The osmotic sensitivity of rat growth plate chondrocytes in situ; clarifying the mechanisms of hypertrophy
Abstract
Bone elongation is predominantly driven by the volume expansion of growth plate chondrocytes. This mechanism was initially believed to be “hypertrophy”, describing a proportional increase of cell water and organelles. However, morphometrical analysis subsequently assumed the increase to be “swelling”, resulting in a disproportionate increase of cell water (osmotically active fraction). Histological approaches were performed on fixed tissue, and for the “swelling” assumption to be valid, the osmotic sensitivity of living cells before and during volume increase should differ. To test this, analysis of images acquired by 2-photon laser scanning microscopy (2PLSM) were used to determine the osmotic sensitivity, and osmotically active/inactive proportions of in situ chondrocytes from 15 living rat growth plates exposed to varying media osmolarities (∼0–580 mOsm). The dimensions of cell volume swelling in hypotonic media were different to the preferential lengthening seen in vivo, confirming the complexity of directional cell volume increase. Boyle–van't Hoff analysis of cell volume over the range of media osmolarity indicated no significant difference (Student's t-test) in the osmotically inactive fraction, 39.5 ± 2.9% and 47.0 ± 4.3% (n = 13) for proliferative and hypertrophic zones, respectively, or the sensitivity of volume to changes in media osmolarity (proliferative 15.5 ± 0.8 and hypertrophic zone 15.5 ± 1.2%volume · Osm). The osmotic fractions did not change as chondrocytes progress from proliferative to hypertrophic regions of the growth plate. Our data suggest cell volume increase by hypertrophy may play a greater role in cell enlargement than swelling, and should be re-evaluated as a mechanism responsible for growth plate chondrocyte volume increase and hence bone elongation. J. Cell. Physiol. 214: 621–629, 2008. © 2007 Wiley-Liss, Inc.
Bone elongation is driven by the proliferation, matrix deposition, and cell volume expansion of chondrocytes in the growth plate (Wilsman et al., 1996), a thin layer of cells adjacent to bone formation at either end of a growing bone. These cells are arranged into columns aligned with the direction of bone lengthening. Proximal, proliferating, flattened cells dramatically increase in volume (termed hypertrophy); when at their largest (distal end of the columns) they are sacrificed for new bone formation and hence bone lengthening. The rate of bone elongation is almost entirely dependent upon the rapidity and magnitude of this cell volume expansion (Buckwalter et al., 1986; Hunziker and Schenk, 1989; Breur et al., 1991, 1994, 1997; Hunziker et al., 1994; Kuhn et al., 1996; Wilsman et al., 1996; Vanky et al., 1998) which can account for 80% of daily bone lengthening (Wilsman et al., 1996).
The rate of bone lengthening differs between species, animal age, between bones of the same animal, and even the growth plates either end of the same bone can grow at different rates (Buckwalter et al., 1986; Hunziker and Schenk, 1989; Breur et al., 1991, 1994; Hunziker et al., 1994; Wilsman et al., 1996). Therefore, the rate of bone elongation and hence cell volume increase is highly regulated, yet an understanding of the mechanisms which underpin cell enlargement are not well understood, but crucial to target future research into growth plate disorders (Breur et al., 1992; de Luca, 2006).
The enlargement of terminal growth plate chondrocytes was initially described as hypertrophy (Stump, 1925), hence described as “hypertrophic,” and suggestive of a proportional increase in cell constituents. More recently, however, stereological analysis of fixed tissue has shown a disproportionate increase in cell compartments (Brighton et al., 1973, 1982; Buckwalter et al., 1986). These observations (Buckwalter et al., 1986) showed an increase in cell organelle volumes (endoplasmic reticulum, Golgi membranes, and mitochondria) was responsible for ∼15% of the total cell volume increase between proliferative zone chondrocytes (PZC) and hypertrophic zone chondrocytes (HZC), the majority (85%) of volume increase arising from cytoplasm and nucleoplasm expansion (Buckwalter et al., 1986). The apparent preferential increase of cell cytoplasm and nucleoplasm led to the assumption that cell volume increase was primarily due to “swelling” (fluid accumulation).
A cell can be considered to have two osmotic compartments, an osmotically active and inactive fraction. The osmotically active fraction describes freely mobile water within the cell, and it is this fraction that would increase dramatically if a cell increased in volume by “swelling” alone. Conversely, if a cell showed a general increase in total volume in proportion to its intracellular constituents, “hypertrophy,” then the osmotically active and inactive fractions will increase in proportion. If, as stereological approaches have suggested, cell volume increase occurs primarily due to fluid accumulation rather than hypertrophy, the osmotically inactive/active fractions of the PZC and HZC should differ (Fig. 1).

Schematic representation of the osmotically inactive (black) and osmotically active (white) fractions of a PZC differentiating to a HZC by either “hypertrophy” or “swelling.” PZC volume increase by a concomitant increase in cell compartments (“hypertrophy”) will result in a similar osmotically active fraction for the resulting HZC, whereas fluid accumulation (“swelling”) would result in an increase of the osmotically active fraction. By exposing cells to a range of extracellular osmolarities, a Boyle–van't Hoff plot can be constructed and the osmotically active and inactive fractions (y-intercept) estimated by extrapolation. This can allow the identification of the mechanism of cell volume increase between PZCs and HZCs.
Osmotic fractions can be determined by measuring cell volume change in response to a known osmotic challenge, usually described as the Boyle–van't Hoff relationship (McGann et al., 1988). Exposing cells to a range of extracellular osmolarities, a Boyle–van't Hoff plot can be constructed and the osmotic active and inactive fractions estimated by extrapolation.
Such an approach has been used in articular cartilage to ascertain the osmotic sensitivity of isolated (McGann et al., 1988) and in situ bovine and human chondrocytes (Bush and Hall, 2001, 2005). In the present study, we have used the same technique to test the assumption that volume increase of HZCs results from “swelling” and therefore an increased osmotically active fraction.
Two-photon laser scanning microscopy (2PLSM) has been used to study live in situ chondrocytes with excellent chondrocyte viability and vital dye stability (Bush et al., 2007). Utilizing 2PLSM, we have investigated the osmotic characteristics of 7-day-old rat proximal tibial growth plate chondrocytes. Living in situ PZCs and HZCs were exposed to a range of media osmolarities, images acquired, and their volumes determined using three-dimensional image reconstruction software. In addition to the osmotically active and inactive fractions, determined by their Boyle–van't Hoff relationship, the cell dimension (length, width, and depth) increases due to osmotically induced swelling were compared to the preferential cell lengthening which occurs in vivo along the growth plate (Breur et al., 1994).
Our work suggested that stereological techniques have markedly under-estimated the contribution of “non-osmotic volume” to early chondrocyte enlargement, as we found no significant difference in the osmotic sensitivity (osmotic and non-osmotic fractions) of cells between proliferative and hypertrophic regions. Furthermore, osmotic challenge induced cell swelling equally in all axes, unlike the preferential longitudinal increase seen with hypertrophy in vivo, confirming that directional chondrocyte enlargement during bone elongation is a more complex and directional event than simple cell swelling (Smits et al., 2004).
Materials and Methods
Biochemicals and solutions
The vital fluorophore calcein-AM was obtained from Cambridge Bioscience, Cambridge, UK and stock solutions made to 1 mM with dimethylsulphoxide. The standard tissue culture medium was serum- and phenol red-free Dulbecco's modified Eagle's medium buffered with (N-[2-hydroxyethylpiperazine]-N′-[2-ethanesulphonic acid]; HEPES; 10 mM) containing penicillin (50 units · ml−1) and streptomycin (50 µg · ml−1; 280 mOsm · kg−1 H2O; pH 7.4), all purchased from the Sigma-Aldrich Co., Ltd. (Poole, Dorset, UK). To alter media osmolarity for hyper- or hypotonic solutions, the required amount of NaCl or ddH2O was added, respectively, pH was checked and adjusted to 7.4. Media osmolarity were confirmed using a freezing-point Micro Osmometer Model 3300 (Advanced Instruments, Norwood, MA).
Growth plate cartilage preparation
Fifteen Sprague-Dawley rat pups (7-day-old) were humanely killed by decapitation following UK Home Office guidelines for other experiments. Tibia were removed under aseptic conditions and cleaned of soft tissue. A sagittal bisection of each tibia from the anterior surface was performed using a fresh scalpel blade. Tissue was immediately placed into standard culture media and maintained at 37°C (CO2 (5%):air (95%)) until required. Samples were incubated with calcein-AM (5 µM, 30 min at 37°C) to load the cells with the fluorescent dye calcein. The distal tibia was then attached to the base of a 35 mm plastic culture dish using a small drop of cyano-acrylate glue, at a point distant from that being observed (proximal tibia growth plate). Explants were mounted with the bisected surface uppermost, so that cells along the entire length of the growth plate could be easily visualized on an upright 2PLSM.
Successive osmotic challenges were performed by manually aspirating and replacing with media of the required osmolarity, with a complete change of media (including wash) being performed within 15 sec. Although in situ articular chondrocyte volume changes after osmotic challenge have been shown to be complete within 90 sec, because of the large size of HZCs, a 5 min incubation period was allowed before image acquisition. To inhibit any possible chondrocyte volume regulation activity, all solutions were kept at 4°C (Bush and Hall, 2001, 2005).
Acquisition of in situ chondrocyte images by 2-photon laser scanning microscopy
An upright Zeiss Axioskop LSM510 NLO (Carl Zeiss Ltd., Welwyn Garden City, Herts, UK) laser scanning microscope with a near infra-red (NIR) pulsed excitation light source was used to acquire fluorescent images of in situ calcein-loaded, growth plate chondrocytes. Cells were visualized using an Achroplan ×63/0.95 numerical aperture ceramic water dipping lens or a Plan-Neofluar ×10/0.3 numerical aperture dry objective. Intracellular calcein fluorescence (excitation max 494 nm, emission max 517 nm) was imaged with a 2-photon excitation wavelength of 800 nm (Bush et al., 2007), provided by a 10 W Verdi/Mira manually tuneable (range 700–1,000 nm) NIR pulsed titanium sapphire laser (Coherent Inc., Santa Clara, CA). Calcein fluorescence was detected using internal (de-scanned) detectors, emitted light passing through a 500–550 nm band-pass filter. The emission light path routinely contained a BG39 filter to block any reflected NIR light. The focal nature of 2-photon excitation allowed the confocal detection pinhole to be opened to its widest extent (1,000 µm) maximizing the system sensitivity. Laser power and detector sensitivity were adjusted to provide optimum image quality without excessive dye bleaching or pixel saturation. Scanning speed was typically 0.6 Hz with 2 frame integration of a 512 × 512 pixel image, representing ∼150 µm2 when using the 63× ceramic dipping lens, with serial 1 µm z-step optical sections.
Image analysis
Correctly acquired 2PLSM data sets, can provide three-dimensional information that can be reconstructed using imaging software to give quantitative data about the volume of living in situ chondrocytes (Bush and Hall, 2001; Bush et al., 2007). In order to correctly determine the volume of a cell, it was essential to accurately determine the chondrocyte edge. This was achieved using a threshold segmentation method as previously described (Bush and Hall, 2001; Bush et al., 2007). Individual cells were “isolated” as regions of interest and a 45% threshold used for 2PLSM to identify the boundary between the cell (foreground) and matrix (background) using Volocity software (Improvision, Coventry, UK). This threshold was confirmed by frequent calibrations using 10.16 ± 0.1 µm diameter fluorescent latex beads (Polyscience Inc., Warrington, PA).
Measurements of chondrocyte dimensions were made by eye using Volocity software. The maximal cell length (parallel to the direction of bone elongation) was measured from the optical section with the longest cell cross section. Cell width measurements were obtained along an axis perpendicular to the direction of growth but parallel to the cut surface, and depth the axis perpendicular to both the growth plate axis and cut surface (see Fig. 2 for diagrammatic representation).
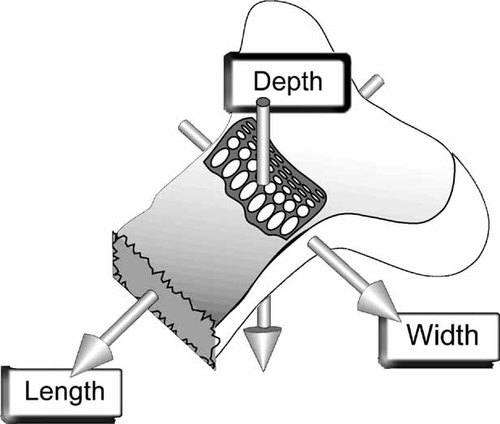
A diagrammatic representation of the chondrocyte dimensions measured with respect to the growth plate. Growth plates were “revealed” after a sagittal bisection of the anterior surface. In situ cell lengths corresponded to the axis parallel to the longitudinal axis of the bone, widths were measured parallel to the cut surface but perpendicular to cell length, and depths measured in an axis down from the cut surface.
Statistical analysis
Data were expressed as means ± SEM obtained from a number of joints (n). Differences between the means of two groups were determined by Student's unpaired t-tests and trends analyzed by one- and two-way analysis of variance (ANOVA). Where the direction of change was known, such as an increase in cell size with reduced osmolarity, P < 0.1 was considered significant (one-tailed), for all other tests P was only considered significant when <0.05 (two-tailed). Where significant differences were reported using a one-way ANOVA, a further post hoc Student–Neuman Keul's all-pairs comparison was performed. Linear relationships were tested using linear regression. All statistical analysis was performed using SigmaStat (Jandel Scientific, Ekrath, Germany).
Results
Overview of live chondrocyte morphology and volume along the growth plate
Live cell imaging of in situ growth plate chondrocytes allowed examination of cell morphology without the shrinkage artefacts associated with histological tissue fixation techniques (Buckwalter et al., 1986). Using 2PLSM imaging of calcein-loaded cells, the volume and morphological characteristics of living growth plate chondrocytes were examined in situ. Figure 3 shows an overview of adjacent fields of view to illustrate the complex arrangement of cells within a hind leg proximal tibia (sagittal bisection) of 7-day-old rats. Groups of cells arranged in longitudinal columns originated from the top of the image down toward the point of ossification. The columns tended to be near upright in the center of the growth plate, but deviated from the vertical toward the bone edge. Due to the technical difficulty in replicating identical cutting planes, and hence identical growth plate length and orientation between samples, cell volume could not be expressed simply in terms of distance along the growth plate. Instead, each growth plate was divided into eight equal sections (S1–S8). The top of S1 was taken as the first visible group of proliferating cells and the bottom of S8 taken from the point of clear tissue mineralization (see overlay on Fig. 3).
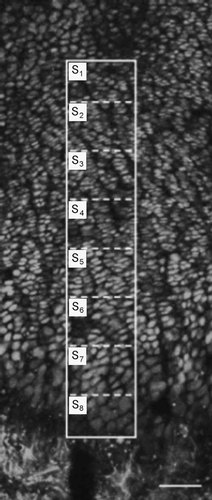
A projected image of calcein-loaded in situ growth plate chondrocytes from a sagittally bisected rat proximal tibia. Using a low magnification (10×) objective lens, sequential optical sections were acquired by 2PLSM as described in Materials and Methods. Chondrocytes are shown as bright cellular structures arranged in columns with the auto-fluorescence of new bone mineralization at the lower edge. The overlaid box with divisions (dashed lines) shows the classification of the growth plate into eight equal sections from the top of proliferating cells (S1) up to the mineralization front (S8). Scale bar 100 µm.
The change in cell size from top (proliferative) to bottom (hypertrophic) of the columns, even from an image of low magnification, can be seen clearly (Fig. 3). Adjacent images down columns of cells at a higher magnification (Fig. 4A) confirmed the increase in cell size along the growth plate. An example of the volume data that can be extracted from 2PLSM three-dimensional images is shown in Figure 4B for the images shown in Figure 4A, with the cells surface rendered and color coded according to their volume.
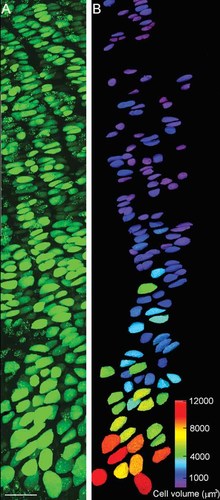
Adjacent projected images of calcein-loaded growth plate chondrocytes in situ using a high power (63×) dipping objective lens (A), from a sagittally bisected rat proximal tibia. Sequential optical sections were acquired using a 2PLSM as described in Materials and Methods. (B) Volume analysis (see Materials and Methods) was performed on selected chondrocytes from (A), and surface rendered cells color coded according to volume. Scale bar 50 µm.
Growth plate chondrocyte volume
Cell volumes increased ∼10-fold, from 1,172 ± 209µm3 in S1 to 10,584 ± 1,315 µm3 in S8, (see Fig. 5A). Cell volumes in sections S1 through to S5 were all significantly different from S7, and S1 through to S6 all significantly different to S8 (P < 0.05; n = 3; Student's unpaired t-test). The volume increase was partially due to an ∼165% increase in the cell diameter (width and depth), but predominantly the result of a near 400% increase in cell length (Fig. 5B).
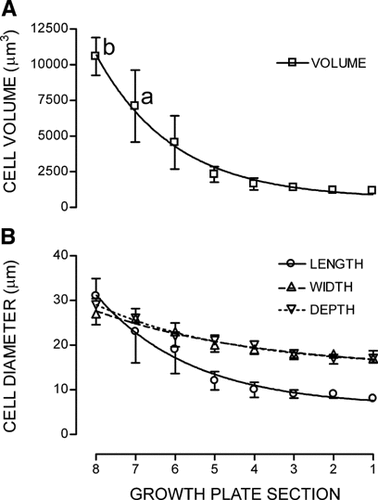
The change in cell dimensions of in situ growth plate chondrocytes along the length of the growth plate measured by 2PLSM (see Materials and Methods) for (A) cell volume (µm3) and (B) cell length, width, and depth (µm). The growth plate were divided into eight equal segments S1–S8, from the early PZCs to late HZCs, respectively. Notations “a” and “b” denote significant differences in cell volume between cells from S1 to S5 and S7, and cells from S1 to S6 and S8, respectively (Student's paired t-test; P < 0.05). Data points expressed as mean ± SEM (n = 3).
The standard error bars in Figure 5A indicate the heterogeneity of cell volumes within each section, cells ranging from 275 to 2,586 µm3 in S1 and a near 9,000 µm3 range between 6,861 and 15,435 µm3 in S8. Although the trend was for increasing cell volume along the growth plate, the largest cell (26,862 µm3) was recorded in S6. The large range of cell volumes within each segment reflected the difficulty in identifying cells to a specific section, as well as an inherent variability in cell size along the growth plate.
Similarly, the pattern of volume increase of cells along the growth plate shown in Figures 4B and 5, was as reported by Breur et al. (1994) with the majority of volume expansion occurring in the final third of the growth plate. This gave confidence in the techniques employed before exposing cells in situ to osmotic challenge.
Cell volume changes in response to varying media osmolarity
Cell osmotic sensitivity was performed by determining chondrocyte volumes before and after exposure to solutions of differing osmolarities (∼0–580 mOsm). Due to the large variability of resting cell volumes, both within separate zones and with distance along the growth plate (see above), absolute volume changes in response to osmotic challenge also showed wide variations. To clarify any differences, cell volume data were therefore normalized, with chondrocyte volume in 280 mOsm media taken as the control value (100%).
In order to successfully determine cell volumes, the software employed required cells not to be touching, hence fields of view were chosen in which a number of cells were clearly distinct, separated by non-fluorescent extracellular matrix. On average, each field of view contained eight complete cells from which volumes could be ascertained. Due to the similarity in cell volumes along the growth plate between S1 and S5, not being significantly larger until S6 (Fig. 5A), fields of view for PZCs were chosen from areas pertaining to S1–3.
Bisecting the tibia to allow visualization of the growth plate resulted in some cell death at the cut surface, even when using a fresh scalpel blade (Huntley et al., 2005). The bigger the cell, the greater the likelihood of physical damage by cutting, and we observed the largest HZCs were liable to perish during tissue preparation (unpublished observations). The large terminal HZCs (S8) adjacent to the area of mineralization which survived bone bisection, also tended to load and/or retain calcein poorly. Such cells had insufficient fluorescence to clearly differentiate intracellular calcein from the surrounding matrix and tended to resist changes in osmotic environment poorly, all resulting in errors when calculating cell volumes (Bush et al., 2007). In addition, the fluorescence intensity decreased in a non-linear fashion with depth into the tissue. Although this was less than 1% total fluorescence/µm (data not shown), cells with a large z-axis diameter (>25 µm) nevertheless showed an ∼20% reduction in fluorescence with image depth. Such a large reduction in fluorescence intensity may result in inaccurate volume calculations. Calibration images of 25 µm fluorescently labeled beads returned the expected volumes (∼8,200 µm3; data not shown), but for images of large cells with poor calcein fluorescence, we could not guarantee volumes were accurately determined over repeated measurements.
Due to the possible errors associated with volume measurements of the largest cells (S8), only cells from regions S6 and S7 were used to determine HZC osmotic sensitivity. These cells were on average nearly fourfold larger than PZCs which were selected from regions S1–3 with resting volumes of 1,314 ± 180µm3 (n = 10; 96 cells in total) and 4,808 ± 733µm3 (n = 13; 81 cells in total), respectively.
Cells from both S1–3 and S6–7 freely reduced in volume in hypertonic media. Neither chondrocyte group showed any apparent restriction to swelling by the surrounding extracellular matrix with hypotonic solutions, even when media were reduced to 0 mOsm (Fig. 6).
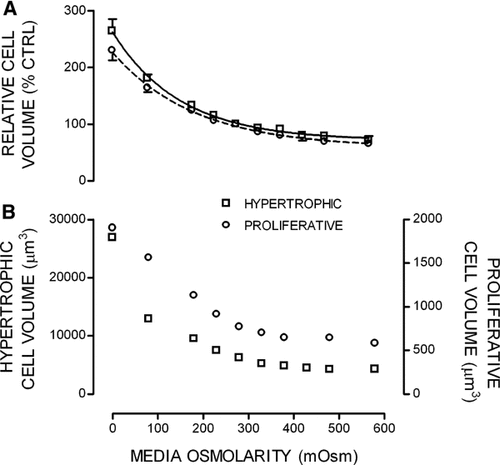
The relative volume changes of chondrocytes within growth plate cartilage measured in situ using 2PLSM as a function of medium osmolarity. Cell volumes were determined over a range of medium osmolarities (∼0–580 mOsm) at 4°C. A: Shows grouped data for PZCs (S1–3) and HZCs (S6–7). Data are shown as means ± SEM from 10 and 13 rats for PZCs and HZCs, respectively. B: Absolute volumes (µm3) of a typical in situ HZC (right axis) and PZC (left axis) in response to altered medium osmolarity are shown.
Two-way ANOVA showed a significant cell volume change from the control on exposure to varying media osmolarity (P < 0.01) for cells in both S1–3 and S6–7. Two-way ANOVA also revealed a significant difference (P < 0.01) between S1–3 and S6–7 across experimental osmolarities; showing significant differences at 480, 380, and 80 mOsm (Student's unpaired t-test; P < 0.05).
Normalizing the data reduced the variability between measured volumes, allowing trends to be analyzed statistically, but these relative values can disguise the extremely large absolute volume changes that occur. To highlight the changes occurring in individual cells, the osmotic sensitivity for a single PZC and HZC is shown in Figure 6B. The increase in volume shown by this PZC was from 768 µm3 in 280 mOsm media to 1,900 µm3 in near 0 mOsm, whereas the HZC volume increased from 6,236 µm3 in 280 mOsm media to 26,878 µm3 in near 0 mOsm media.
Osmotic sensitivity of in situ growth plate chondrocytes
When the mean relative volume changes of tibial growth plate cells obtained were plotted against the reciprocal of the media osmolarity (Osm−1) between 180 and 580 mOsm, a linear Boyle–van't Hoff relationship was observed (Fig. 7). Statistical analysis using two-way ANOVA revealed a significant difference between S1–3 and S6–7 (P < 0.01), but a comparison of the y-intercept, which represents the osmotically inactive fraction at a theoretical infinite osmolarity, indicated no significant difference between S1–3 and S6–7 cells of 39.5 ± 2.9% (PZCs; n = 10) and 47.0 ± 4.3% (HZCs; n = 13; Student's unpaired t-test; P = 0.237). In addition, there was no significant difference in the osmotic sensitivity (slope of the line; Student's unpaired t-test; P = 0.822) between cells from S1–3 and S6–7 of 15.5 ± 0.8 and 15.5 ± 1.2%volume · Osm−1, respectively.
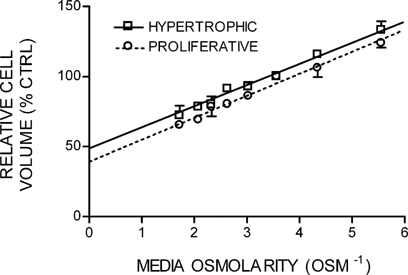
The relative volumes of in situ growth plate chondrocytes measured by 2PLSM plotted against the reciprocal of the medium osmolarity over a range of 180–580 mOsm. The linear regression coefficients (r2 = 0.9838 and 0.9892 for S1–3 and S6–7 chondrocytes, respectively) indicated a Boyle–van't Hoff relationship, with the cells behaving as near perfect osmometers. Linear regression curves were extrapolated to the y-intercept, which represents the osmotically inactive volume. Data points expressed as mean ± SEM (n = 10 and 13 for PZCs and HZCs, respectively).
To further investigate the relationship between cell volume and Boyle–van't Hoff regression-derived values, initial resting volumes (in 280 mOsm media) were compared with the osmotically active fraction for all cells (data not shown). Linear regression analysis showed little change between control cell volumes ranging between 340 and 23,719 µm3, a reduction in osmotically active fraction of only 0.6% for every 1,000 µm3 resting cell volume increase was observed. However, the poor correlation coefficient of the regression fit (r2 = 0.0586) illustrated the variability of osmotic sensitive fraction values. The data show no difference between the osmotic sensitivity of PZC and HZC, suggesting a “hypertrophic” rather than “swelling” mechanism being responsible for cell volume increase.
In situ growth plate chondrocyte length, depth, and width changes with swelling
During bone elongation, growth plate chondrocytes in vivo show a differential enlargement of their axis, with an increasing cell length in relation to their width and depth. In order to ascertain if cell swelling due to changing media osmolarity showed a similar preferential increase in length over width and depth, cell dimensions were measured over the “cell swelling” range of medium osmolarities (280 to ∼0 mOsm). Resting cell dimensions along the growth plate are shown as length:width and depth:width ratios in Figure 8. The ratio of depth to width remained constant along the entire length of the growth plate, indicating cell width and depth increased in concert. However, the cell length confirmed the expected increase (Buckwalter et al., 1985; Breur et al., 1994, 1997), with the ratio of length to width increasing significantly (Student's paired t-test; P < 0.05) from 0.46 ± 0.02 (S1) to 1.20 ± 0.2 (S8) (n = 3).
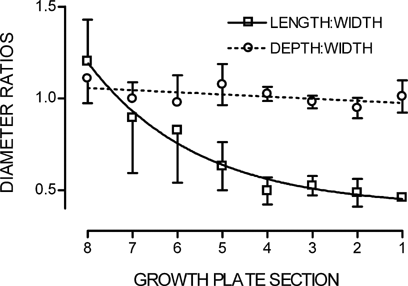
Length:width and depth:width linear ratios of in situ chondrocytes measured by 2PLSM along the growth plate length (S1–S8). Data points expressed as mean ± SEM (n = 3). Lines fitted by linear regression and curve fit regression (see Materials and Methods) for depth:width and length:width, respectively.
When cell diameters from zones S1–3 and S6–7 were measured over the range of hypo-osmotic challenge, the ratios of depth:width for both S1–3 and S6–7 showed no change from unity with volume increase (data not shown). However, unlike cells in vivo undergoing a volume increase along the growth plate, the length:width ratio for S1–3 or S6–7 following acute hypo-osmotic challenge did not significantly differ between 280 and ∼0 mOsm media (Student's paired t-test; P = 0.121 and P = 0.354, respectively), see Figure 9.
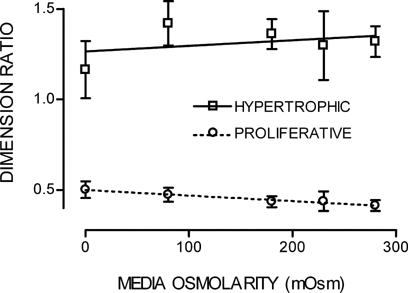
Length:width linear ratios of in situ S1–3 and S6–7 chondrocytes measured by 2PLSM in response to hypo-osmotic challenge (280 to ∼0 mOsm media). Lines fitted by linear regression. Data points expressed as mean ± SEM (n = 3).
Discussion
In the present study, we have used 2PLSM to determine the passive osmotic response of in situ growth plate chondrocytes to changes in medium osmolarity. The data showed that over a wide range of media osmolarity, the osmotic sensitivity of chondrocytes was not significantly different between cells from the proliferative or hypertrophic regions of the proximal tibial growth plate. Cells from both regions exhibited unrestricted shrinkage/swelling over a wide range of media osmolarities; however, changes to cell dimensions (length, width, and depth) were not similar to those seen from cells as they undergo their volume increase in vivo. There was no difference in the osmotically active/inactive properties of cells from either PZC or HZC, in contrast to the assumptions derived from stereological studies of growth plate chondrocyte volume increase by “swelling,” and are therefore worthy of further consideration.
Apart from a brief report (Huntley et al., 2003), the wealth of literature reporting in situ growth plate chondrocyte volumes was obtained using stereological techniques on fixed tissue (Hunziker et al., 1987, 1999; Hunziker and Schenk, 1989; Breur et al., 1991, 1994). The volumes reported here for PZCs and HZCs (Figs. 3 and 4) fall within the range of stereological-derived values albeit for rat tibia from different aged animals (Hunziker et al., 1987, 1999; Hunziker and Schenk, 1989; Breur et al., 1991, 1994). This gives confidence in our methods of live cell volume determination by 2PLSM image acquisition and subsequent analysis techniques. Although the older animals used for earlier histological studies (generally 4 weeks) tend to show a more “classical” growth plate architecture with clearly defined complete cell columns, the younger animals used in this study are near the age of maximal growth rate (day 10; Hughes and Tanner, 1970) with comparable cell volumes. This would suggest that the rate of cell volume enlargement would be higher in 7-day-old rat pups than the animals used for stereological analysis.
Once satisfied with the validity of using this preparation and 2PLSM to accurately determine quantitatively growth plate chondrocytes volume in situ, our attention turned to the osmotic response of these cells. All cells showed a clear reduction in volume with hyper-osmotic challenge, and hypotonicity increased chondrocyte volume even when media osmolarity reduced from 80 to ∼0 mOsm media, with little apparent restriction to swelling from the surrounding matrix (Fig. 5). A concern, when subjecting a hypotonic challenge to cells adjacent to the cut edge of a bisected growth plate, is the possible loss of matrix integrity allowing preferential swelling at the cut edge. However, when chondrocyte swelling was analyzed with respect to the changes in linear cell dimensions (length, width, and depth), there was no preferential increase in cell length, width, or depth (data not shown). The lack of preferential swelling in any axis is unlike the changes seen in situ (Fig. 7; Buckwalter et al., 1985; Breur et al., 1994) where cell lengthening occurs. This confirms that the preferential increase in cell length, as they expand along the growth plate in vivo, is not due to a simple cell swelling process (Smits et al., 2004).
In order to determine osmotic sensitivity data, the Boyle–van't Hoff relationship of cell volume with respect to reciprocal osmolarity was plotted. This raised a dilemma as to which value for the osmolarity should be used. An accurate understanding of the true osmotic challenge experienced by the cells in situ is unknown, being determined by the glycosaminoglycan (GAG) content of the extracellular matrix. GAGs will preferentially attract cations, but matrix/bone restriction to swelling will limit the volume of osmotically obliged water that can follow. Hence, the osmolarity of the extracellular matrix will be higher than the explant bathing media. Unlike the extensive studies of bovine cartilage extracellular osmolarity (Urban et al., 1993), no such data are available for growth plate cartilage. The concentration and the type of GAG have been shown to vary from proliferative to hypertrophic zones (Byers et al., 1992; Pavasant et al., 1996; Hunziker et al., 1999), but the impact on matrix osmolyte content may be limited (Wuthier, 1977) with the osmolarity throughout the growth plate showing little variation. An extracellular osmolarity of 400 mOsm has been suggested for entire bovine rib growth plate explants bathed in 280 mOsm media (Farnum et al., 2002). If correct, and assuming the absolute changes in media osmolarity result in similar changes in extracellular osmolarity, the Boyle–van't Hoff relationship would still show a matched slope, albeit a shallower gradient compared with Figure 6. Osmotically inactive fractions (y-intercept) would be reduced for both zones, 17.7 ± 3.8% and 24.8 ± 6.0% (n = 13) for PZCs and HZCs cells, respectively, but again showing no significant difference (Student's unpaired t-test; P > 0.05). Without any published evidence of specific extracellular osmolarity, we plotted the reciprocals of the known bathing media osmolarities against cell volume.
The similarity of osmotic sensitivity (slope) and the osmotically inactive fraction (y-intercept) derived from the Boyle–van't Hoff relationship between PZCs and HZCs (Fig. 6) indicates a similar relative cell water content for all cells. This was confirmed by additional analysis of the osmotically inactive fraction for individual cells with respect to their resting volume. For HZCs to maintain the osmotically active/inactive fractions of PZCs, any increase in size must be by classical “hypertrophy,” and not “swelling” which would have resulted in increased water content and corresponding changes in the osmotic fraction (see Fig. 1). This appears to be in direct contradiction to the assumptions of cell enlargement by “swelling” from stereological studies (Brighton et al., 1973, 1982; Buckwalter et al., 1986). For example, Buckwalter et al. (1986) reported an increase in cell volume of 3,265 µm3 between lower PZCs and upper HZCs (605–3,870 µm3, respectively). A significant proportion of the increase in cell volume (2,900 µm3) was suspected to be fluid accumulation (Buckwalter et al., 1986). Assuming the osmotically active fraction for proliferative zone cells was near to that in this study, and that seen for bovine in situ articular chondrocytes (McGann et al., 1988; Bush and Hall, 2001) of ∼60%, an increase in cell fluid content of 2,900 µm3 as the cell undergoes volume increase equates to an osmotically inactive fraction of ∼80% in HZCs, much greater than obtained here (53%).
A key question that is raised as a result of this study is why there was such a large difference between the present work determining the osmotic active fractions (Fig. 6) and stereological-derived cell fluid content? It is unlikely that the limitations of stereological analysis would cause such large differences, the relative contribution of large structures (organelles and cytoplasm) being adequately measured. Shrinkage artifacts associated with fixed tissue, if present, would have reduced the apparent osmotically active component; this would increase the apparent “dry matter” not reduce it. A possible explanation is that stereological measurements may have not reported all osmotically inactive material, possibly through the lack of image resolution. For example, stereological studies made no specific mention of cytoskeletal proteins (Brighton et al., 1973, 1982; Buckwalter et al., 1986), whereas HZCs of chick proximal tibiotarsi growth plates show intense cytoplasmic staining of the cytoskeletal element β7-tubulin compared with PZCs (Farquharson et al., 1999). Osmotically inactive “dry mass” in HPZs cytoplasm may therefore have been underestimated, and only revealed when studying the osmotic sensitivity of live cells by 2PLSM. Analysis of protein content per cell would be needed to confirm this hypothesis.
Due to the fragility of the largest cells (S8) when exposed to osmotic challenge, their osmotic characteristics were not analyzed. It is possible that the volume increase of these late HZCs between S7 and S8 is driven by swelling, and it is these cells which were reported in stereological studies. However, the HZC volumes reported in previous morphological studies were similar to those exposed to osmotic challenge here suggesting similar cell populations were examined in the present study. To provide insight into the osmotic potential required to increase the volume of a HZC from region S6–7 to one situated in S8 by “swelling” alone, their response to osmotic challenge can be examined further. When S6–7 cells were exposed to bathing medium of ∼0 mOsm, their volumes increased by 264–12,693 µm3. This increase in response to a Δ280 mOsm hypo-osmotic challenge compared closely to the volume increase seen in cells from regions S6 to S8 of 4,554 ± 1,877 µm3 and 10,584 ± 1,315 µm3, respectively (Fig. 4), an ∼230% increase. This suggests an ∼280 mOsm reduction in media osmolarity can elicit the increase in cell volumes observed between early–mid and late HZCs. For such a mechanism to occur in vivo, plasma osmolarity would therefore have to be reduced from ∼300 (Kennedy et al., 1964) to ∼20 mOsm, which is clearly implausible.
We also cannot discount that the driving force for cell volume increase is by swelling, followed by consolidation through the production of “dry matter.” A PZC increasing in volume by 10-fold through “hypertrophy,” with a concomitant increase in osmotically inactive volume would require ∼3,600 µm3 (assuming a 40% osmotically inactive fraction) increase in “dry matter.” Transport of the components required for synthesis of osmotically inactive structures (e.g., amino acids, simple sugars, etc.) may cause a transient osmotic gradient inducing cell swelling. Indeed, transporter (with receptor) mRNA expression shows a near tripling in the number of relevant up-regulated genes (Wang et al., 2004). Increased free amino acid and sugar content has also been shown between mid PZCs and HZCs, suggestive of an accumulation of osmolyte-driven cell swelling (Farnum et al., 2002). Amino acids and simple sugars are well known as regulators of articular chondrocyte volume (Hall, 1995; de Angelis et al., 1999; Hall and Bush, 2001). Farnum et al. (2002) estimated that ∼4.6 × 10−3 nmol/cell of intracellular solute would be required to elicit the volume expansion assuming the majority of cell volume increase was due to swelling, and “free” organic osmolytes accounted for 6–7% of the total osmolytes required. However, this value may be a significant underestimate, the uptake may be much greater had more molecules been incorporated into proteins; as our study suggests, the total volume increase is not predominantly brought about by swelling but instead by an increase in non-osmotically active cellular “dry matter.”
In order to fully understand the mechanisms underlying “hypertrophic” growth plate chondrocyte volume expansion, further studies are clearly required. Intracellular concentrations of osmolytes and the osmotically inactive fraction will help determine the relative contribution of swelling/hypertrophic pressures on cell volume. Static measurements of extra- and intracellular constituents will not completely resolve the conundrum of the nature of growth plate cell volume increase. Kinetic measurements of osmolyte transport and osmotically inactive material synthesis will also be needed to help provide the answers to the question of how growth plate chondrocytes undergo hypertrophy. Such work ideally requires the observation of living cells in situ, and this study has demonstrated the advantages of 2PLSM over conventional histological approaches.
Using 2PLSM and image analysis techniques, we were able to show the osmotic sensitivity of cells in proliferative and hypertrophic zones were not significantly different. This suggests that homeostasis of intracellular composition is maintained as cells progress from proliferative to hypertrophic zones of the growth plate.
Acknowledgements
This work was supported by the BBSRC (BB/C513985/1). We also thank Dr. Mike Cousin for his contribution of experimental resources.




