Intracellular phospholipase A2 expression and location in human macrophages: Influence of synthetic material surface chemistry
Abstract
Phospholipase A2 (PLA2) enzymes participate in a potent inflammatory pathway through the liberation of arachidonic acid upon hydrolysis of membrane glycerophospholipids. The presence of implanted polycarbonate-urethane (PCNU) materials, used in several medical applications, has the ability to influence inflammatory responses of human macrophages that are recruited to a tissue-material interface; however, the specific inflammatory pathways that are activated upon macrophage attachment to PCNU are largely unknown. Previous studies suggested the participation of PLA2 pathways in material degradation with the use of chemical inhibitors, such as aristolochic acid (ARIST), however not accurately defining the specific PLA2 enzymes involved. The current study aimed to establish specific groups of PLA2 involved in the macrophage foreign body response to PCNU. ARIST was assessed for specific effects on secretory PLA2 (sPLA2) protein expression and non-specific effects on key proteins, β-actin and monocyte-specific esterase, implicated in the macrophage attack on PCNU materials. Macrophage attachment to PCNU materials induced increased intracellular expression of cytosolic PLA2 (cPLA2), but not sPLA2, relative to tissue culture polystyrene (TCPS) as detected by immunoblot analysis, demonstrating an early and delayed stimulation during the time course of increased cPLA2 protein expression. Laser scanning confocal microscopy images indicated a change in location of cPLA2 in macrophages adherent to PCNU surfaces compared to TCPS. This study has illustrated changes in macrophage cPLA2 expression in response to cell-attachment to PCNU surfaces, demonstrating that the macrophage foreign body response to biomaterials induces a potent inflammatory pathway, which may lead to tissue damage near the site of material implantation. J. Cell. Physiol. 214:136–144, 2008. © 2007 Wiley-Liss, Inc.
One of the most important pathways initiated during an inflammatory response is the release of arachidonic acid (AA) from cell membranes by phospholipase A2 (PLA2) enzymes. The PLA2 superfamily consists of a group of enzymes that hydrolyze the sn-2 ester bond of cell membrane glycerophospholipids to release AA and lysophospholipids as products (Dennis, 1994). To date, 14 groups of PLA2 enzymes have been characterized with classification based on the substrate hydrolyzed from the sn-2 ester bond of phospholipids, amino acid sequence of the mature protein as well as sequence homology (Six and Dennis, 2000). The three classical categories of PLA2 are secretory (sPLA2), cytosolic Ca2+-dependant (cPLA2) and Ca2+-independent (iPLA2) PLA2 (reviewed in Six and Dennis (2000)). The sPLA2 enzymes are low MW (13–18 kDa) proteins with an active site histidine. They have a requirement for mM Ca2+ and are secreted as indicated by the name. cPLA2 enzymes are higher MW proteins (61–114 kDa) with a µM requirement for Ca2+, an active site serine and a high preference for AA in the sn-2 position of phospholipids. The final group, iPLA2, was classified as such since it was the first PLA2 cloned with no dependence for Ca2+. Since PLA2 enzymes are pivotal to the inflammatory response, their role in human macrophages and the foreign body response to implanted biomaterials is important to define (Labow et al., 2001b; Matheson et al., 2002; Dinnes et al., 2005).
Materials used for the manufacture of implanted medical devices (such as pacemaker lead insulation, artificial heart valves, and indwelling catheters) as well as tissue engineering and drug delivery applications, consist of a wide range of synthesized elastomeric polymers depending on the properties desired for their intended use. Polyurethanes (PU) are one of the most common types of biomaterials in use today since they are relatively stable and biocompatible, however these materials still maintain the ability to elicit immune responses as well as being shown to be susceptible to material degradation over time (reviewed in Santerre et al. (2005)). Whether biodegradation is an event that is desired or not (i.e., long-term implants vs. short-term degradable materials for tissue engineering applications), the initiation of an immune or inflammatory response can result in negative and even harmful effects either at an implant site or the site of regenerating tissue.
The model polymers used in the current study represent an important family of PU, referred to as polycarbonate-based polyurethanes (PCNU). They were synthesized with either 1,6-hexane diisocyanate (HDI) or 4,4′-methylene bisphenyl diisocyanate (MDI), a polycarbonate (PCN) [poly(1,6-hexyl carbonate)diol] soft segment, and 1,4-butanediol (BD) in varied stoichiometric ratios to examine sensitivity of PLA2 responses to PCNU chemistry differences. PCNU materials were originally developed in order to overcome hydrolytic and oxidative degradation experienced with traditional polyester- and polyether-based PU materials (Stokes, 1988; Stokes et al., 1995). Although, some PCNUs are more stable to hydrolytic and oxidative degradation than polyester- and polyether-based PUs respectively (Pinchuk, 1994; Stokes et al., 1995; Tanzi et al., 1997; Christenson et al., 2004), these materials remain susceptible to some biodegradation (Labow et al., 2001a, 2002). Both neutrophils and monocyte-derived macrophages (MDM) are recruited to a material site as part of the foreign body response. Although both cell types participate in the foreign body response, the MDM contribution is much greater since neutrophils only survive a short time and MDM demonstrate 5–10 times greater degradative capacity towards PU materials (Labow et al., 2001b). In addition, there is significant evidence that the MDM is also the greatest cellular contributor to in vivo PU degradation. These cells have been shown to be the most abundant cell type adherent to explanted long-term in vivo implant materials, as well as remaining at the cell-material interface for the lifetime of the device (Kao et al., 1995). In addition, MDM have the capacity to fuse and form foreign body giant cells (FBGC), a hallmark of chronic inflammation (Anderson, 2000). Studies have shown that PCNU materials induced increases in FBGC formation in comparison to a control tissue culture polystyrene (TCPS) surface (Matheson et al., 2004). These findings provide supporting evidence that PCNU materials have the capacity to induce inflammatory responses. Despite this, very few specific inflammatory pathways have been characterized with the goal of advancing our understanding the foreign body response to PU materials.
Previous studies have demonstrated that chemical inhibitors of various PLA2 enzymes reduced neutrophil (Labow et al., 2001c) and macrophage-mediated PCNU degradation (Dinnes et al., 2005). Inhibitors of each sPLA2, cPLA2, and iPLA2 enzymes resulted in a reduction of macrophage-mediated PCNU degradation, with differences in inhibition elicited by only slight differences in PCNU chemistry. PLA2 inhibitors caused a reduction in PCNU degradation demonstrating a potential contribution of PLA2 enzymes in the material degradation pathways within macrophages. In addition, there was a difference in PLA2 activation, as measured by 3H-AA release, between PCNU materials and TCPS, with significantly more 3H-AA being released from macrophages on PCNU (Dinnes et al., 2005). Although cPLA2 is primarily responsible for AA release (reviewed in Leslie (2004)), both group IVA cPLA2 and group V sPLA2 have demonstrated the capacity to mobilize AA from phospholipid membranes of macrophages (Shirai et al., 2005). Since AA release was increased from PCNU-adherent macrophages, group IVA cPLA2 and group V sPLA2 enzymes were investigated here to determine if the increase seen in AA release was correlated to changes in protein expression for either enzyme when macrophages interact with PCNUs.
The current study assessed the three model PCNU materials described above for their effect on PLA2 expression in human macrophages and compared these effects to TCPS as the control tissue culture surface. The effect of the sPLA2 inhibitor, aristolochic acid (ARIST), on PLA2 expression was compared to a cytoskeletal protein, β-actin, and an enzyme shown to be related to the degradation of PCNU, monocyte-specific esterase (MSE) (Labow et al., 2002; Matheson et al., 2004). Changes in macrophage protein expression of both cPLA2 and sPLA2 induced by material chemistry were determined by immunoblotting at various time points of cell adherence to TCPS and the model PCNUs. In addition, changes in location of cPLA2 on PCNU versus TCPS surfaces were investigated by immunofluorescence staining of U937 cells and imaged with laser scanning confocal microscopy.
Materials and Methods
Unless otherwise specified, all reagents were purchased from Sigma-Aldrich Canada Ltd (Oakville, ON, Canada).
Preparation of material surfaces
The model PCNUs used in this study were synthesized with a PCN soft segment, BD chain extender and either HDI or MDI as the diisocyanate as described in detail previously (Tang et al., 2001). Three PCNUs were used for this study (Table 1); (1) a PCNU containing a linear aliphatic diisocyanate (HDI) with a stoichiometric ratio of 4:3:1 (HDI/PCN/BD—referred to as HDI431), (2) a HDI-containing PCNU with a stoichiometric ratio of 3:2:1 (HDI/PCN/BD—referred to as HDI321), and (3) a PCNU containing an aromatic diisocyanate (MDI) with a stoichiometric ratio of 3:2:1 (MDI/PCN/BD—referred to as MDI321). The final partial structure of each model PCNU used, in addition to the structure of the reference TCPS surface are presented in Figure 1.
| Reagent | Stoichiometry | Acronym |
|---|---|---|
| HDI/PCN/BD | 4:3:1 | HDI431 |
| HDI/PCN/BD | 3:2:1 | HDI321 |
| MDI/PCN/BD | 3:2:1 | MDI321 |
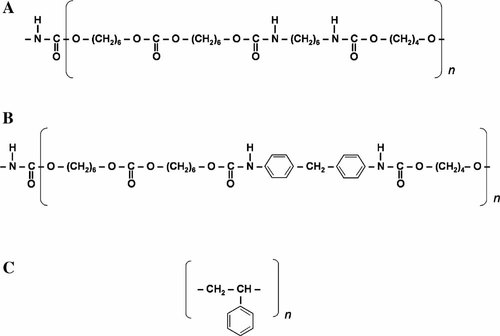
Chemical structure of material surfaces used in this study. A: HDI-based PCNU, (B) MDI-based PCNU, (C) tissue culture polystyrene.
The basis for selection of these model PCNU materials arises from previous studies analyzing these model HDI- and MDI-based PCNUs with varying ratios of hard segment (HDI or MDI and BD) to soft segment (PCN) in addition to comparing hard segment chemistry differences (HDI vs. MDI). Although HDI- and MDI-based PCNUs are relatively similar in chemical structure (see Fig. 1), surface characterization data previously reported on may reflect relative differences in stability of these materials and “activatibility” towards macrophages illustrated previously (Labow et al., 2001a, 2005a; Matheson et al., 2002, 2004; Dinnes et al., 2005). X-ray photoelectron spectroscopy (XPS) analysis of HDI431, HDI321, and MDI321 (Tang et al., 2002) using C1s high resolution at an incident angle of 15°, generated results regarding the content of carbonate (OCOO) and urethane (NHCOO) groups for the top 1 nm of each material. Comparing the NHCOO/OCOO ratio can estimate the potential for interacting groups via hydrogen bonding. NHCOO groups have a greater potential to form hydrogen bonds. Therefore, MDI-based PCNU, which showed a greater NHCOO/OCOO ratio than HDI-based PCNUs, will be more stable due a greater number of NHCOO groups from the hard segment that is also composed of the large aromatic moieties providing steric hinderance to hydrolytic cleavage. Data supporting that MDI321 is a more structured material at the surface relative to HDI431 and HDI321 include atomic force microscopy (AFM) phase images (Revenko et al., 2001). AFM images suggest greater phase mixing with an MDI-based PCNU therefore allowing hydrogen bonding to occur, making MDI321 more resistant to degradation by hydrolysis. Furthermore, the smaller aliphatic HDI moieties allow access to enzymes for hydrolytic cleavage. Studies have illustrated that HDI-based PCNU are highly degradable by both hydrolytic enzymes (Labow et al., 2002; Tang et al., 2002) and macrophages (Matheson et al., 2004; Labow et al., 2005a). In order to compare these degradable material surfaces to a “reference” material, TCPS (non-degradable) was analyzed in this study since this is a material surface used most often in the scientific community for growth and cultivation of numerous cell types. Water contact angle data generated for HDI431, HDI321, and MDI321 (Tang et al., 2003) were not significantly different, however, indicated that each of these materials is relatively hydrophobic in comparison to typical TCPS. Contact angle data have not been previously reported on by our group, however are included here to indicate the relative hydrophobicity of these model PCNUs in comparison to TCPS surfaces used in standard cell culture. Water contact angle analysis was performed as outlined in detail previously (Tang et al., 2003), introducing a bead of HPLC-grade water to the TCPS cover slip surface and using a standard goniometer apparatus (NRL Goniometer, Rame-Hart, Inc., Mountain Lake, NJ). The contact angle data of TCPS relative to previously published contact angle results for HDI431, HDI321, and MDI321 (Tang et al., 2003) are presented in Table 2.
| Material surface | Advancing angle | Receding angle |
|---|---|---|
| TCPS | 55 | 16 |
| HDI431 | 85a | 55a |
| HDI321 | 79a | 53a |
| MDI321 | 83a | 58a |
- a Tang et al. (2003).
The synthesized PCNUs were dissolved overnight in a 10% dimethylacetamide solution (w/v), centrifuged and filtered (0.45 µm Teflon filter; Chromatographic Specialties, Toronto, ON, Canada), then coated as a thin layer (100 µl) onto 15 mm diameter glass cover slips (Fisher Scientific, Ottawa, ON, Canada) under sterile conditions in a laminar flow hood, as previously described (Labow et al., 2001b). The slips were dried overnight at 50°C, purged for 24 h and then dried under vacuum for 72 h. Prior to using the PCNU slips or TCPS multiwell plates for an experiment, they were hydrated by incubating with phosphate buffered saline (PBS; 0.0027 M KCl, 0.0015 M KH2PO4, 0.137 M NaCl, 0.008 M Na2HPO4) at 37°C and 5% CO2 with 100% humidity overnight. This process equilibrates the polymer slips with an aqueous environment and removes trace solvent that may possibly interfere with the experiment.
U937 cell culture
U937 cells (American Type Culture Collection (ATCC™ catalogue # CRL—1,593.2)) were employed as a well characterized model for MDM-mediated PCNU biodegradation (Matheson et al., 2002). U937 cells were maintained as a promonocytic cell suspension and fed every second day with RPMI 1640 medium containing 10% fetal bovine serum (Invitrogen Canada, Inc., Burlington, ON, Canada), 1 mM sodium pyruvate (Invitrogen) with 100 U/ml penicillin and 0.1 mg/ml streptomycin (Invitrogen), as described in detail previously (Matheson et al., 2002). Only cells that were between passage 7 and 20 were used for differentiation into macrophage-like cells by culturing a 1 × 106 cells/ml suspension in TCPS 12-well plates with phorbol 12-myristate 13-acetate (PMA) (1 × 10−7 M) for 72 h. At this time point cell division had ceased as determined by DNA analysis (see below), the cells were adherent and the parameters (maximal protein synthesis and esterase activity) that characterize a fully differentiated U937 cell for this in vitro model system occurred (Matheson et al., 2002). The cells were removed from the TCPS surface by gentle pipetting and were resuspended at a concentration of 1 × 106 or 2 × 106 cells/ml and reseeded onto the required material surface for the experiment.
Effect of aristolochic acid (ARIST) on protein expression
Differentiated U937 cells were re-seeded (1 ml of a 2 × 106 cells/ml suspension) into 24-well plates alone (TCPS control) or into wells containing HDI431-, HDI321-, or MDI321-coated glass slips. The cells were allowed to adhere for 1 h after which the medium was replaced with or without the sPLA2 inhibitor ARIST [200 µM] (Rosenthal et al., 1989; Dinnes et al., 2005). The cells were then incubated further for 48 h after which the cells were lysed for DNA determination (see below) and prepared for immunoblot analysis.
DNA determination
Whole cell lysates were prepared and analyzed for DNA as described in detail previously (Labow et al., 2001b; Matheson et al., 2004). Cold Triton X-100 buffer (180 µl) (0.05% Triton-X 100, 10 mM EDTA in PBS) was added to each well and the cells were lysed for 1 h on ice. Hoechst dye (number 33258; Amersham Biosciences., Baie d'Urfe, QC, Canada) was diluted with TRIS buffer (0.01 M Tris, 0.001 M EDTA, 0.2 M NaCl, pH 7.4) immediately prior to analysis. Cell lysates collected for DNA (10 µl) were added to the dye (100 µl) in 96 well microtiter plates (Microfluor 2 Black; VWR, Mississauga, ON, Canada). The lysates were compared to a DNA standard (Calf Thymus DNA) and each standard well to be analyzed also contained the same amount of 0.05% Triton X-100 buffer as in the lysate samples. The samples were analyzed using a fluorescence microplate reader (POLARstar Galaxy, BMG Labtechnologies, Durham, NC) with an excitation and emission wavelength of 360 and 460 nm respectively.
Immunoblotting
Proteins from whole cell lysate samples were separated by SDS–PAGE using a Protean II Cell System (Bio-Rad Laboratories Ltd., Mississauga, ON, Canada) with 5% stacking and 10% (cPLA2), 12% (β-actin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), MSE) or 15% (sPLA2) separating gel. U937 cells stimulated with PMA are non-dividing, terminally differentiated cells, therefore samples for electrophoresis were loaded into each well based on the amount of DNA in each sample. Two hundred and fifty nanograms of DNA were loaded into each well except for gels that were immunoblotted for cPLA2 and sPLA2, which required 1,000 ng of DNA to be loaded per well (as a result of the low abundance of these proteins). GAPDH was used as an additional control to ensure equal loading of protein in each well. Proteins from the resulting gels were transferred to nitrocellulose membranes (0.45 µm, Bio-Rad) for 75 min at 130 V. The membranes were then blocked for 1 h in Tris-buffered saline (TBST; 10 mM Tris, 0.1 M NaCl, 0.1% Tween-20, pH 7.5) containing either 5% non-fat dry skim milk or 5% bovine serum albumin. The membranes were then probed for 1 h with rabbit anti-cPLA2 (Research Diagnostics, Inc., Concord, MA), mouse anti-sPLA2 (group V) (Cayman Chemical Co., Ann Arbor, MI), rabbit anti-porcine esterase (Rockland Immunochemicals, Inc., Gilbertville, PA—reacts with MSE), mouse anti-β-actin or mouse anti-GAPDH (Chemicon International, Inc., Temecula, CA) and subsequently washed eight times with TBST. The membranes were then incubated with either anti-rabbit IgG (Rockland) or anti-mouse IgG (Pierce Biotechnology, Inc., Rockford, IL) conjugated to horseradish peroxidase, for 1 h followed by eight washes with TBST. Protein bands were visualized using a chemiluminescence detection kit (Pierce). The intensity of the protein bands were quantified using Quantity One® software (Bio-Rad).
Immunofluorescence and laser scanning confocal microscopy
Differentiated U937 cells, as described above, were re-seeded at a concentration of 1 × 106 cells/ml into 24-well plates containing either HDI431-coated glass slips or TCPS cover slips (Sarstedt, Inc., Montreal, QC, Canada) and allowed to adhere for 30 min or 2 h. The adherent cells were washed three times with warmed PBS and fixed at room temperature with 4% paraformaldehyde (Fisher Scientific) in PBS for 10 min. The cells were then blocked for 1 h with 10% rabbit serum (Cedarlane Laboratories Ltd., Hornby, ON, Canada) in PBS, followed by a 1 h incubation with mouse anti-cPLA2 (group IVA)(Santa Cruz Biotechnology, Inc., Santa Cruz, CA) in 5% rabbit serum in PBS. The slips were then washed three times in 5% rabbit serum in PBS followed by incubation for 45 min with rabbit anti-mouse Alexa Fluor® 488 (Invitrogen). The slips were finally washed three times in PBS and mounted onto microscope slides with 90% glycerol. The cells were visualized with an Olympus 100X oil immersion objective with a numerical aperture of 1.4, on an Olympus IX80 laser scanning confocal microscope operated by FV1000 software (version 1.4a). The Alexa Fluor® 488 fluorochrome was excited with the 488 nm line of the argon multi-line laser.
Statistical analysis
Statistical analyses were performed using a one-way ANOVA. The SAS® program (version 9.1) for windows was used and values were considered significant with a P-value of <0.05.
Results
Specific inhibition of intracellular sPLA2 protein expression by ARIST
Previous studies compared several chemical inhibitors of PLA2 to assess their effect on U937 cell-mediated 14C-PCNU degradation and inhibition of PCNU-mediated U937 cell 3H-AA release (Dinnes et al., 2005). The sPLA2 inhibitor ARIST demonstrated the greatest reduction of both U937 cell-mediated 14C-PCNU degradation and PCNU-induced 3H-AA release by U937 cells. To assess the specificity of ARIST towards sPLA2 versus cPLA2, immunoblot analysis for intracellular cPLA2 and sPLA2 protein from lysates of cells cultured with and without ARIST was performed to determine the effect of ARIST on intracellular protein expression. Figure 2A shows representative blots of cell lysates from U937 cells cultured on each material surface with and without ARIST for 48 h. Lanes 1, 3, 5, and 7 of each blot represent U937 cell lysates from TCPS, HDI431, HDI321, and MDI321 respectively from cells cultured with media alone. Lanes 2, 4, 6, and 8 of each blot represent U937 cell lysates from TCPS, HDI431, HDI321, and MDI321 respectively from cells cultured with ARIST. The first blot demonstrates lysates probed with anti-cPLA2 and the second blot probed with anti-sPLA2. ARIST had no significant effect on cPLA2 protein expression as indicated by quantification of blots from multiple experiments (Fig. 2B). However, as seen in the second blot (Fig. 2A), ARIST had a significant effect on sPLA2 protein expression in U937 cells adherent to TCPS, HDI321 and MDI321 each demonstrating 50–60% banding intensity of sPLA2 protein relative to cells cultured with media alone (Fig. 2B). ARIST had no significant effect on sPLA2 protein expression in U937 cells adherent to HDI431.
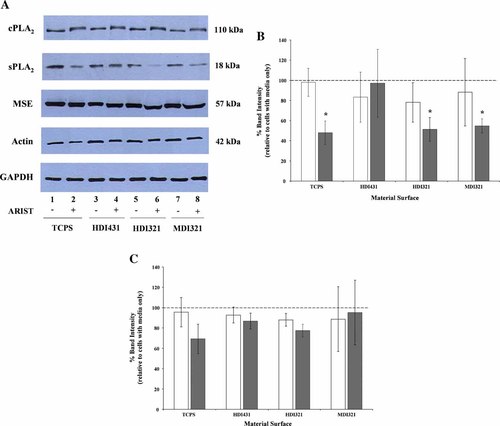
PLA2 inhibitor ARIST decreases U937 cell sPLA2 expression. PMA-differentiated U937 cells were seeded onto TCPS (control) or PCNU surfaces for 1 h. The culture medium was replaced with either fresh medium or medium containing ARIST (sPLA2 inhibitor) as described in Materials and Methods. Cells were then incubated further for 48 h before collection of cell lysates in preparation for immunoblotting. A: Representative blots of U937 cell lysates with and without ARIST. Blots were probed with anti-cPLA2, anti-sPLA2, anti-MSE, or anti-β-Actin. Cell lysates were loaded by equal amounts of DNA and blots were stripped and reprobed with anti-GAPDH as an additional loading control. Lane 1,2—TCPS; 3,4—HDI431; 5,6—HDI321; 7,8—MDI321. (+) with PLA2 inhibitor ARIST (200 µM), (−) no ARIST. Quantification of immunoblots (from Fig. A) from TCPS or PCNU adherent U937 cells are shown in (B, C). Protein bands were quantified using Quantity One. Data from repeated experiments (n = minimum of 3) were pooled and the data were statistically analyzed by a one-way ANOVA (*P < 0.05). B: Shows the relative amounts of cPLA2 (open bars) or sPLA2 (gray bars). C: Shows MSE (open bars) or β-actin (gray bars). All bars represent ARIST treated cell samples relative to their respective cells with the media only sample (dotted line), which was assigned a value of 100%.
In order to ensure that ARIST was not eliciting non-specific effects on key intracellular proteins, immunoblot analysis was performed on these U937 cell lysates to probe for β-actin and MSE. Immunoblot analysis determined that ARIST did not have significant effects on intracellular MSE protein as seen in Figure 2A (third blot) and quantified in Figure 2C. No significant effect on β-actin expression (fourth blot) was detected as outlined in the quantification of blots from multiple experiments (Fig. 2C).
Effect of PCNU materials on intracellular cPLA2 and sPLA2 protein expression
Immunoblotting analysis of lysates from U937 cells cultured for 2 or 48 h on PCNU (HDI431, HDI321, and MDI321) and TCPS was performed to probe for group IVA cPLA2 and group V sPLA2. These time points were chosen based on established biodegradation assays (Labow et al., 2001a, b; Matheson et al., 2002; Dinnes et al., 2005) in which cells were re-seeded onto the candidate surface for 1 h (since the majority of cells have become adherent by approximately 1 h post re-seeding). The culture medium was then replaced with or without the reagent to be tested and then incubated further for 48 h for degradation analysis. Representative immunoblots showed that at 2 h post re-seeding to PCNU surfaces, cPLA2 protein expression was increased relative to cells cultured on TCPS (Fig. 3A) for all three PCNUs. Two hours after U937 cell attachment to each PCNU surface, cPLA2 protein was significantly increased by 3- to 3.5-fold on each PCNU relative to TCPS. By 48 h post cell attachment the expression of cPLA2 protein, although still increased on PCNU relative to TCPS, was no longer significant on all surfaces (data not shown). Representative sPLA2 blots (Fig. 3A), verified by quantification from multiple experiments (Fig. 3B), indicated that intracellular sPLA2 protein expression was not significantly altered by the material surface to which U937 cells were adherent.
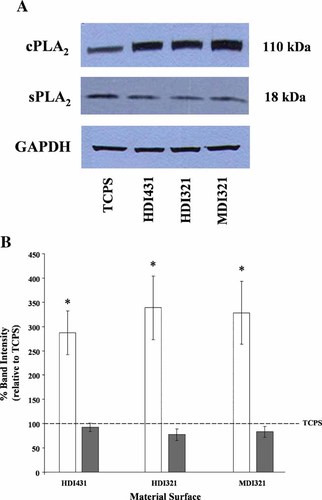
PCNU material surfaces induce increased intracellular U937 cell cPLA2 expression. A: PMA-differentiated U937 cells were seeded onto TCPS (control), HDI431, HDI321, or MDI321 for 2 h, as described in detail in Materials and Methods. Cell lysates were collected for immunoblotting and loaded by equal amounts of DNA. Resulting blots were probed with anti-cPLA2 and anti-sPLA2 antibodies, then stripped and reprobed with anti-GAPDH as an additional loading control. B: Quantification of protein bands from immunoblots (from A) of TCPS or PCNU adherent U937 cells. Protein bands were quantified using Quantity One, data from repeated experiments (n = minimum of 3) were pooled and data statistically analyzed by one-way ANOVA (*P < 0.05). Relative amounts of cPLA2 (open bars) or sPLA2 (gray bars) are indicated for U937 cells adherent to TCPS, HDI431, HDI321, and MDI321.
PCNU materials induce early changes in cPLA2 protein expression
Group IVA cPLA2 protein expression was clearly altered by the material surface to which U937 cells were adherent (Fig. 3A). Since there was such a large increase (∼3-fold) in cPLA2 protein expression at 2 h post cell attachment, earlier time points were investigated to determine how early this response was initiated by cPLA2 as a result of cell attachment to PCNU. At 2 h, the altered cPLA2 protein expression was comparable on each model PCNU material (Fig. 3B), therefore further experiments exploring earlier time points of PLA2 protein expression were carried out between only HDI431 and TCPS. Previous studies which demonstrated that PCNU materials induced increased 3H-AA release compared to TCPS illustrated that the greatest PLA2 activation occurred within 10 min of U937 cell attachment (Dinnes et al., 2005). Accordingly, a time course of protein expression between 10 and 60 min post cell attachment to HDI431 and TCPS was analyzed to assess the initial point at which PCNU had the ability to induce detectable changes in either cPLA2 or sPLA2 protein expression. Further to the results from 2 h, sPLA2 intracellular protein expression (Fig. 4A) was not significantly altered in HDI431 adherent U937 cells compared to TCPS (Fig. 4B). However, a slight increase in cPLA2 protein expression was detectable as early as 10 min post cell attachment to HDI431 compared to TCPS (Fig. 4A) with significantly increased expression (∼2- to 2.5-fold increase) at 20 and 30 min (Fig. 4B). cPLA2 protein expression returned to comparable expression levels seen in TCPS-adherent U937 cells by 45 min post cell attachment (Fig. 4A,B).
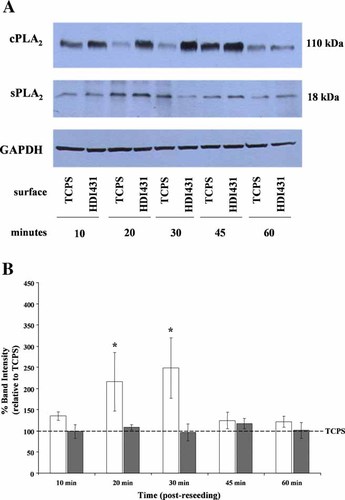
PCNU material surfaces induce early changes in U937 cell cPLA2 expression. PMA-differentiated U937 cells were cultured on TCPS or HDI431 for 10, 20, 30, 45, or 60 min. At the end of each time point, cell lysates were collected and prepared for immunoblotting analysis as described in detail in Materials and Methods. A: Representative immunoblots of U937 cell lysates probed with anti-cPLA2 or anti-sPLA2 antibodies. Cell lysates were loaded using equal amounts of DNA. Blots were also stripped and reprobed with anti-GAPDH as an additional loading control. B: Quantification of protein bands from immunoblots (from A) from TCPS or HDI431 adherent U937 cells. Protein bands were quantified using Quantity One, data from repeated experiments (n = minimum of 3) were pooled and data statistically analyzed by ANOVA (*P < 0.05). Relative amounts of cPLA2 (open bars) or sPLA2 (gray bars) indicated for U937 cells adherent to TCPS and HDI431 for a time course of 10–60 min.
cPLA2 location was altered by U937 cell adherence to a PCNU surface
Considering the significant changes in group IVA cPLA2 expression in response to material chemistry differences of the substrate on which U937 cells were cultured, cellular location of cPLA2 was assessed using laser scanning confocal microscopy to investigate material surface influences on cPLA2 protein mobilization. U937 cells that were cultured for either 30 min or 2 h on TCPS or HDI431 were immunofluorescently labeled with anti-group IVA cPLA2 and visualized by laser scanning confocal microscopy. These time points, 30 min and 2 h respectively, were chosen based on the fact that a peak of cPLA2 expression was detected at 30 min (Fig. 4B) and 2 h (Fig. 3B) on PCNU relative to TCPS. Fluorescent images illustrated a change in location of cPLA2 in response to adherence to HDI431 compared to TCPS. At both 30 min and 2 h, cPLA2 was located diffusely throughout the cytoplasm in TCPS adherent cells (Fig. 5A,C respectively). In contrast, U937 cells that were adherent to HDI431 for both 30 min and 2 h exhibited a change in location of cPLA2 towards the cell membrane (Fig. 5B,D respectively) compared to U937 cells adherent to TCPS (Fig. 5A,C).
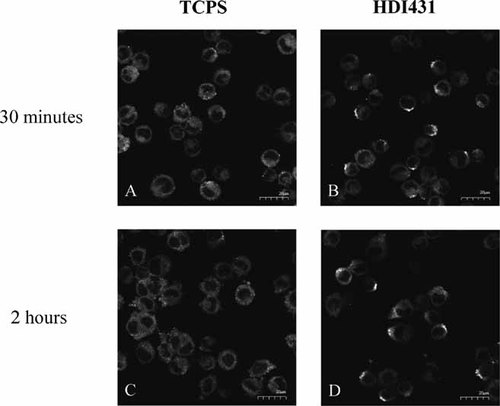
Intracellular cPLA2 expression patterns are influenced by material surface chemistry. PMA-differentiated U937 cells were seeded onto TCPS cover slips (control) or HDI431 coated glass cover slips for 30 min or 2 h. As described in detail in Materials and Methods, cells were then fixed with 4% paraformaldehyde and labeled with anti-group IVA cPLA2 and rabbit anti-mouse Alexa Fluor® 488. Representative images were obtained using a laser scanning confocal microscope.
Discussion
The current study has been able to establish the effect of PCNU surfaces on specific PLA2 protein expression. Earlier studies showed that inhibitors of each category of PLA2 enzymes induced a reduction in U937 cell-mediated degradation of PCNUs (Dinnes et al., 2005). In addition, PCNU materials elicited an increase in AA release from U937 cells relative to a TCPS control, and this stimulated AA release was also primarily inhibited by the chemical inhibitors of sPLA2 and cPLA2 (Dinnes et al., 2005). Group IVA cPLA2 and Group V sPLA2 were previously shown to be involved in AA release from macrophages with cPLA2 being the main regulator (Shirai et al., 2005). sPLA2 may potentially enhance this cPLA2-mediated AA mobilization (Murakami and Kudo, 2001), thereby generating an immediate and delayed AA mobilization response. In order to define the specific groups of PLA2 responsible for the early PCNU-mediated macrophage response, group IVA cPLA2 and group V sPLA2 were investigated for alterations in protein expression in response to cell-attachment to PCNU versus TCPS surfaces. iPLA2 was not investigated since inhibitors of iPLA2 elicited the least effect on 14C-PCNU degradation (Dinnes et al., 2005), in addition to the fact that iPLA2 does not appear to contribute to AA release in U937 and other cells (Hsu et al., 2000), most probably acting as a homeostatic enzyme regulating glycerophospholipid composition (Fonteh and Wykle, 2004). Several studies have illustrated that PU materials induce chronic inflammation (Peltroche-Llacsahuanga et al., 2001; Ma et al., 2002; Lee et al., 2003; Rhodes et al., 2005), however, few studies have investigated specific signaling pathways that may contribute to PU-induced inflammation.
Specific inhibition of intracellular sPLA2 protein expression by ARIST
Chemical inhibitors are often not as specific as claimed therefore experiments were carried out to determine if the inhibition of U937-cell mediated 14C-PCNU degradation elicited by ARIST (Dinnes et al., 2005) was a consequence of non-specific effects on protein expression. Particularly, expression of proteins implicated in macrophage-mediated degradation processes in addition to both sPLA2 and cPLA2 protein expression was evaluated. β-actin is a key cytoskeletal protein in many cell types but particularly motile cells such as macrophages (Buccione et al., 2004). Since β-actin is so central to cell shape and attachment, as well as cell spreading and adhesion (Dadsetan et al., 2004; Dinnes et al., 2007), protein expression of β-actin was assessed by immunoblotting to ensure no significant influences on β-actin protein expression were occurring in response to this inhibitor. Target substances can be released from macrophages into the extracellular space formed between the cells and the material surface that may contribute to extracellular matrix/biomaterial degradation (Wright and Silverstein, 1984). Candidate enzymes that have been well studied and implicated in macrophage-mediated PU degradation are esterases, specifically MSE (Labow et al., 2001a, 2002, 2005b). MSE is an esterase that has demonstrated high degradative capacity toward PCNU materials, in addition to being newly synthesized and secreted from MDM in response to cell attachment to these surfaces (Matheson et al., 2004). Since this enzyme is central to macrophage-mediated degradation of PCNU, MSE protein expression was assessed in cells cultured with ARIST to ensure inhibition of degradation seen previously was not a result of effects on MSE protein expression.
Since both β-actin and MSE protein expression were unaltered in the presence of ARIST (Fig. 2), it can be concluded that inhibition of 14C-PCNU degradation elicited by U937 cells was not a result of non-specific effects on β-actin or intracellular MSE and was due to the effect of ARIST on PLA2. Supporting this result was the previous study where the pattern of inhibition by ARIST of 14C-PCNU degradation by U937 cells (Dinnes et al., 2005) was similar to the ARIST inhibition of sPLA2 protein expression in HDI321 and MDI321 adherent cells (Fig. 2B). The more prominent effect on both 14C-PCNU degradation (Dinnes et al., 2005) and sPLA2 protein expression (Fig. 2A,B) was demonstrated on PCNU with a stoichiometric ratio of 3:2:1 of the starting components, regardless of the hard segment chemistry (HDI or MDI), in comparison to HDI431. This suggests the presence of a hard segment dependant response, possibly related to the strong polar chemistry contained within the hard segment, since polar groups have been shown to influence both degradation and adhesion (Santerre et al., 2005).
The comparable results between ARIST influence on U937 cell-mediated 14C-PCNU degradation and inhibition of sPLA2 protein expression without non-specific effects on cPLA2 expression indicate that ARIST inhibition of 14C-PCNU degradation was indeed via sPLA2 down-regulation. It is important to note that although ARIST inhibited U937 cell-mediated PCNU degradation, this was not due to the enzymatic activity of sPLA2 on the material itself. No PCNU degradation, as measured by radiolabel release, occurred when sPLA2 from bovine liver was incubated with 14C-PCNUs (data not shown). It is unclear what the inhibitory effect of ARIST on sPLA2 expression had on the cells' response to the PCNU surfaces, since sPLA2 expression was unchanged in U937 cells adherent to PCNUs (Fig. 3). Although ARIST is generally classified as inhibiting sPLA2, at the concentration used in this study (Rosenthal et al., 1989; Dinnes et al., 2005) it may be a more general inhibitor of PLA2 activity. In a study where monocyte chemotaxis to MCP-1 was assessed in the presence of 100 µM ARIST, >80% inhibition was observed (Carnevale and Cathcart, 2001). This process was then shown to involve cPLA2 and iPLA2 activity (Carnevale and Cathcart, 2001).
Effect of PCNU materials on intracellular cPLA2 and sPLA2 protein expression
As illustrated in Figure 3, each model PCNU stimulated an increase in cPLA2 protein expression 2 h post-cell attachment in U937 cells, relative to cells on TCPS (control surface), with no significant effect on intracellular sPLA2 protein expression. In addition, sPLA2 protein could not be detected in conditioned media by immunoblot analysis (data not shown). Although sPLA2 is typically a protein that is secreted in response to stimulation, sPLA2 has also been indicated to be internalized via a PLA2 receptor shown to be located on the plasma membrane (Ohara et al., 1995; Hanasaki and Arita, 2002), therefore potentially contributing again to the intracellular pool of sPLA2 protein.
PCNU materials induce early changes in cPLA2 protein expression
It is possible that the lack of induced expression of sPLA2 protein observed at 2 h (Fig. 3) could have been due to an early burst of AA release occurring within minutes of cell attachment to the PCNU surface (Dinnes et al., 2005). At 2 h, there was no significant difference in the altered cPLA2 protein expression in the cell lysates between each model PCNU material (Fig. 3B). Therefore further experiments exploring earlier time points of PLA2 protein expression were only carried out for one PCNU (HDI431) and TCPS. HDI431 has been shown to be the most biodegradable model PCNU (Labow et al., 2002) in addition to inducing the greatest amount of macrophage fusion and multinucleation (Matheson et al., 2004), therefore likely to be the model PCNU able to initiate the greatest inflammatory response. As mentioned previously, surface characterization of the model PCNUs used here by XPS and AFM analysis (discussed above) illustrated that HDI-based PCNUs are more phase-separated than MDI-based PCNUs, with a greater ratio of OCOO groups at the surface of HDI-based PCNUs and smaller aliphatic HDI groups all contributing to less stability of this material to cleavage. Although these surface characteristics reflect the susceptibility of these materials to degradation by enzymes and macrophages, these surface differences between the PCNUs do not appear to be directing any differences in cPLA2 protein expression in PCNU-adherent macrophages. However, the differences in cPLA2 expression agree with the differences in water contact angle (relative hydrophobicity) on PCNUs versus TCPS (advancing and receding angle TCPS << PCNU), with greater cPLA2 expression on the hydrophobic PCNU surfaces relative to the hydrophilic TCPS. TCPS is a hydrophilic surface, however, gas plasma treatment typical of TCPS surfaces for cell culture would further contribute to the large difference in water contact angle between TCPS and these PCNU surfaces.
The significant induction of cPLA2 protein expression that was detected at 30 min post cell attachment to HDI431 versus TCPS (Fig. 4) that then diminishes again until 2 h can be explained by an immediate and delayed AA release response that has been reported due to cell activation (Fonteh and Wykle, 2004). This effect has been suggested to be mainly initiated by cPLA2 and possibly sustained or enhanced by sPLA2 (Murakami and Kudo, 2001). Regardless, these results have clearly demonstrated a role for cPLA2 in the inflammatory signaling pathways induced in the macrophage foreign body response to PCNU materials.
cPLA2 location was altered by U937 cell adherence to a PCNU surface
The role of cPLA2, upon cell activation, is to hydrolyze membrane phospholipids to release AA as an initial precursor to several potent inflammatory mediators. cPLA2 requires phosphorylation for activation in addition to possessing a requirement for µM levels of Ca2+ necessary for translocation to cell membranes (Qiu et al., 1998). Under resting conditions cPLA2 is typically constitutively expressed and located throughout the cytoplasm (Schievella et al., 1995), as was indicated in TCPS adherent U937 cells (Fig. 5A,C). Although not confirmed through co-localization to cytosolic compartments, upon adherence to PCNU (HDI431), cPLA2 appeared to localize to the edges of the cytoplasm perhaps clustering towards the plasma membrane (Figs. 4D and 5B). This would allow access to cell membrane phospholipids for AA release as has been extensively reported on (Dennis, 2000; Balsinde et al., 2002; Dinnes et al., 2005). Previous studies assessing various forms of cell activation have indicated localization of cPLA2 to the plasma membrane (Durstin et al., 1994; Schievella et al., 1995; Klapisz et al., 1999), nuclear envelope (Fatima et al., 2005), endoplasmic reticulum (Schievella et al., 1995), in addition to the golgi apparatus (Evans and Leslie, 2004).
Conclusions
The current study has provided evidence for the first time that signaling via cPLA2 rather than sPLA2, occurred when macrophages, cells pivotal to the innate immune response, adhere to PCNU surfaces. There was an increase in the intracellular expression of cPLA2 that was time dependent along with a translocation of cPLA2 that appeared to approach the plasma membrane where the hydrolysis of membrane phospholipids via PLA2 enzymes would release AA, a potent inflammatory mediator of the innate immune response. Understanding the macrophage response at the cell-material interface is becoming increasingly important whether PCNU materials are used for long-term implants, drug delivery systems or regenerative medicine techniques. The initiation of an inflammatory response can result in negative and even harmful effects to the surrounding tissue and alter the desired outcome.
Acknowledgements
The authors would like to thank Dr. Meilin Yang for the synthesis of polymers used in this study. Dr. Yang assisted Soroor Sharifpoor in the water contact angle measurements of TCPS. Assessments of the effect of commercial preparations of sPLA2 on PCNU degradation were performed by summer student Sarah McDonald. Donna Lee M. Dinnes is a CIHR Strategic Training Fellow in Cell Signaling in Mucosal Inflammation and Pain (STP-53877).