Functional interleukin-7/interleukin-7Rα, and SDF-1α/CXCR4 are expressed by human periodontal ligament derived mesenchymal stem cells
Abstract
Hematopoiesis in the bone marrow (BM) is maintained by specific interactions between both hematopoietic and non-hematopoietic stromal cells, which are mesenchymal stem cells (MSCs) capable of giving rise to several cell types. The human periodontal ligament (PDL), a tissue of ectomesenchymal origin, has been shown to also be a source of MSCs. We have investigated whether MSCs expanded from the PDL of healthy volunteers express characteristics similar to BM-derived stem cells using structural, immunocytochemical and molecular approaches. Their ability to support the growth of hematopoietic progenitors was also analyzed. The PDL-MSCs exhibited a fibroblast-like morphology and their chromatin was dispersed, indicating active gene transcription. The mesenchymal-related antigens CD90, CD29, CD166, CD105, and CD44 were homogeneously detected by cytofluorimetric analysis, whereas membrane CXCR4 was expressed only by a minority of cells. The PDL-MSCs differentiated in vitro into osteogenic and adipogenic cells. Immunolocalization of IL-7, IL-7Rα, SDF-1α, and CXCR4 resulted in a diffuse but specific labeling. RT-PCR analysis confirmed the expression of the above-mentioned transcripts. The cells spontaneously produced high levels of IL-7 and SDF-1α and were able to support the development and long-term maintenance of BM precursor cells more efficiently than murine stromal cells and similarly to normal BM human stromal cells. We examined IL-7 and SDF-1α secretion pathway during adipogenic and osteogenic differentiation. IL-7 increased during osteogenic and adipogenic differentiation, while the SDF-1α secretion was downregulated during osteogenic differentiation but increased during adipogenic induction. Our study provides evidence that in human PDL there is an accessible niche of MSCs showing the features of BM-derived MSCs. J. Cell. Physiol. 214: 706–713, 2008. © 2007 Wiley-Liss, Inc.
In vivo, and in vitro, hematopoiesis occurs in association with the complex network of cell types found in the stroma including hematopoietic and mesenchymal stem cells (MSCs) (Dexter and Lajtha, 1974; Gordon et al., 1987; Roberts et al., 1988; Sadahira and Mori, 1999). MSCs are the stromal cell population in the bone marrow (BM) containing precursors for several lineages including fibroblasts, endothelial cells, and adipocytes, and are engaged in the support of hematopoiesis providing both contact and non-contact signals (Gordon et al., 1987; Roberts et al., 1988). Recent studies reported that MSCs are able to regenerate tissues of the mesenchymal lineage, but also to transdifferentiate into cells derived from other embryonic layers, including neurons. These results have encouraged research about the possible use of ex vivo expanded MSCs populations for tissue engineering and gene therapy applications (Bianco and Robey, 2001; Trubiani et al., 2005).
Postnatal stem cells identified in various tissues reside in specialized microenvironments or niches endowed with the capacity to maintain stem cell proliferation, and to provide signals for their migration and differentiation. These unique attributes involve a complex array of both paracrine and autocrine signaling molecules, specific cell–cell and cell–extracellular matrix interactions, and biochemical and mechanical stimuli (Kortesidis et al., 2005). The expression of a chemokine receptor, CXCR4, is required for engraftment of hemopoietic and MSCs in the BM and for its repopulation (Lapidot, 2001); a small proportion of MSCs display membrane CXCR4 which directs their migration to the stem cell rich endosteal region (Lapidot et al., 2005). CXCR4 is a G protein-coupled 7-transmembrane receptor for the chemokine stromal cell-derived factor (SDF-1α or CXCL12), required for maintenance and renewal of stromal cells (Bleul et al., 1996; Oberlin et al., 1996; Kortesidis et al., 2005). SDF-1α was first identified as a pre-B-cell growth factor and was later demonstrated to be involved in migration, survival, and activation of different cell types. SDF-1α has highly conserved nucleotide and amino acid sequences and is widely expressed, suggesting a key biologic role. In fact, SDF-1α and CXCR4 are required for the embryonic development of the nervous, hematopoietic, and cardiovascular systems, and play an essential role in homing of hematopoietic stem/progenitor cells (Peled et al., 1999; Wynn et al., 2004; Zagzag et al., 2005) as well as in the metastatic colonization of bone and BM by breast and prostate cancer cells (Muller et al., 2001).
The receptor for another cytokine, IL-7 (IL-7Rα) is present on stromal cells (Sudo et al., 1989). IL-7 primarily acts as a growth and antiapoptotic factor for B and T cell precursors in the BM, thus representing a critical factor in lymphopoiesis. Recent evidence shows that IL-7 acts also in the periphery as a master regulator of T cell homeostasis, expanding both the naive and memory T cell populations (Mackall et al., 2001; Tan et al., 2001). IL-7 is constitutively expressed in the thymus, both in epithelial and dendritic cells, as well as in keratinocytes and in fetal and adult liver (Pillai et al., 2004; Isgro et al., 2005).
Recently, MSCs from various sources have been investigated for their versatility in tissue repair, since they can differentiate into multiple lineages (Bianco and Robey, 2001; Trubiani et al., 2005). The occurrence of stem cells with MSCs characteristics has been reported in periodontal ligament (PDL) and dental pulp (Seo et al., 2004; Pierdomenico et al., 2005; Shi et al., 2005; Trubiani et al., 2005; Ivanovski et al., 2006; Nagatomo et al., 2006). Tooth development occurs through mutually inductive signaling between oral epithelial and ectomesenchymal cells originating from migrating neural crest cells (Thesleff and Sharpe, 1997; Pispa and Thesleff, 2003). The whole tooth structure is held in place in the surrounding bone by a fibrocellular layer of PDL derived from the dental follicle, which is also of ectomesenchymal origin (Seo et al., 2004). It has been demonstrated that MSCs obtained from PDL can be successfully exploited for periodontal tissue regeneration (Seo et al., 2004; Shi et al., 2005; Trubiani et al., 2005; Gronthos et al., 2006; Inanc et al., 2006; Ivanovski et al., 2006; Nagatomo et al., 2006). These MSCs exhibit morphological and phenotypic features similar to dental pulp and BM stromal cells (Pierdomenico et al., 2005) but their molecular characteristics remain to be fully defined.
In the present study, we have further characterized PDL-derived MSCs by evaluating their pattern of expression of SDF-1α/CXCR4, IL-7/IL-7Rα, and their ability to support hematopoietic progenitors. Moreover, we successfully induced PDL-derived MSCs to differentiate towards osteoblasts or adipocytes, and we examined the production of IL-7 and SDF-1α during osteogenic and adipogenic induction.
Materials and Methods
Isolation and culture of PDL-MSCs
Human PDL biopsies were carried out after informed consent on five volunteers aged 20–35 years. All subjects were exempt from systemic and oral diseases. Explants were obtained from alveolar crestal and horizontal fibers of the PDL by scraping with a Gracey's curette the roots of non-carious third molar teeth (Carranza and Ubios, 1996). The PDL-MSCs were obtained and cultured in MSCM medium (Cambrex Company, Walkersville MD 21793-0127) according to the manufacturer's directions provided (Trubiani et al., 2005).
Cells migrated from the explants and on day 7, adherent cells which were 80–90% confluent as determined by phase contrast microscopy, were isolated using 0.1% trypsin solution and plated in tissue culture polystyrene flasks at 5 × 103 cells/cm2. Primary cultures of PDL mainly consisted of colonies of bipolar fibroblastoid cells which, after subcultivation, proliferate with a population-doubling time of 48 h reaching a confluent growth-arrested condition.
Single-cell suspensions of the developing adherent layer were prepared by trypsin/ ethylenediaminetetraacetic acid (EDTA) and cell growth was evaluated by the trypan blue dye exclusion test.
Light and electron microscopy analysis
On the second passage glass-adherent cells were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer pH 7.4 for 30 min at 4°C, postfixed with 1% osmium tetroxide, dehydrated in a graded series of ethanol, and embedded in Epon. The same monolayers were reembedded and sectioned perpendicularly after the coverslip was removed. Semithin sections were stained with toluidine blue and used for light microscope analysis. Images were observed with a Nikon Eclipse E 600 light microscope (Nikon) and captured using a Nikon Digital Camera Dmx 1200 operated with ACT-1 software. Thin sections were stained with uranyl acetate and lead citrate (all from Electron Microscopy Science, Rome, Italy), and observed with a JEM 1010 electron microscope (JEOL Ltd., Tokyo, Japan).
Flow cytometry analysis
Human PDL-MSCs at the 2nd passage were treated with 0.1% trypsin-EDTA, harvested and suspended in PBS with 1:100 dilution of mouse monoclonal antibodies directed to the following human antigens, either conjugated with fluorescein isothiocyanate (FITC): HLA-DR, CD2, CD3, CD7, CD14, CD26, CD29, CD31, CD34, CD38, CD44, CD45, CXCR4; HER-2, CD117 or with phycoerythrin (PE): CD33, CD68, CD90, CD105, CD166, S-100. The labeled cells were acquired and analyzed using a FACStar-plus flow cytometry system running the CellQuest software (Becton Dickinson (BD), San Jose, CA). All antibodies were from BD.
Osteogenic differentiation of PDL-MSCs
To induce osteogenic differentiation the sub-confluent cells were cultured in α-MEM (Sigma-Aldrich Inc, Milano, Italy) supplemented with 10% FBS, 10 mM β-glycerophosphate (Sigma), 0.5 mM ascorbic acid (Sigma), and 10−8 M dexamethasone (Sigma), and cultured for 4 weeks, replacing the medium every 2–3 days (Pierdomenico et al., 2005). To assess osteogenic differentiation, the cells were fixed in 10% formalin and stained with acid fuchsin and toluidine blue (Piattelli et al., 1997).
Adipogenic differentiation of PDL-MSCs
To induce adipogenic differentiation, 20 × 103 cells/cm2 were cultured in DMEM high glucose (Sigma) supplemented with 10% FBS, 0.5 mM isobutyl-methylxanthine (Sigma), 200 µM indomethacin (Sigma), 10−6 M dexamethasone (Sigma) and 10 µg/ml insulin (Sigma). The medium was replaced every 2–3 days, and after 3 weeks living cells were analyzed by contrast phase microscopy (Pierdomenico et al., 2005).
Immunofluorescence staining and confocal laser scanning microscope analysis
In these sets of experiments, PDL-MSCs cultures were fixed for 10 min with 4% paraformaldehyde solution in 0.1% cacodylate buffer at room temperature, after the cells were incubated with 10% BSA for 10 min, followed by incubation with the primary antibodies anti-human IL-7 (1:50), IL-7R α (1:50) (Sigma), SDF-1α (1:5), CXCR4 (1:50), HLA-DR (1:50, acting as negative control) (Santa Cruz Biotechnology; Santa Cruz, CA).
After 6 h incubation, the slides were rinsed with HBSS, and incubated for 60 min with (1:100) goat FITC-conjugated secondary antibody anti-mouse IgG (Santa Cruz Biotechnology).
Subsequently samples were counterstained with a 0.1% propidium iodide (PI) solution for 10 min. Intracellular staining was visualized by a Zeiss LSM510 META confocal system (Jena, Germany), connected to an inverted Zeiss Axiovert 200 microscope equipped with a Plan Neofluar oil-immersion objective (40×/1.3 NA). Images were collected using an argon laser beam with excitation lines at 488 nm and a helium-neon 543 nm source. To separate emissions of the two fluorochromes HTF 488/543 and NTF 545 primary and secondary dichroic mirrors respectively were used. Detector bandpass filters were set over 505–530 and 565–615 ranges for the green (FITC) and red (PI) emissions. The image series obtained from the PI and FITC signals were electronically merged and pseudo stained. The green and red digital images, respectively, were merged to detect any overlapping distribution of the two fluorochromes (yellow).
Reverse-transcription-polymerase chain reaction (RT-PCR) analysis
Total RNA from the MSCs was extracted using a SV total RNA isolation Kit (Promega; Mannheim, Germany), as recommended by the manufacturer. For RT-PCR, cDNA was synthesized using RETROscript (Ambion, Inc.; Austin, TX) with oligo-dT. The mRNA expression levels of CXCR4, SDF-1α, and IL-7 in the MSCs were analyzed using the specific primers, indicated in Figure 2E. Amplification was performed for 35 cycles (94°C 1 min, 59°C 1 min, 72°C 1 min). Amplified products were analyzed by 2% agarose gel electophoresis and visualized by ethidium bromide staining. Semiquantitative analysis of transcripts was acquired by GEL DOC 1000 Bio Rad instrument (BIO RAD, Inc.; Hercules, CA).
Spontaneous IL-7 and SDF-1α production from MSCs
MSCs were cultured in 24-well plates in RPMI, supplemented as described above, and incubated at 37°C in humidified air at 5% CO2. At weekly intervals, cultures were fed by demipopulation of the non-adherent cells and replacement of 500 µl of fresh, supplemented RPMI. The cultures were maintained until stromal confluence, then the cells were collected by trypsinization and cultured at a concentration of 1 × 106 cells/ml in a total volume of 1 ml/well. Supernatants at the I, III, and V passage collected after 24 h of culture and supernatants of PDL-MSCs induced to osteogenesis and adipogenesis was performed by enzyme-linked immunosorbent assay (ELISA) to evaluate IL-7 (R&D System, Minneapolis, MN) and SDF-1α (Ray Biotech, Inc., Norcross, GA) expression.
Long-term culture-initiating cell (LTC-IC) assay with periodontal MSCs
To analyze the capacity of the PDL-MSCs to support hematopoietic immature progenitor cell growth, we used the LTC-IC assay, according to a modification of the described methods (Dexter and Lajtha, 1974), in parallel with stromal cells obtained from the BM aspirate of a healthy subject and the murine M210B4 cell line (Isgro et al., 2005).
BM aspirate was initially collected into a tube containing EDTA as anticoagulant (Sigma–Aldrich). The BM sample was diluted 1:3 with PBS 1× plus EDTA 5 mM, and then separated after centrifugation by Ficoll (Lymphoprep, Nycomed Pharma, Oslo, Norway), resuspended in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), L-glutamine (2 mmol/L) and penicillin (250 U/ml) (all from Invitrogen Life Technologies s.r.l., Milan, Italy) and cultured until stromal confluence.
The murine stromal cell line M210B4, the PDL derived cell line and the stromal cell line from the normal BM were then trypsinized, irradiated (8,000 rad), washed and placed in six-well plates. Total BM mononuclear cells (BMMCs) (1 × 106 cells, in duplicate cultures) were applied on the pre-established, irradiated stromal feeder layer (from the murine cell line, from PDL-MSCs and from the healthy donor's stromal BM cells) and cultured at 37°C for 5 weeks.
The half-medium liquid was changed weekly. After 5 weeks, the adherent and the non-adherent cells were harvested by treatment with trypsin (Invitrogen Life Technologies), washed and replaced in duplicate in methylcellulose to evaluate the number of cells able to give rise to secondary colonies. The number of CFC (colonies forming cells) generated after 5 weeks of cultures on stromal cells gives an indirect, but consistent, measurement of the content of LTC-IC (Dexter and Lajtha, 1974).
Statistical analysis
The results were expressed as mean ± standard deviations of three or more experiments performed in duplicate. Values of P ≤ 0.05 were considered statistically different. Statistical analyses were performed by using Stat View 5.0 software (SAS Institute, Cary, NC).
RESULTS
Structural analyses
By light microscope observation the cells showed a fibroblast-like spindle shape and oval nuclei containing two or three nucleoli (Fig. 1A). The fine structure of human PDL-MSCs was analyzed by transmission electron microscopy. The cells were flattened and parallel sections showed a large cytoplasm containing extensive rough endoplasmic reticulum profiles, abundant mitochondria, some residual lysosomal bodies containing electron-dense material and bundles of filaments. In nuclei, the chromatin was dispersed indicating active gene transcription. Some membrane contacts with neighbor cells and numerous filopodia and occasionally desmosome-like junctions were observed (Fig. 1B, square and inset).
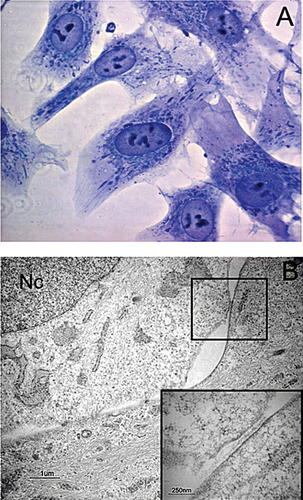
Light and electron microscopy analysis of human PDL-MSCs. A: Toluidine blue-stained cells analyzed by light microscopy. The cells show a fibroblastic-like morphology with a spindle shape and oval nuclei containing two or more nucleoli. B: Ultrastructural appearance of PDL-MSCs cells. The cells show a large cytoplasm with bundles of filaments, a well developed rough endoplasmic reticulum and normally shaped mitochondria. The nucleus (Nc) shows a decondensed homogeneous chromatin. The adjacent cells present junctional complexes; a desmosome-like junction in a square and at higher magnification in inset. Sections parallel to the culture surface. Original magnification: A, 100×; B, 11,000×, inset 38,000×. Bar: 1 µm (B) and 250 nm (inset).
Flow cytometry analysis
Homogeneous expression of the mesenchymal-related antigens CD90 (98.94%), CD29 (98.72%), CD166 (98.88%), CD105 (99.09%), CD44 (98.64%) was detected in PDL-MSCs, as previously for MSCs derived from extracted teeth, BM, and dental pulp (Pierdomenico et al., 2005; Trubiani et al., 2005; Ivanovski et al., 2006; Nagatomo et al., 2006). PDL-MSCs did not express HLA-DR, CD2, CD3, CD7, CD14, CD15, CD26, CD31, CD33, CD34, CD38, CD45, HER-2, CD117.
High cytoplasmic granularity and large size, as reported in previous works (Pierdomenico et al., 2005; Trubiani et al., 2005) was detected by light scatter. Only a small proportion of PDL-MSCs (3.7%) expressed surface CXCR4 (Fig. 2), despite constant intracellular positivity (see below).
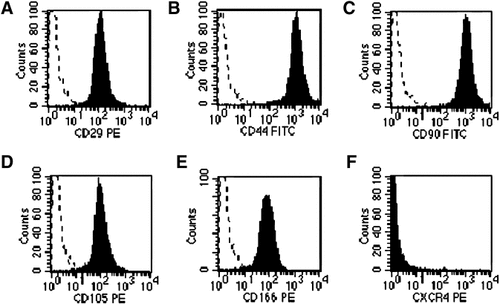
Immune phenotype of PDL-MSCs. Section A–F: cytofluorimetric analysis of the surface expression of the mesenchymal-related antigens: CD90, CD166, CD29, CD105, CD44, and CXCR4. All antigens were present, but CXCR4 was detectable only in a few cells. Broken lines indicate isotype control staining. The data are representative of five separate experiments.
PDL-MSCs differentiation
MSCs isolated from PDL were induced to differentiate into osteoblasts and fat cells using selective culture media as reported in Section Materials and Methods (Pierdomenico et al., 2005). In particular, during osteogenic differentiation the confluent cells, developing in an adherent layer, organize as small round nodules that subsequently mineralize as demonstrated by acid fuchsin and toluidine blue positive staining (Fig. 3A), whereas after adipogenic induction, the spindle-shaped MSCs exhibited many typical lipid vacuoles within the cells (Fig. 3B). The above features were not observed in PDL-MSCs cultures grown in regular culture medium.
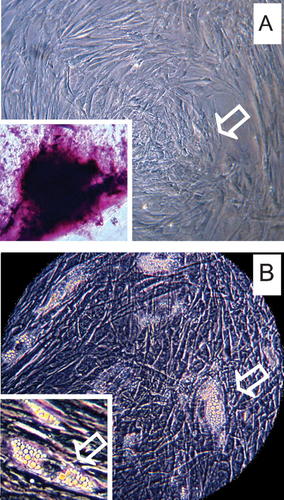
Ability of MSCs to differentiate into multiple lineages. A: osteogenic differentiation, indicated by bone nodule formation and acid fuchsin and toluidine blue staining (inset). B: adipogenic differentiation with the accumulation of lipid vacuoles in the cytoplasm of PDL-MSCs. Insets in the lower left corner of each image show at higher magnification the features of osteogenic and adipogenic induction. Magnification 25× (A,B) and 40× (insets).
Immunocytochemical characterization and RNA expression
Confocal microscope analysis of IL-7 (Fig. 4A), and SDF-1α (Fig. 4B) revealed a diffuse fluorescence localized in the cell cytoplasm, while CXCR4 (Fig. 4C) was detected in both cytoplasmic and cell surface compartments. For FITC-labeled anti-IL-7Rα (Fig. 4D), a specific immunostaining both at cytoplasmic and cell surface level was evidenced. At higher magnification the presence of large patches of immunoreactivity along the cell surface were observed.
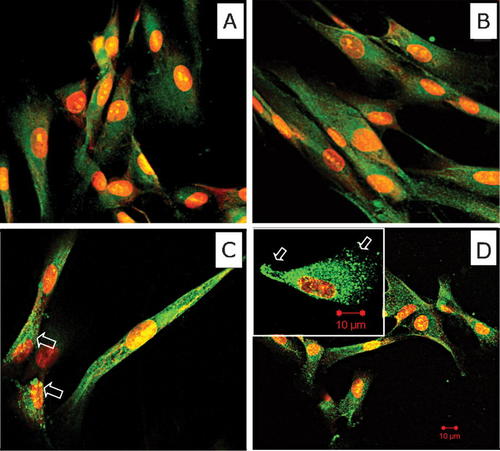
Confocal microscopy analysis of MSCs. Cells stained for IL-7(A), SDF-1α (B), and CXCR4 (C) show a diffuse cytoplasmic fluorescent green signal. Only few cells are positive for CXCR4 at the membrane surface (white arrow). In (D), the monolayer was stained with anti IL-7Rα antibody. PDL-MSCs showed the immune reactivity distributed at cytoplasmic and cell surface compartment. At higher magnification the presence of large areas of positivity are obviously present on the cell membrane (inset). In all parts, the nuclei were counterstained with PI. Original magnification: 40×, inset 100×. Bar: 10 µm.
Active production of the transcripts for CXCR4, SDF-1α, and IL-7, was confirmed by semiquantitative RT-PCR analysis of total RNA from PDL-MSCs (Fig. 5).
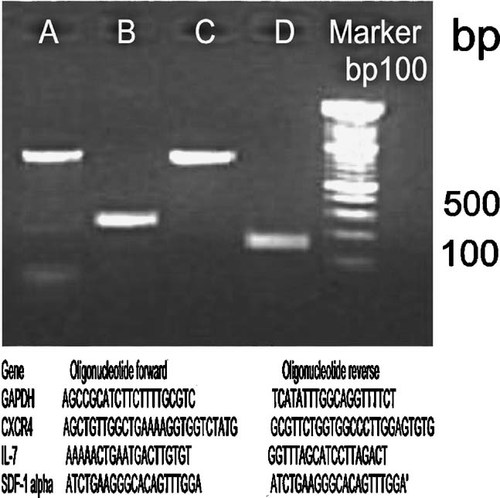
RT-PCR analysis. mRNA expression in 2nd passage of PDL-MSCs cultures using the following primer sets: lane A, GAPDH (763 bp); lane B, CXCR4 (260 bp); lane C, IL-7 (761 bp); lane D, SDF-1α (174 bp). Lane 5, molecular weight (100 bp). Primer sets used are indicated.
Spontaneous cytokine production from PDL-MSCS cultures
PDL-MSCs spontaneously produced significantly higher levels of IL-7 at passages I (10.9 pg/ml ± 0.1), III (9.5 pg/ml ± 0.1), and V (9 pg/ml ± 0.6) (Fig. 6A) than BM stromal cells from normal subjects (0.17 ± 0.1 pg/ml) (normal values from reference 20). High production of SDF-1α was also measured in the culture supernatants from the same passages, at I (4,560 pg/ml), III (4,129 pg/ml), and V passages (3,470 pg/ml) (Fig. 6B). Supernatants of PDL-MSCs cultures after induction to either adipocyte or osteoblast differentiation with selective media, were analyzed for both SDF-1α and IL-7 content. The results were compared with un-induced MSCs cultures. We observed a higher production of IL-7 in both differentiation induced cultures: osteoblasts: 20 pg/ml (Fig. 6C), adipocytes: 120 pg/ml (Fig. 6E), whereas SDF-1α was increased only in supernatants of adipocyte differentiating cells (20,000 pg/ml; Fig. 6F), with reduced levels in osteoblast cultures (400 pg/ml; Fig. 6D).
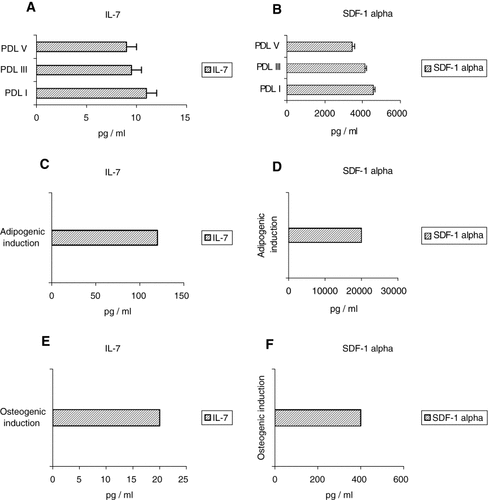
IL-7 and SDF-1α levels in PDL-MSCs. The histograms demonstrate the spontaneous production measured in the culture supernatant. The cells produced high levels of IL-7 (A) and SDF-1α (B) at I, III, and V passage in culture. The secretion of selected cytokines (IL-7 and SDF-1α) was also measured after 4 weeks in osteogenic differentiation (C,D), and after 3 weeks of induction in adipocyte-differentiated cultures (E,F). Samples from five different subjects were analyzed.
LTC-IC assay
We then investigated the ability of MSCs expanded ex vivo from PDL to support the survival and growth of hematopoietic progenitors by the LTC-IC assay, and compared them to the murine stromal M210B4 cell line and human normal BM stromal cells. This was determined by formation of secondary colonies of normal BMMCs cultured for 5 weeks.
PDL-MSCs supported the development and long-term maintenance of the BM precursor cells at least to the same extent as murine stromal cells and human BM-MSCs (Fig. 7).
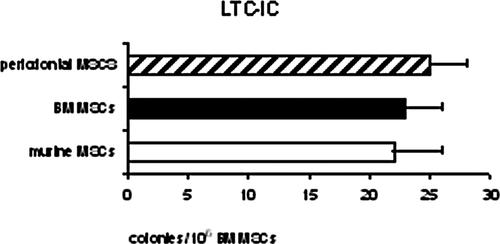
LTC-IC assay. The content of primitive progenitors was evaluated by the LTC-IC assay. The number of secondary colonies formed by human normal BM mononucleated cells after growing for 5 weeks on MSCs derived from periodontal ligament is shown, compared to those obtained after growing on the M210B4 murine stromal cell line or normal BM stromal cells.
Discussion
We have phenotypically and functionally characterized human mesenchymal cell lines derived from PDL. PDL contains postnatal MSCs able to regenerate several tissues of mesenchymal origin, such as bone, cartilage, and adipose tissue, as well as cementum/PDL-like structures which may assist in periodontal tissue repair (Seo et al., 2004; Shi et al., 2005). The resemblance of such MSCs cultured from PDL, with stromal cells in the BM has been noted previously (Trubiani et al., 2005; Ivanovski et al., 2006; Nagatomo et al., 2006) but detailed analysis of their in vitro functions, including support of hematopoiesis, has not been performed. In this study, we addressed this issue and analyzed ultrastructural and functional activities of human PDL-MSCs.
The spindle-like cells from the II passage onwards exhibit prominent nucleoli, rough endoplasmic reticulum, a well developed Golgi apparatus, and dispersed chromatin, all cellular features consistent with active transcription and not characteristic of a quiescent state. They also indicate the responsiveness of MSCs to growth stimuli contained in the selective culture medium. At higher magnification the cytoplasmic processes of PDL-MSCs can be seen to make contact with neighbor cells generating numerous filopodia (Trubiani et al., 2005) and junctional intercellular complexes as adherens junctions are observed. Intercellular adhesion and communication are essential components of tissue differentiation, morphogenesis, and are intimately related to the processes of cell signaling and cell motility (Gumbiner, 1996). Intercellular adhesion and gap junctions are demonstrated also in periodontal tissue and in undifferentiated embryonic cells (Kevin et al., 2000; Sathananthan et al., 2001; Baharvand and Matthaei, 2003).
PDL-MSCs display a phenotype expressing many known putative markers of MSCs (CD29, CD44, CD90, CD105, and CD166) and at the same time the absence of other hematopoietic and lineage-specific markers (CD2, CD3, CD7, CD14, CD26, CD31, CD33, CD34, CD38, CD45, HLA-DR, CD68, Her-2, CD117). Immunocytochemistry detected abundant intracellular SDF-1α and its receptor CXCR4, which was also present on the cell membrane in a small fraction of the PDL-MSCs by cytofluorimetric analysis. RT-PCR analysis confirmed the presence of transcripts for both molecules; it has been reported that SDF-1α and CXCR4 can be simultaneously induced in cancer cells (Broxmeyer et al., 2003). This is partly at variance with data on BM stromal cells, which express membrane CXCR4 only in a few cells, but are invariably positive for intracytoplasmic staining, perhaps due to internalization of the receptor with its ligand, which is thought to be bound from the environment (Lapidot et al., 2005). The membrane expression of CXCR4 should require further signals, which are not provided in our culture system, for example, the resecretion of SDF-1α by endothelial cells in the BM (Broxmeyer et al., 2003). This may reflect the characteristic binding of exogeneous SDF-1α by CXCR4 followed by internalization and transcytosis, demonstrated for BM stromal cells (Dar et al., 2005). Engraftment of hematopoietic stem cell transplants is dependent on the presence of SDF-1α and CXCR4 for homing and repopulation of the recipient BM, thus their expression on PDL-MSCs renders these cells ideally suited to provide support for clinical autologous transplantation (Trubiani et al., 2005). The low number of surface staining cells is critical for their homing to a favorable niche for survival (Tan et al., 2001).
An interesting observation concerns the detection of high amounts of IL-7 secreted in supernatants at several culture passages of PDL-MSCs. The levels observed are >1 log higher that those reported in normal BM cultures and in patients with HIV-1 infection or primary immunodeficiencies (Isgro et al., 2002).
The presence of cells able to produce IL-7 in the developing tooth has not been reported so far (Pispa and Thesleff, 2003), and PDL-MSCs are likely candidates for this function, in addition to the reported regenerative potential for dental structures (Shi et al., 2005; Ivanovski et al., 2006; Nagatomo et al., 2006). Constitutive secretion of IL-7 is uncommon even in tissues rich for their inducible production, such as the thymus (Zubkova et al., 2005) where mRNA level correlates with atrophic changes, and BM stroma, where IL-7 dependent B cell development depends on induction by contact with pre-B lymphocytes (Sudo et al., 1989). However, BM stromal cells are poor producers of IL-7 as shown by failure to detect IL-7 mRNA expression in unstimulated MSCs derived from the BM (Kim et al., 2005) and low secreted levels in normal BM, previously reported (Isgro et al., 2005). In addition, IL-7 was distinctly absent among the many cytokines measured by protein array in umbilical cord blood-derived MSCs under normal growth conditions (Liu and Hwang, 2005). Since MSCs express IL-7 receptor and IL-7, a typical stromal derived cytokine, seems to be necessary for their function (Iwata et al., 2002; Pillai et al., 2004) it has been proposed that IL-7 drives MSCs self renewal. The finding of high amounts of secreted IL-7 in PDL-MSCs cultures points to its role both in autocrine growth and in providing survival and differentiation signals for the surrounding odontogenic structures, since PDL itself can regenerate starting from these MSCs. However, mechanistic and functional studies on the relevance of the IL-7/IL-7Rα pathway have yet to be performed.
The peculiar production of IL-7 renders such cells an attractive candidate to promote engraftment of BM transplant and accelerate lymphoid reconstitution (Appasamy, 1999). Transfection of BM stroma with a retroviral vector containing the IL-7 gene has resulted in the generation of CD 90+CD105+ mesenchymal cells spontaneously secreting high amounts of IL-7 in the pg/ml range (Di Ianni et al., 2005), similar to levels measured in our PDL-MSCs supernatants at different culture passages.
We easily induced PDL-MSCs to differentiate to a certain degree, showing characteristic features of specialized cells of mesenchymal origin, such as osteoblasts and adipocytes. A central issue in this respect is the ability of the PDL-derived MSCs to act as efficiently as BM stromal cells in supporting long-term maintenance of hematopoietic primitive precursor cells. The ability of PDL-MSCs to perform as well as BM stroma on BMMC precursors represents additional evidence of the plasticity of these cell lines. Moreover, the modulated expression of IL-7 and SDF-1α during adipogenic and osteogenic differentiation processes are consistent with the functionality of these secreted molecules, and in particular confirming the downregulation of SDF-1 gene expression during osteogenesis (Kortesidis et al., 2005). Since intracellular signaling of SDF-1 α involves several kinases (Okabe et al., 2005), we are currently addressing the role of Zap-70, which is expressed in our PDL-derived MSCs (data not shown) in mediating modifications of potential release of cytokines. Better definition of the full range of activities of MSCs derived from an easily accessible source as PDL is presently the focus of our joint research effort.
Acknowledgements
The authors are indebted to Massimo Valentini M.D. and Ms. Marika D'Urbano for flow cytometric data analysis.




