CD11b cells in the S100A9 deficient group (−/−), but a high number of infiltrating leukocytes in the wild-type pancreatitis group (+/+) 4 h after the last injection of caerulein. Neutrophils were significantly reduced in S100A9 knockout animals at the indicated time points. Repetition of the same experiment with a fluorogenic secondary antibody confirmed these results. Numerical values indicate means ± SEM from five mice and micrographs are representative for five animals. Asterisks (*) indicate 5% significance level (P < 0.05).
Acinar cell function in S100A9 deficient mice
In order to exclude that an impaired responsiveness to supramaximal caerulein stimulation in acinar cells from S100A9 deficient mice is responsible for the ameliorated course of pancreatitis we measured caerulein induced trypsinogen activation in isolated pancreatic acini. Pancreatic acini either from S100A9 deficient or S100A9 wild-type mice were freshly isolated by collagenase digestion and stimulated with a supramaximal caerulein concentration of 10 nM for 30 min. The intracellular trypsinogen activation was measured employing the trypsin specific fluorogenic substrate (Ile-Pro-Arg-R110) by cytofluorometry.
As shown in Figure 5, acinar cells from S100A9 deficient mice responded to the secretagogue with the same extent of trypsin activation as acinar cells from S100A9 wild-type mice. This gives evidence that acinar cells of S100A9 deficient mice are functionally intact and that the significantly reduced TAP levels in S100A9 deficient mice are indicative of less severe pancreatitis rather than of impaired acinar cell function in these animals. These data also suggest that early leukocyte infiltration in the course of pancreatitis has a profound impact even on intraacinar cell signaling events such as those involved in premature trypsinogen activation.
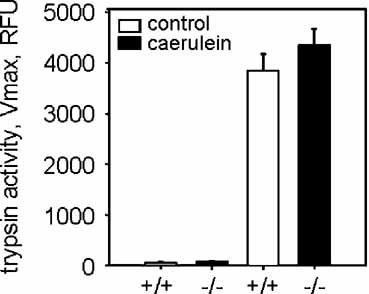
Trypsinogen activation in isolated acini after supramaximal CCK stimulation for 30 min. Dispersed pancreatic acini were generated from S100A9 deficient mice and wild-type animals as described under Methods Section and incubated with supramaximal concentration of the secretagogue CCK (Cholecystokinin, 10 nM). Acini were then homogenized and trypsin activity was determined employing the fluorogenic substrate Ile-Pro-Arg coupled to a Rhodamin residue which is specifically cleaved by active trypsin. Acini from S100A9 deficient mice and from wild-type animals showed the same response to supramaximal CCK stimulation indicating that a lack of S100A9 does not affect CCK mediated signaling pathways in pancreatic acini. Data points represent means ± SEM of four or more animals in each group at the indicated time point.
Effects of S100A9 deficiency on pancreatitis-associated lung injury
Acute pancreatitis is a systemic inflammatory disease with manifestations in different organ systems. As a measure of extrapancreatic organ damage we determined leukocyte infiltration into the lung by quantification of MPO activity in lung homogenates and immunohistochemical staining of the leukocyte marker CD11b in lung sections. An elevated MPO activity in lung homogenates of caerulein treated S100A9 wild-type mice indicated systemic inflammation. Lung tissues of S100A9 knockout mice showed a significantly reduced MPO activity during acute pancreatitis (Fig. 6A). Representative images of immunohistochemical and immunofluorescence detection of CD11b in lung of S100A9 deficient and wild-type mice showed reduced infiltration of leukocytes in caerulein treated S100A9 deficient mice (Fig. 6B). Consequently, deletion of S100A9 thus resulted not only in reduced pancreatic organ damage but also in a less severe systemic inflammation.
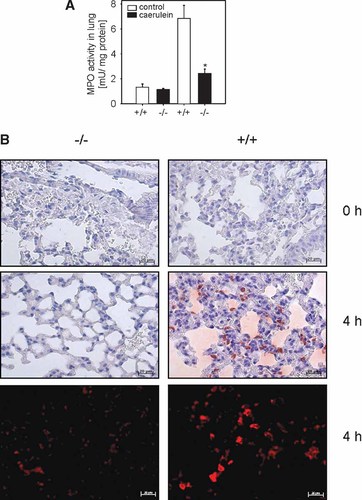
Effect of S100A9 deficiency on myeloperoxidase activity (MPO) in the lung during caerulein induced pancreatitis (50 µg/kg/h intraperitoneally) 4 h after the last injection of caerulein. MPO as a marker for leukocyte infiltration into the lung is indicative for a systemic inflammatory response syndrome during the course of acute pancreatitis. MPO was significantly reduced in the S100A9 deficient mice compared to S100A9 wild-type animals (A). To give a second line of evidence confirming the MPO data in homogenized lung tissue we performed immunostaining for the leukocyte marker CD11b (X300). B: Representative micrographs with no leukocyte infiltration in untreated wild-type and knockout animals and reduced infiltration in the S100A9 deficient group (−/−), but a high level of infiltrating leukocytes in the wild-type pancreatitis group (+/+) 4 h after the last injection of caerulein. Neutrophils transmigrating to the site of inflammation were significantly reduced in S100A9 knockout animals at the indicated time point. Repetition of the same experiment with a fluorogenic secondary antibody confirmed these results.
Calcium dependent dissociation of acinar cell–cell contacts by S100A8/A9
The secretagogue-induced pancreatitis in S100A9 deficient mice showed significantly reduced severity of pancreatitis parameters such as edema formation and leukocyte infiltration. Both processes require the dissociation of pancreatic cell–cell contacts. We therefore analyzed the effect of S100A9 on the integrity of pancreatic epithelial cell–cell contacts in a recently established and standardized in vitro assay (Mayerle et al., 2005; Schnekenburger et al., 2005). This technique allows to study the maintenance and to quantitate the disassembly of cell–cell contacts within isolated pancreatic acini. The dissociation of cell–cell contacts in acini is indicated by an increase in dissociated single acinar cells. The impact of calcium on the dissociation of pancreatic acini was studied by incubation of pancreatic acini with purified S100A8/A9 in the presence (2 mM calcium) or the absence of calcium. Incubation with S100A8/A9 effectively disrupted acinar cell–cell contacts in a concentration—dependent manner. In calcium free medium the most prominent effect was observed whereas the addition of 2 mM calcium directly abolished the effect of S100A8/A9 on acinar cell–cell contact dissociation (Fig. 7).
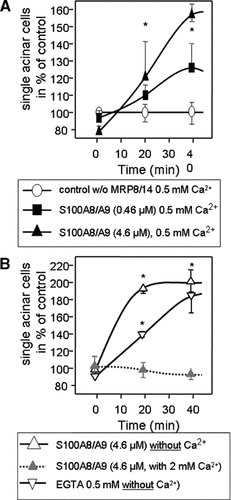
S100A8/A9 plays a crucial role in the calcium dependent cell–cell contact dissociation of pancreatic acinar cells. Isolated dispersed acini were generated from the pancreas of rats as described in Materials and Methods Section and incubated with different concentrations of S100A8/A9 as well as with varying calcium concentrations or EGTA. The increase of single cells indicates their dissociation from the intact acini due to resolution of cell–cell contacts. Note the dose dependent break up of cell-contacts induced by S100A8/A9 14 in the presence of physiological calcium concentrations which, in itself, don't have an effect on cell–cell contacts (control) (Panel A). The effect of the calcium chelator EGTA alone did not surpass the effect which was achieved by incubation with purified S100A8/A9 complex (Panel B). The effects on cell–cell-contact dissociation could be prevented in both cases (EGTA and S100A8/A9) by adding excess calcium to the incubation buffer (Panel B). The values reported denote means ± SEM of triplicates, representative of five experiments.
These experiments demonstrated that the cell-contact dissociating effect of S100A8/A9 is highly dependent on the calcium-binding function of the complex. It also explains via which mechanism leukocytes employ S100A8/A9 to propagate their own transmigration into the interstitial space of the pancreas and how they directly dissociate the calcium-dependent adherens junctions between acinar cells.
In vivo dissociation of pancreatic cell–cell contacts by S100A8/A9
The dissociation of isolated pancreatic acinar cells by purified S100A8/A9 already suggests a crucial function of the heterodimer for the tissue infiltration of leukocytes. To study the in vivo effect of S100A8/A9 on pancreatic cell–cell contact integrity we used an Evans Blue extravasation assay. Evans blue binds to plasma proteins and thus remains within the confines of the vascular bed until gaps between pancreatic cells, are formed and the dye can enter the interstitial space. Evans blue extraction from pancreatic tissue therefore reflects cell–cell contact integrity and its accumulation between acinar cells correlates directly with the degree of cell–cell contact dissociation (Saria and Lundberg, 1983). We injected 100 µg purified S100A8/A9 in 200 µl buffer (10 mM Tris/HCl pH 8, 150 mM NaCl, 1 mM DTT) into the tail vein of C57BL/6 mice (n = 5 in all groups). The control group received 200 µl buffer without S100A8/A9. Two hours after S100A8/A9 application the mice received 30 mg/kg Evans Blue. A second control group received 7 hourly intraperitoneal injections of supramaximal caerulein (50 µg/kg/h) and Evans Blue 6 h after the first caerulein injection. Evans Blue extravasation into the interstitial space was analyzed by fluorometry of homognized pancreatic tissue after formamide extraction. The development of acute pancreatitis over 6 h involves the formation of pancreatic edema and resulted in accumulation of Evans Blue in the interstitial space of the exocrine pancreas (Fig. 8). After injection of S100A8/A9 the mean Evans Blue concentration in pancreatic tissue rose to 286.8 µg/mg compared to only 145.6 µg/mg tissue after supramaximal caerulein stimulation. These data show that S100A8/A9 can directly dissociate pancreatic cell–cell contacts in vivo and in the absence of pancreatitis. They further confirm our hypothesis that S100A8/A9 plays a critical role for the tissue infiltration of leukocytes during acute pancreatitis.
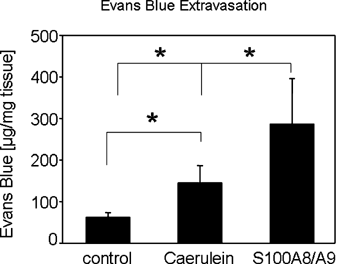
Pancreatic cell–cell contact dissociation after S100A8/A9 injection in vivo. C57BL/6 mice were injected with 100 µg purified S100A8/A9 into the tail vein or buffer as control. Two hours after S100A8/9 application the mice were injected with 30 mg/kg Evans Blue. A second control group received 7 hourly injections of supramaximal caerulein concentrations (50µg/kg/h) and was injected with Evans Blue 6 h after the first caerulein injection. Quantification of Evans Blue leakage by formamide extraction of pancreatic tissue and measurement of Evans Blue absorbance at 620 nm. The induction of an acute pancreatitis resulted in the influx of Evans Blue in into the parenchymal organ. S100A8/A9 injection caused an even stronger Evans Blue extravasation and was sufficient for the disruption of pancreatic cell–cell contacts. The values reported are means ± SEM of triplicates, representative of five animals per group.
Discussion
Acute pancreatitis is a multiphase disease process initiated by premature activation of pancreatic zymogens within acinar cells. The progression of an inflammatory cascade depends on the rapid infiltration of leukocytes into pancreatic tissue. We and others have shown that the transmigration of leukocytes into the pancreas occurs as early as 1 h after induction of experimental pancreatitis in rats (Gukovskaya et al., 1997; Keck et al., 2002; Mayerle et al., 2005). Moreover, inhibition of leukocyte infiltration, reduced the severity of acute pancreatitis in rodents and neutrophil depletion can ameliorate pancreatic edema, vacuole formation in acinar cells, serum amylase and lipase as well as the extent of pancreatic necrosis (Sandoval et al., 1996; Frossard et al., 1999; Gukovskaya et al., 2002).
In addition to the long established mechanism of ICAM-1 mediated transendothelial leukocyte transmigration (Frossard et al., 1999; Aurrand-Lions et al., 2002; Vestweber, 2002) we have recently identified two novel pathways involving epithelial cell–cell contact regulation during acute pancreatitis that both affect E-cadherin mediated cell adhesion. First, we identified supramaximal stimulation-induced tyrosine phosphorylation of the cadherin/catenin complex as an early event in the dissociation of acinar cell–cell contacts and pancreatic edema formation (Schnekenburger et al., 2005). Second, we could demonstrate that the cleavage of acinar cell E-cadherin by PMN elastase secreted from infiltrating leukocytes during acute pancreatitis permits the progressive tissue infiltration of inflammatory cells. The specific inhibition of PMN elastase in vivo prevented the cleavage of E-cadherin, reduced leukocyte transmigration and significantly ameliorated the severity of pancreatitis (Mayerle et al., 2005).
Here we present a third mechanism involved in the process of leukocyte transmigration during the initial phase of acute pancreatitis: secretion of two calcium binding proteins by leukocytes, S100A8 and S100A9, which directly dissociate acinar cell–cell contacts via a calcium-dependent process.
S100A8 and S100A9 are predominantly expressed in cells of the myeloid lineage. They form heteromeric complexes which are released into the extracellular environment at the site of inflammation and in response to cell activation (Rammes et al., 1997). Consistent with this finding high plasma levels can be found in various inflammatory disorders such as chronic bronchitis, cystic fibrosis, and rheumatoid arthritis (Nacken et al., 2003). Although the exact function of the S100 proteins was previously unknown, they were suggested to exert regulatory activity toward migration or adherence of inflammatory cells (Newton and Hogg, 1998; Srikrishna et al., 2001; Ehlermann et al., 2006; Anceriz et al., 2007). In addition, S100A8 and S100A9 have also been suggested to exert chemotactic properties (Ryckman et al., 2003; Vandal et al., 2003) but this has been disputed by others (Sroussi et al., 2006, 2007). Studies employing genetically modified animals for S100A8 and S100A9 function initially resulted in no deeper understanding of the role of S100A8/A9 because S100A8 knock out mice were not viable and found to be resorbed during early embryogenesis (Passey et al., 1999). In S100A9 knock out animals, on the other hand, also S100A8 expression was absent from peripheral leukocytes but the animals were viable and had no spontaneous or apparent phenotype (Hobbs et al., 2003; Manitz et al., 2003).
In order to study the role of S100A8/A9 in leukocyte transmigration and cell–cell contact regulation we used an in vivo model in which both events can be experimentally affected in a well controlled and reversible manner—acute pancreatitis—using previously generated S100A9 knockout mice. Pancreatitis induced by supramaximal secretagogue stimulation is characterized by rapid morphological and biochemical alterations in the exocrine pancreas that are comparable to morphological and biochemical characteristics of acute pancreatitis in humans. Local and systemic changes include hyperamylasemia, a systemic inflammatory response and an associated lung injury (Niederau et al., 1985). Moreover, as in human pancreatitis, significant intrapancreatic trypsinogen activation precedes acinar cell injury (Lerch et al., 2003). Pancreatic S100A9 expression during acute experimental pancreatitis was found to be restricted to infiltrating leukocytes, and leukocyte infiltration as well as S100A9 expression reached a maximum 48 h after the first caerulein injection.
The analysis of pancreatitis parameters in S100A9 knockout mice showed reduced pancreatic tissue damage, reduced intrapancreatic trypsin activation and the absence of edema formation whereas C57BL/6 wild-type mice developed all characteristics of fully established acute pancreatitis. The prominent reduction in TAP release and thus trypsinogen activation suggested a crucial function of infiltrating leukocytes not only for the progression of acute pancreatitis toward systemic inflammation but also in the initiation of the intra-acinar cell zymogen activation cascade. These results are in line with previous reports demonstrating reduced TAP activation after either neutrophil depletion or inhibition of leukocyte infiltration (Gukovskaya et al., 2002; Mayerle et al., 2005).
The stimulus-secretion coupling and secretory function of isolated pancreatic acini from S100A9 knockout mice, on the other hand, were not altered in comparison to acini from wild-type animals. This excludes that the deletion of S100A9 affects acinar cells directly and confirms that all events observed during the course of pancreatitis are due to the absence of S100A9 and S100A8 from infiltrating leukocytes.
The most important finding of our study, however, is the direct effect of S100A8/A9 on cell–cell contacts. Using a recently developed and characterized assay for acinar cell–cell contact dissociation (Schnekenburger et al., 2005), we found that purified S100A8/A9 complex dissociated epithelial cell–cell contacts directly, and that it did so in a concentration-dependent manner and in the presence of lower than physiological calcium concentrations. Since this effect was completely reversed by the addition of excess extracellular calcium, we attributed the effect to the calcium-binding properties of S100A8/A9. The two S100 proteins are composed of two distinct helix-loop-helix motifs (EF-hand) flanked by hydrophobic regions at either terminus and separated by a central hinge region. Although the two EF-hands differ in their affinity for Ca2+, it is well established that the heterodimer has a high affinity (Zwadlo et al., 1988). We therefore suggest that S100A8/A9 acts as a calcium scavenger at adherens junctions through its two EF-hands and in this manner chelates calcium away from tight homophilic E-cadherin bindings (Pokutta et al., 1994; Baumgartner et al., 2000, 2003; Prasad and Pedigo, 2005).
We further tested the effect of S100A8/A9 on the integrity of cell–cell contacts in vivo with an assay of Evans Blue accumulation in the pancreatic interstitium as an indicator of cell–cell contact dissociation. Treatment of mice with purified S100A8/A9 alone was sufficient to disrupt pancreatic cell–cell contacts. The effect of purified S100A8/A9 even exceeded the extent of pancreatic cell–cell contact dissociation observed with supramaximal caerulein stimulation although the two processes are difficult to compare directly because changes in edema formation and other vascular events may account for the differences. These experiments further confirm our in vitro data and support the notion that S100A8/A9 is a critical component for the infiltration of leukocytes in an inflammatory process such as acute pancreatitis. Most important these data demonstrate, for the first time, a direct in vivo function of S100A8/A9.
Recently, Viemann et al. (2005) demonstrated a decrease in cellular integrity after S100A8/A9 binding to endothelial cells followed by altered gene expression of a number of genes involved in the thrombogenic and inflammatory response. However, the authors used high concentrations of S100A8/A9 (200 µg/ml), and the alteration in gene expression was analyzed after 6 h. As we, by contrast, observed a decrease in cellular integrity already after 40 min and with more physiological S100A8/A9 protein concentrations we don't expect and did not detect (data not shown) altered protein expression to be the reason for of the decreased intercellular integrity.
The observed reduced number of leukocytes could be due to an impaired migration of S100A9-depleted (−/−) PMN as reported by Vogl et al. (2004). These authors found that S100A9−/− mice display diminished recruitment of granulocytes into the granulation tissue during wound healing and attributed the effect to the promoting effect of S100A8/A9 on the polymerization of microtubules.
In conclusion, our studies using S100A9 knockout mice and purified S100A8/A9 protein complexes in an experimental model of pancreatitis give evidence that the two S100 proteins from leukocytes are critical for inflammatory cell transmigration into epithelial tissue. We further show that the absence of S100A9 (and S100A8) from leukocytes greatly reduces severity of pancreatitis, the tissue inflammatory response and even intra-acinar events such as premature trypsinogen activation. Moreover, the S100A8/A9 complex directly dissociates epithelial cell–cell contacts in a highly calcium dependent manner and due to its calcium binding property. Therefore, our data not only provide the first experimental evidence for a direct effect of secreted S100A8/A9 in an in vivo-model but also suggest that S100A8/A9 may be an ideal target for the development of treatment strategies against pancreatitis.
Acknowledgements
This work was supported by grants from the Deutsche Forschungsgemeinschaft (Le 625/8-1 and 9-1 to M.M.L., SFB 293 B7 to J.S. and M.M.L., KE 820/2-4 to C.K. MA 4115/1-2 to J.M.), BMBF-NBL3 Programme and DFG GRK840 to M.M.L and J.M., Deutsche Krebshilfe to M.M.L., J.M. and J.S. and the IZKF Münster (IZKF H3 to J.S. and M.M.L., IZKF Ker3_086_04 to C.K., and IZKF Na2/009/04 to W.N.). We wish to thank U. Breite, H. Heitmann, U. Naumann and S. Rackow for expert technical assistance.




