Estrogen-mediated activation of non-genomic pathway improves macrophages cytokine production following trauma-hemorrhage
Abstract
Although 17β-estradiol (E2) attenuates the alterations in Kupffer cells and splenic macrophages (MΦ) cytokine production following trauma-hemorrhage, the mechanism by which this occurs remains unknown. Utilizing a cell-impermeable E2 conjugated with BSA (E2-BSA), we examined the non-genomic effects of E2 on the above two cell population cytokine production, MAPK and transcription factors activation following trauma-hemorrhage. Male Sprague–Dawley rats underwent trauma-hemorrhage (mean BP 40 mmHg for 90 min, then resuscitation). E2, E2-BSA (1 mg/kg E2) with or without an estrogen receptor antagonist (ICI 182,780), or vehicle was administrated during resuscitation. Two hrs thereafter, Kupffer cells and SMΦ production of IL-6, TNF-α, and IL-10, activation of MAPK (p38, ERK-1/2, and JNK), and transcription factors (NF-κB and AP-1) were determined. IL-6, TNF-α, and IL-10 productive capacity, MAPK, and transcription factors activation increased in Kupffer cells while they decreased in SMΦ following trauma-hemorrhage. However, E2 administration normalized all of these alterations. Although E2-BSA also attenuated the alterations in cytokine production/transcription factors, the values were higher in Kupffer cells and lower in SMΦ compared to shams. In contrast, E2-BSA prevented trauma-hemorrhage-mediated changes in MAPK activation to the same extent as E2. Co-administration of ICI 182,780 abolished E2-BSA effects. Although some MAPK inhibitors suppressed cytokine production, the inhibitor effectiveness was dependent on cytokine, cell type and animal condition (trauma-hemorrhage or sham). Thus, E2 effects on Kupffer cells and SMΦ cytokine production and transcription factors activation following trauma-hemorrhage are mediated at least in part via non-genomic pathway and these non-genomic effects are likely mediated via MAPK pathways. J. Cell. Physiol. 214: 662–672, 2008. © 2007 Wiley-Liss, Inc.
Previous studies have shown that the capacity of Kupffer cells to produce various proinflammatory cytokines increases, while that of splenic macrophages decreases in male animals following trauma-hemorrhage (Ayala et al., 1996; Angele et al., 2000; Chow et al., 2005). These alterations were associated with the increased susceptibility to subsequent sepsis (Angele et al., 2000; Schneider et al., 2000; Diodato et al., 2001; Knoferl et al., 2002; Wu et al., 2004; Moore et al., 2005). However, such alterations in the production of pro-inflammatory cytokines and mortality from subsequent sepsis were not observed in proestrus female animals under those conditions (Angele et al., 2000). Additional studies have shown that the protection against trauma-hemorrhage in proestrus females is due to the high levels of female sex steroids under those conditions (Angele et al., 2000; Meldrum, 2006). Studies have also shown that administration of a single dose of 17β-estradiol (E2) following trauma-hemorrhage in ovariectomized animals and males normalizes immune cell functions (Angele et al., 2000; Knoferl et al., 2001).
Two major pathways, generally named genomic and non-genomic pathways, are known to mediate E2 effects on cells. E2 has traditionally been described to mediate its effects via intracellular receptors located in the cytoplasm or on the nuclear membrane and thus studies have investigated the role of transcription factors in the regulation of target genes (Kuiper et al., 1996). However, recent findings indicate that E2 also initiates a rapid signaling pathways in the cytoplasm and regulate cellular functions, which is referred to as the non-genomic pathway (Ho and Liao, 2002; Simoncini and Genazzani, 2003).
E2 conjugated to BSA (E2-BSA) has been shown to be a plasma membrane impermeable compound and thus has been used to study the role of surface E2 receptors in producing the salutary effects of E2 on cellular functions. Furthermore, this E2-BSA conjugates has also been used to study the non-genomic effects of E2 (Razandi et al., 1999; Russell et al., 2000; Kow and Pfaff, 2004).
Although E2 produces salutary effects on immune cells following trauma-hemorrhage, it remains unknown whether those effects of E2 are mediated via genomic or non-genomic pathway. In this study, we determined the role of non-genomic pathway in E2-mediated restoration of Kupffer cell and splenic macrophage cytokine production, by administrating cell impermeable E2-BSA conjugate following trauma-hemorrhage. In addition, since studies have shown that the non-genomic effects of E2 are mediated by mitogen-activated protein kinases (MAPK) pathways (Razandi et al., 2000; Simoncini et al., 2000; Watters et al., 2000; Zhang and Shapiro, 2000; Flores-Delgado et al., 2001; de Jager et al., 2001; Setalo et al., 2002), we examined the effects of E2-BSA as well as E2 on MAPK pathways in Kupffer cells and splenic macrophages. By using selective MAPK inhibitors in vitro, we also determined which MAPK pathway is involved in cytokine production by Kupffer cells and splenic macrophages. Additionally, to elucidate further the role of downstream molecules in E2-mediated restoration of Kupffer cell and macrophage functions, we examined the effect of E2-BSA and E2 on the activation of transcription factors nuclear factor kappa B (NF-κB; Coimbra et al., 2006) and activator protein 1 (AP-1) following trauma-hemorrhage.
Materials and Methods
Animals
Adult male (275–325 g) Sprague–Dawley rats (Charles River Laboratories, Wilmington, MA) were used in this study. All experiments were performed in adherence to the National Institutes of Health Guidelines for the Use of Experimental Animals and approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
Trauma-hemorrhage procedure
A nonheparinized rat model of trauma-hemorrhage, as described previously (Wang et al., 1997), was used in this study. Briefly, rats (275–325 g) were fasted overnight before the experiment but allowed water ad libitum. The rats were anesthetized by isoflurane (Attane, Minrad, Bethlehem, PA) inhalation before the induction of soft tissue trauma (i.e., 5-cm midline laparotomy). The abdominal incision was then closed in two layers, and polyethylene catheters (PE-50, Becton-Dickinson, Sparks, MD) were placed in both femoral arteries and the right femoral vein. The rats were then placed into a Plexiglas box (21 × 9 × 5 cm) in a prone position and allowed to awaken following which they were bled rapidly within 10 min to a mean arterial pressure (MAP) of 35–40 mmHg. The time at which the animals could no longer maintain a MAP of 35–40 mmHg without infusion of some fluid was defined as maximum bleed-out volume. MAP was maintained at 40 mmHg until 40% of the shed blood was returned in the form of Ringer's lactate. The animals were then resuscitated with 4× the shed blood volume with Ringer's lactate over 60 min. Thirty minutes before the end of the resuscitation period, the rats received 17β-estradiol (E2; 1 mg/kg, intravenously), E2-BSA (1 mg/kg E2) with or without estrogen receptor (ER) antagonist ICI 182,780 (3 mg/kg, intraperitoneally at the beginning of resuscitation), or an equal volume of the vehicle (BSA). E2-BSA was filtered before injection to remove any free E2 (Stevis et al., 1999). Following resuscitation, the catheters were removed, the vessels ligated, and skin incisions closed with sutures. Sham-operated animals underwent laparotomy and the same groin dissection, which included the ligation of the femoral artery and vein, but neither hemorrhage nor resuscitation was carried out. At 2 h following trauma-hemorrhage or sham operation, the rats were anesthetized with isoflurane and exsanguinated to collect samples.
Kupffer cell isolation
Kupffer cells were isolated by an in situ collagenase digestion method (Suzuki et al., 2006b). Briefly, the liver was perfused with oxygenized HBSS (Invitrogen, Grant Island, NY) for 10 min to wash the blood and was perfused with 0.04% collagenase (Sigma, St. Louis, MO) for 5 min. After the digestion and mechanical disruption of the liver, the cell suspension was filtered through a sieve and centrifuged at 50g for 3 min to separate parenchymal from nonparenchymal cells. Nonparenchymal cells were collected and centrifuged over 16% HistoDenz (Sigma) for 45 min (2000g, 4°C). The cells at the interface were collected and washed twice by centrifugation with HBSS (450g, 10 min, 4°C). The cell fractions were then washed, counted, and suspended (1 × 106 cells/ml) in William's E medium (Invitrogen) in 24-well plates. After 2 h of incubation (37°C at 5% CO2), nonadherent cells were removed, and 1 ml of fresh William's E medium containing 10% fetal bovine serum (Invitrogen) and 1% penicillin–streptomycin (Invitrogen) was added to the adhered Kupffer cells.
Isolation of splenic macrophages
Spleens were removed aseptically and placed into 50-ml conical tube with cold PBS (Suzuki et al., 2006a). The spleens were then gently ground between frosted microscope slides to produce single-cell suspension, and centrifuged at 400g, at 4°C for 15 min. The erythrocytes were lysed with Buffer EL (Qiagen, Valencia, CA). The remaining cells were then washed, counted, and suspended (1 × 107 cells/ml) in RPMI 1640 and incubated in 24-well plates. After 2 h of incubation (37°C at 5% CO2), nonadherent cells were removed, and 1 ml of fresh RPMI 1640 containing 10% fetal bovine serum and antibiotics was added to the adhered splenic macrophages.
Measurement of cytokine production
Kupffer cells and splenic macrophages were incubated at 37°C and 5% CO2 for 24 h with 1 µg/ml LPS (from E. coli, 055: B5, Sigma). Using a separate groups of trauma-hemorrhage or sham animals, Kupffer cells and splenic macrophages were isolated and were treated with the selective inhibitors of MAPK pathway, SB203580 (5 µM) for p38, PD98059 (20 µM) for ERK1/2, and SP600125 (20 µM) for JNK (Calbiochem, La Jolla, CA) 30 min before LPS stimulation (Alexander et al., 2004). The supernatants were then harvested and analyzed for the presence of IL-6, TNF-α and IL-10 using DuoSet ELISA system (R&D, Minneapolis, MN) according to the manufacturer's instructions.
Measurement of p38, ERK1/2, and JNK protein and phosphorylation levels
As described previously (Li et al., 2005), Kupffer cells and splenic macrophages were incubated with 1 µg/ml LPS for 10 and 30 min respectively, and lysed in a lysis buffer. For the analysis of p38, ERK1/2, and JNK protein and phosphorylation, lysates were analyzed using SDS–PAGE and transferred to Immobilon P membranes (polyvinylidene difluoride; Millipore, Bedford, MA) by using a Semidry Trans-Blot system (Bio-Rad, Richmond, CA). The membranes were saturated with blocking buffer (10 mM Tris, 150 mM NaCl, and 0.05% Tween-20 supplemented with 5% dry milk) for 1 h at room temperature and incubated with the antibody to p38 protein, phospho-p38, ERK1/2 protein, phospho-ERK1/2, JNK protein and phospho-JNK (Cell Signaling, Beverly, MA) at 4°C overnight. The membranes were then washed five times with TBST (Tris-buffered saline supplemented with 0.05% Tween-20) followed by incubation with a secondary antibody conjugated with horseradish peroxidase for 1 h at room temperature. The membranes were washed five times with TBST and probed using enhanced chemiluminescence dye, and phosphoproteins were autoradiographed.
Measurement of NF-κB and AP-1 DNA binding activity
Kupffer cells and splenic macrophages were incubated with 1 µg/ml LPS for 1 h and nuclear extracts were prepared as previously described (Schreiber et al., 1989). NF-κB and AP-1 DNA binding activity was measured using Trans-AM ELISA-based kits (Active Motif, Carlsbad, CA) according to the manufacturer's instructions (Sorokina et al., 2004). Briefly, the nuclear extracts of Kupffer cells and splenic macrophages were incubated in a 96-well plate coated with an oligonucleotide containing the NF-κB or AP-1 consensus binding site. Activated transcription factors from the nuclear extracts specifically bound to the respective immobilized oligonucleotide were detected using the antibodies to NF-κB p65, c-Fos, or c-Jun followed by a secondary antibody conjugated to horseradish peroxidase in an ELISA-like assay.
Statistical analysis
Data are presented as mean ± SEM. Statistical differences between groups were determined by one-way ANOVA followed by Student–Newman–Keuls Method or Dunnett's Method. The differences were considered significant if P < 0.05.
Results
Cytokine production
IL-6, TNF-α, and IL-10 production by Kupffer cells increased following trauma-hemorrhage (Fig. 1A). However, E2 administration following trauma-hemorrhage normalized the production of these cytokines by Kupffer cells. Although the increase of IL-6, TNF-α, and IL-10 production by Kupffer cells was also attenuated by E2-BSA administration following trauma-hemorrhage, the Kupffer cells capacity to produce these cytokines still remained significantly higher than shams.
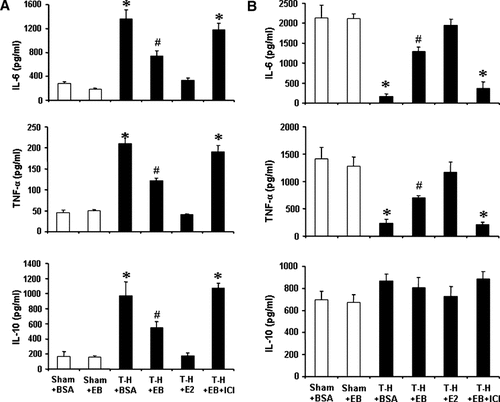
IL-6, TNF-α, and IL-10 production by Kupffer cells (A) and splenic macrophages (B) from sham animals receiving BSA (Sham + BSA), shams receiving E2-BSA (Sham + E), trauma-hemorrhage (T-H) receiving BSA (T-H + BSA), T-H treated with E2-BSA (T-H + EB), T-H treated with E2 (T-H + E2), and T-H treated with E2-BSA and ICI 182,780 (T-H + E + ICI). Kupffer cells and splenic macrophages were isolated 2 h after T-H and resuscitation and cultured with LPS (1 µg/ml) for 24 h. Cytokine levels in culture supernatants were determined by ELISA. Data are mean ± SEM of six to seven animals in each group. *P < 0.05 compared to sham; #P < 0.05 compared to sham and T-H + BSA.
IL-6 and TNF-α production by splenic macrophages decreased following trauma-hemorrhage (Fig. 1B). However, E2 administration following trauma-hemorrhage normalized the production of these cytokines by splenic macrophages. Although the suppression of IL-6 and TNF-α production by splenic macrophages was also attenuated by E2-BSA administration following trauma-hemorrhage, the splenic macrophages capacity to produce these cytokines still remained significantly lower than shams. In contrast, IL-10 production by splenic macrophages was not significantly different in all groups.
Administration of ICI 182,780 along with E2-BSA abolished the effects of E2-BSA on both Kupffer cells and splenic macrophages cytokine productive capacities.
P38, ERK1/2, and JNK phosphorylation
To determine MAPK activation, phosphorylation of p38, ERK1/2, and JNK in Kupffer cells (Figs. 2-4, respectively) and splenic macrophages (Figs. 5-7, respectively) with or without LPS stimulation was evaluated. The phosphorylation of p38, ERK1/2, and JNK in unstimulated Kupffer cells was increased following trauma-hemorrhage (Part A in Figs. 2-4). Stimulation of these cells with LPS resulted in an increase in the three MAPK phosphorylation in Kupffer cells from animals in all groups (Part A in Figs. 2-4) compared to unstimulated cells; however, LPS-induced phosphorylation of p38, ERK1/2, and JNK was significantly higher in Kupffer cells from rats subjected to trauma-hemorrhage compared to those from sham-operated rats (Part B in Figs. 2-4). Administration of E2-BSA or E2 following trauma-hemorrhage prevented the increase in p38, ERK1/2, and JNK phosphorylation in Kupffer cells. There was no significant difference in p38, ERK1/2, and JNK protein expression in Kupffer cells from rats subjected to trauma-hemorrhage compared to rats subjected to sham operation (Part C in Figs. 2-4).
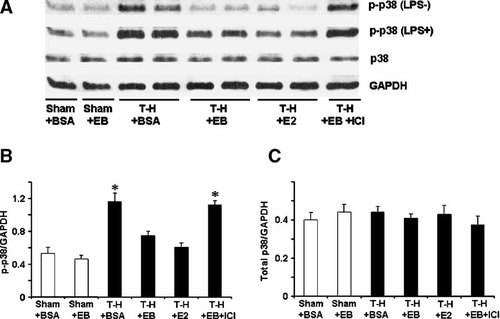
p38 phosphorylation and protein expression in Kupffer cells with LPS stimulation (LPS + ) or without stimulation (LPS−) from sham animals treated with BSA (Sham + BSA), sham treated with E2-BSA (Sham + EB), trauma-hemorrhage (T-H) treated with BSA (T-H + BSA), T-H treated with E2-BSA (T-H + EB), T-H treated with E2 (T-H + E2), and T-H treated with E2-BSA and ICI 182,780 (T-H + EB + ICI). Kupffer cells were isolated 2 h after T-H and resuscitation, stimulated with 1 µg/ml LPS for 10 min, and lysed. Lysates were then analyzed for p38 phosphorylation (p-p38) and protein expression (A). Blots were reprobed for GAPDH for equal protein loading in various lanes. p38 blots obtained from five animals were analyzed using densitometry, and densitometric values for phosphorylation and total protein were normalized to GAPDH and are shown as mean ± SEM in (Parts B, C) respectively. *P < 0.05 compared to sham.
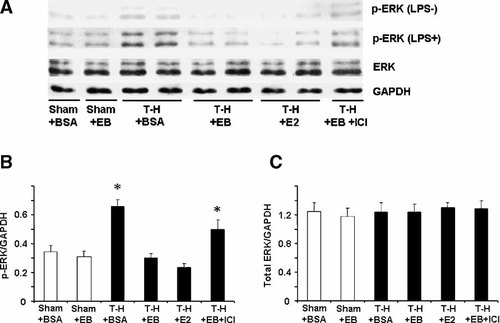
ERK phosphorylation and protein expression in Kupffer cells with LPS stimulation (LPS+) or without stimulation (LPS−) from sham animals treated with BSA (Sham + BSA), sham treated with E2-BSA (Sham + EB), trauma-hemorrhage (T-H) treated with BSA (T-H + BSA), T-H treated with E2-BSA (T-H + EB), T-H treated with E2 (T-H + E2), and T-H treated with E2-BSA and ICI 182,780 (TH + EB + ICI). Kupffer cells were isolated 2 h after T-H and resuscitation, stimulated with 1 µg/ml LPS for 10 min, and lysed. Lysates were then analyzed for ERK phosphorylation (p-ERK) and protein expression (A). Blots were reprobed for GAPDH for equal protein loading in various lanes. ERK blots obtained from five animals were analyzed using densitometry, and densitometric values for phosphorylation and total protein were normalized to GAPDH and are shown as mean ± SEM in (Parts B, C) respectively. *P < 0.05 compared to sham.
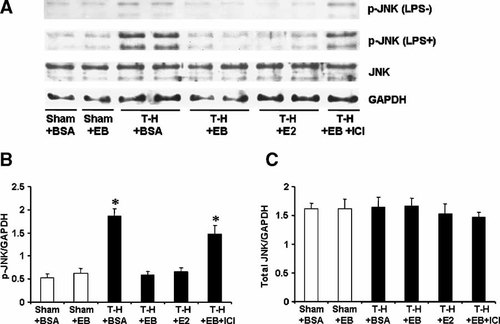
JNK phosphorylation and protein expression in Kupffer cells with LPS stimulation (LPS+) or without stimulation (LPS−) from sham animals treated with BSA (Sham + BSA), sham treated with E2-BSA (Sham + EB), trauma-hemorrhage (T-H) treated with BSA (T-H + BSA), T-H treated with E2-BSA (T-H + EB), T-H treated with E2 (TH + E2), and T-H treated with E2-BSA and ICI 182,780 (TH + EB + ICI). Kupffer cells were isolated 2 h after T-H and resuscitation, stimulated with 1 µg/ml LPS for 10 min, and lysed. Lysates were then analyzed for JNK phosphorylation (p-JNK) and protein expression (A). Blots were reprobed for GAPDH for equal protein loading in various lanes. JNK blots obtained from five animals were analyzed using densitometry, and densitometric values for phosphorylation and total protein were normalized to GAPDH and are shown as mean ± SEM in (Parts B, C) respectively. *P < 0.05 compared to sham.
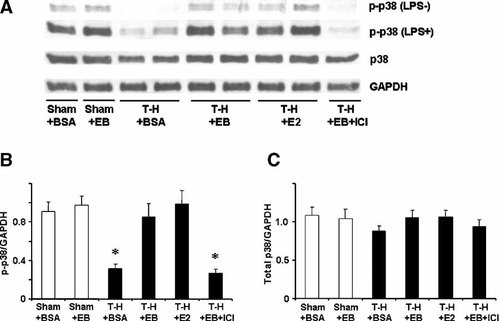
p38 phosphorylation and protein expression in splenic macrophages with LPS stimulation (LPS+) or without stimulation (LPS−) from sham animals treated with BSA (Sham + BSA), sham treated with E2-BSA (Sham + EB), trauma-hemorrhage (T-H) treated with BSA (T-H + BSA), T-H treated with E2-BSA (T-H + EB), T-H treated with E2 (T-H + E2), and T-H treated with E2-BSA and ICI 182,780 (T-H + EB + ICI). Splenic macrophages were isolated 2 h after T-H and resuscitation, stimulated with 1 µg/ml LPS for 30 min, and lysed. Lysates were then analyzed for p38 phosphorylation (p-p38) and protein expression (A). Blots were reprobed for GAPDH for equal protein loading in various lanes. p38 blots obtained from five animals were analyzed using densitometry, and densitometric values for phosphorylation and total protein were normalized to GAPDH and are shown as mean ± SEM in (Parts B, C) respectively. *P < 0.05 compared to sham.
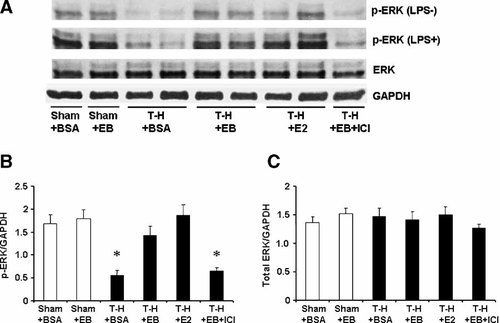
ERK phosphorylation and protein expression in splenic macrophages with LPS stimulation (LPS+) or without stimulation (LPS−) from sham animals treated with BSA (Sham + BSA), sham treated with E2-BSA (Sham + EB), trauma-hemorrhage (T-H) treated with BSA (T-H + BSA), T-H treated with E2-BSA (TH + EB), T-H treated with E2 (T-H + E2), and TH treated with E2-BSA and ICI 182,780 (T-H + EB + ICI). Splenic macrophages were isolated 2 h after T-H and resuscitation, stimulated with 1 µg/ml LPS for 30 min, and lysed. Lysates were then analyzed for ERK phosphorylation (p-ERK) and protein expression (A). Blots were reprobed for GAPDH for equal protein loading in various lanes. ERK blots obtained from five animals were analyzed using densitometry, and densitometric values for phosphorylation and total protein were normalized to GAPDH and are shown as mean ± SEM in (Parts B, C) respectively. *P < 0.05 compared to sham.
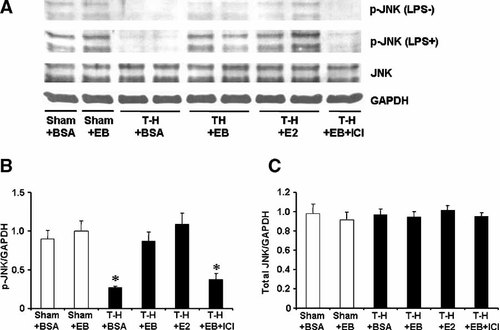
JNK phosphorylation and protein expression in splenic macrophages with LPS stimulation (LPS+) or without stimulation (LPS−) from sham animals treated with BSA (Sham + BSA), sham treated with E2-BSA (Sham + EB), trauma-hemorrhage (T-H) treated with BSA (T-H + BSA), TH treated with E2-BSA (T-H + EB), T-H treated with E2 (T-H + E2), and T-H treated with E2-BSA and ICI 182,780 (T-H + EB + ICI). Splenic macrophages were isolated 2 h after T-H and resuscitation, stimulated with 1 µg/ml LPS for 30 min, and lysed. Lysates were then analyzed for JNK phosphorylation (p-JNK) and protein expression (A). Blots were reprobed for GAPDH for equal protein loading in various lanes. JNK blots obtained from five animals were analyzed using densitometry, and densitometric values for phosphorylation and total protein were normalized to GAPDH and are shown as mean ± SEM in (Parts B, C) respectively. *P < 0.05 compared to sham.
The phosphorylation of p38, ERK1/2, and JNK in unstimulated splenic macrophages was decreased following trauma-hemorrhage (Part A in Figs. 5-7). Stimulation of these cells with LPS resulted in an increase in the three MAPK phosphorylation in splenic macrophages from animals in all groups (Part A in Figs. 5-7); however, LPS-induced phosphorylation of p38, ERK1/2, and JNK was significantly lower in splenic macrophages from rats subjected to trauma-hemorrhage compared to those from sham-operated rats (Part B in Figs. 5-7). Administration of E2-BSA or E2 following trauma-hemorrhage prevented the decrease in p38, ERK1/2, and JNK phosphorylation in splenic macrophages. There was no significant difference in p38, ERK1/2, and JNK protein expression in splenic macrophages from rats subjected to trauma-hemorrhage compared to rats subjected to sham operation (Part C in Figs. 5-7).
Co-administration of ICI 182,780 abolished the effects of E2-BSA on MAPK activation in both Kupffer cells and splenic macrophages.
Effects of MAPK inhibitor on cytokine production
IL-6 production was significantly suppressed by p38 inhibition in Kupffer cells (Fig. 8) and by inhibition of ERK1/2 or JNK pathway in splenic macrophages (Fig. 9) irrespective of whether the rats were subjected to sham or trauma-hemorrhage. TNF-α production was significantly decreased by inhibition of p38 or JNK in both Kupffer cells and splenic macrophages. Moreover, IL-10 production by Kupffer cells was suppressed by p38 inhibition in sham and trauma-hemorrhage groups. JNK inhibition significantly suppressed IL-10 production by Kupffer cells only in trauma-hemorrhage group.
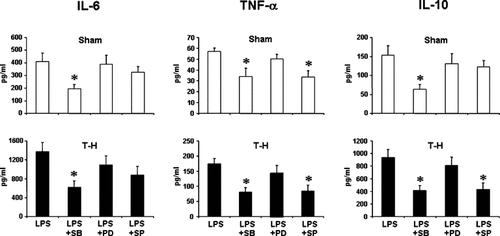
IL-6, TNF-α, and IL-10 production by Kupffer cells from sham and trauma-hemorrhage (T-H) animals. Kupffer cells were isolated 2 h after sham operation or T-H and resuscitation and treated with the selective inhibitors of MAPK pathway, SB203580 (5 µM) for p38, PD98059 (20 µM) for ERK1/2, and SP600125 (20 µM) for JNK 30 min before LPS stimulation (1 µg/ml for 24 h). Cytokine levels in culture supernatants were determined by ELISA. Data are mean ± SEM of six animals in each group. *P < 0.05 compared to LPS alone. SB: SB203580, PD: PD98059, SP: SP600125.
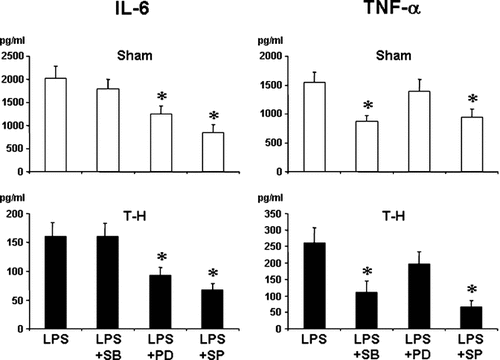
IL-6 and TNF-α production by splenic macrophages from sham and trauma-hemorrhage (T-H) animals. Splenic macrophages were isolated 2 h after sham operation or T-H and resuscitation and treated with the selective inhibitors of MAPK pathway, SB203580 (5 µM) for p38, PD98059 (20 µM) for ERK1/2, and SP600125 (20 µM) for JNK 30 min before LPS stimulation (1 µg/ml for 24 h). Cytokine levels in culture supernatants were determined by ELISA. Data are mean ± SEM of six animals in each group. *P < 0.05 compared to LPS alone. SB: SB203580, PD: PD98059, SP: SP600125.
NF-κB DNA binding activity
To determine the activation of NF-κB and AP-1, DNA binding activity of p65 subunit for NF-κB, and c-Jun and c-Fos for AP-1 in Kupffer cells and splenic macrophages with LPS stimulation was evaluated. DNA binding activity of NF-κB and AP-1 was increased in Kupffer cells (Fig. 10) and decreased in splenic macrophages (Fig. 11) following trauma-hemorrhage. However, E2 administration following trauma-hemorrhage normalized DNA binding activity of these transcription factors. Although E2-BSA administration also attenuated the changes of NF-κB and AP-1 activity, the levels remained significantly different from those observed in sham animals. Co-administration of ICI 182,780 abolished the effects of E2-BSA on the activation of NF-κB and AP-1 in both Kupffer cells and splenic macrophages.
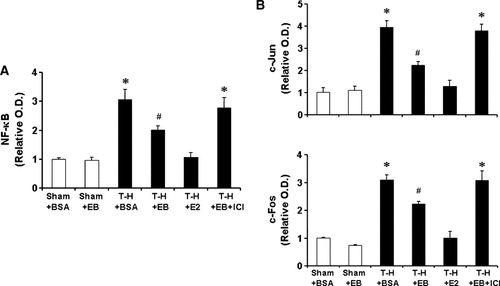
NF-κB and AP-1 DNA binding activity in Kupffer cells with LPS stimulation from sham animals treated with BSA (Sham + BSA), sham treated with E2-BSA (Sham + EB), trauma-hemorrhage (T-H) treated with BSA (T-H + BSA), T-H treated with E2-BSA (T-H + EB), T-H treated with E2 (T-H + E2), and T-H treated with E2-BSA and ICI 182,780 (T-H + EB + ICI). Kupffer cells were isolated 2 h after T-H and resuscitation, stimulated with 1 µg/ml LPS for 1 h, and lysed. Lysates were then analyzed for DNA binding activity of p65 for NF-κB (A), and c-Fos and c-Jun for AP-1 (B) using ELISA-based assay. Data are shown as mean ± SEM. *P < 0.05 compared to sham; #P < 0.05 compared to sham and T-H + BSA. OD, optical density.
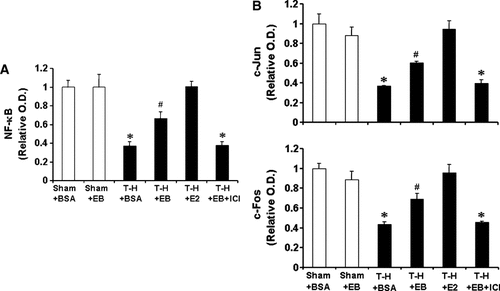
NF-κB and AP-1 DNA binding activity in splenic macrophages with LPS stimulation from sham animals treated with BSA (Sham + BSA), sham treated with E2-BSA (Sham + EB), trauma-hemorrhage (T-H) treated with BSA (T-H + BSA), T-H treated with E2-BSA (T-H + EB), TH treated with E2 (T-H + E2), and T-H treated with E2-BSA and ICI 182,780 (T-H + EB + ICI). Splenic macrophages were isolated 2 h after T-H, stimulated with 1 µg/ml LPS for 1 h, and lysed. Lysates were then analyzed for DNA binding activity of p65 for NF-κB (A), and c-Fos and c-Jun for AP-1 (B) using ELISA-based assay. Data are shown as mean ± SEM. *P < 0.05 compared to sham; #P < 0.05 compared to sham and T-H + BSA. OD, optical density.
Discussion
Our findings demonstrate that Kupffer cells capacity to produce IL-6, TNF-α, and IL-10 increased while the splenic macrophages IL-6 and TNF-α productive capacity decreased following trauma-hemorrhage. However, E2 administration normalized these alterations in cytokine production. Although E2-BSA administration also significantly attenuated the alterations in Kupffer cell and splenic macrophage cytokine production, the capacity of these cytokines production was still higher in Kupffer cells and lower in splenic macrophages compared to shams. This finding suggests that non-genomic effects partially contribute to the total effects of E2 on Kupffer cell and splenic macrophage cytokine production following trauma-hemorrhage.
Our results also show that MAPK activation increased in Kupffer cells and decreased in splenic macrophages following trauma-hemorrhage. In contrast to cytokine production, administration of E2-BSA prevented the trauma-hemorrhage-induced changes in MAPK activation in Kupffer cells and splenic macrophages to the same extent as E2 treatment. These results thus indicate that the effects of E2 on MAPK activation are mediated mainly via the non-genomic pathway. In addition, since co-administration of estrogen receptor antagonist, ICI 182,780 abolished the effects of E2-BSA on Kupffer cells and splenic macrophages, such effects are likely mediated via the estrogen receptors.
In the present study, IL-6 production was significantly suppressed by p38 inhibition in Kupffer cells and by inhibition of ERK1/2 or JNK pathway in splenic macrophages. Moreover, TNF-α production was significantly decreased by inhibition of p38 or JNK in both Kupffer cells and splenic macrophages. Interestingly, JNK inhibition suppressed IL-10 production by Kupffer cells in trauma-hemorrhage animals, but not in shams, while p38 inhibition decreased the capacity of Kupffer cells to produce IL-10 in both trauma-hemorrhage and sham groups. These results suggest that Kupffer cell and splenic macrophage cytokine production are mediated at least in part via MAPK pathways, and which particular pathway is involved depends on cell types and the experimental conditions. The studies of Maung et al. (2005) have also shown that thermal injury-induced increase in TLR4 reactivity in splenic macrophages is mediated, at least in part, by enhanced activation of the p38 signaling pathway.
Our results also demonstrate that NF-κB and AP-1 activity increased in Kupffer cells and decreased in splenic macrophages following trauma-hemorrhage. However, E2 administration normalized those alterations in transcription factors activation. Although E2-BSA administration also significantly attenuated the alterations in the activity of transcription factors in Kupffer cells and splenic macrophages, the levels of transcription factors activation did not return to the levels observed in Kupffer cells and splenic macrophages from sham animals. These findings suggest that similar to cytokine production, non-genomic effects partially contribute to the total effects of E2 on the activation of transcription factors in Kupffer cells and splenic macrophages following trauma-hemorrhage. Our findings collectively suggest that non-genomic pathway of E2 effects on cytokine production by Kupffer cells and splenic macrophages following trauma-hemorrhage is mediated via MAPK pathways. However, both genomic and non-genomic pathways appear to contribute to the total effects of E2 on cytokine production and such effects of genomic and non-genomic pathways are likely mediated via NF-κB and AP-1.
E2 has long been considered to mediate its action via estrogen receptors located on the nuclear membrane (Kuiper et al., 1996). However, a few recent studies have indicated the presence of ER on the plasma membrane (Chen et al., 1999; Razandi et al., 1999; Toran-Allerand et al., 2002; Revankar et al., 2005) and it has been reported that some of the effects of E2 are mediated via these receptors (Ho and Liao, 2002; Simoncini and Genazzani, 2003). These surface receptor-mediated non-genomic effects modulate cell membrane-bound regulatory proteins or cytoplasmic signaling molecules. MAPK pathways are known to be involved in the non-genomic cascade of E2 in various types of cells (Di Domenico et al., 1996; Endoh et al., 1997; Castoria et al., 1999; Nuedling et al., 1999; Flores-Delgado et al., 2001; Jessop et al., 2001; Hwang et al., 2002). For instance, ERK1/2 is activated in cardiomyocytes, colon cancer, breast cancer, and bone, but inhibited in vascular smooth muscle cells and lung myofibroblasts by E2 in a non-genomic manner. Our results also demonstrate that non-genomic pathway of E2 inhibits MAPK in Kupffer cells, while it activated those kinases in splenic macrophages. Other pathways such as the activation of src kinases and PI3K are also implicated in E2 mediated effects (Bellido et al., 1993; Greger et al., 2007), however, whether E2 triggers MAPK activation directly, via src kinases or via PI3k remains to be established.
The present findings collectively indicate that MAPK families (i.e., p38, ERK1/2, and JNK) are involved in the expression of pro- and anti-inflammatory cytokines including IL-6, TNF-α, and IL-10. In addition to MAPK, NF-κB, and AP-1 have also been shown to be implicated in macrophages cytokine production (Williams et al., 1999; Chapman et al., 2000). Furthermore, it is well known that MAPK activate AP-1 (Faris et al., 1996; Whitehurst and Geppert, 1996; Hardy and Chaudhri, 1997; Kaminuma et al., 2002), and recent studies have revealed that NF-κB is also activated by MAPK (Baeza-Raja and Munoz-Canoves, 2004; Kefaloyianni et al., 2006). Thus, NF-κB and AP-1 are likely the downstream factors of MAPK signaling pathways for macrophages cytokine production. Our results suggest that the activation of NF-κB and AP-1 is affected by E2 administration following trauma-hemorrhage via pathways induced by cell permeable E2 (i.e., genomic) as well as via cell impermeable E2-BSA (i.e., non-genomic) pathways. Furthermore, our findings also indicate a role for MAPK in non-genomic effect. Moreover, since genomic pathway has been shown to involve the modulation of the activity of other transcription factors such as NF-κB and AP-1 by activated estrogen receptors to regulate the transcription of genes that lack classic estrogen-responsive elements (ERE; Beato and Klug, 2000), such genomic effects might mediate the effects of E2 on NF-κB and AP-1 in this study.
There are studies indicating the existence of cell membrane estrogen receptors (Simoncini et al., 2004). Two kinds of membrane receptors have been proposed to exist: (1) membrane receptors with a molecular structure related to the classic estrogen receptors, ER-α or ER-β (Chen et al., 1999; Razandi et al., 1999), and (2) non-classic estrogen receptors such as G protein-coupled receptor 30 (GPR30) (Toran-Allerand et al., 2002; Revankar et al., 2005). Our recent studies have shown that the salutary effects of E2 on Kupffer cell functions following trauma-hemorrhage are mediated predominantly via ER-α (Suzuki et al., 2006b), and we have shown in this study that the effects of E2-BSA on Kupffer cells and splenic macrophages are abolished by co-administration of ER antagonist, ICI 182,780. It is therefore likely that E2-BSA acts via membrane ER-α on these cells.
It can be argued that the present study utilized measurement at a single time point, that is, at 2 h after treatment and thus it remains unclear whether the salutary effects of E2 or E2-BSA on macrophage signaling and cytokine production are sustained for periods of time longer than 2 h after treatment. Our previous studies, however, have shown that if the improvement in cell and organ functions by a pharmacological agent was observed early after treatment, those salutary effects are sustained for prolonged intervals, and it also decreased the mortality of animals from subsequent sepsis (Schneider et al., 2000; Diodato et al., 2001). Thus, although a time point other than 2 h was not examined in this study, based on our previous work; it would appear that the salutary effects of E2 or E2-BSA on Kupffer cells and splenic macrophages would be evident even if one measured those effects at another time point following trauma-hemorrhage and resuscitation.
In summary, our results demonstrate that administration of E2 following trauma-hemorrhage normalized Kupffer cell and splenic macrophage cytokine production, and MAPK, NF-κB and AP-1 activation. Although E2-BSA administration following trauma-hemorrhage also normalized MAPK activation, the alterations in cytokine production by Kupffer cells and splenic macrophages, and NF-κB and AP-1 activation in these cells were only attenuated, but not normalized to the sham levels. Moreover, MAPK inhibition in vitro decreased cytokine production by Kupffer cells and splenic macrophages. These findings collectively suggest that genomic and non-genomic pathways are involved in the effects of E2 on Kupffer cell and splenic macrophage cytokine production following trauma-hemorrhage, and that NF-κB and AP-1 mediate such effects of E2. Furthermore, the non-genomic effects of E2 on these cells are mediated via MAPK pathways.




