Endothelin induces functional hypertrophy of peritubular smooth muscle cells†
Francesca Romano and Guido Gambara contributed equally to this work.
Abstract
When chronically stimulated with agonists of contraction, smooth muscle cells (SMCs) undergo cell hypertrophy, a process defined as increase in size and potentiation of the contractile phenotype in the absence of proliferation. Hypertrophic response has long been associated to a number of pathologies of the cardiovascular and respiratory systems. We have investigated the phenotypic and functional response of SMCs to long-term treatment with endothelin. Our model was primary cultures of peritubular smooth muscle cells (PSMC) a testicular cell type target of locally produced endothelin and characterized by an unusual phenotypic stability when cultured in simple medium in complete absence of serum. We report the following responses of PSMC to 4-day exposure to ET-1: (i) increased protein synthesis without induction of cell proliferation; (ii) increase in cell size (evaluated by means of flow cytometry) and increased expression of SM-α-actin, desmin, caldesmon and calponin, markers of the contractile phenotype. In experiments of selective stimulation of either ETA or ETB receptor subtypes, both proved to be involved in inducing the observed hypertrophic responses. The hypertrophic cells exhibit the ultrastructural features of differentiated SMCs and are capable of calcium mediated contractile response when acutely stimulated with ET-1 specifically through ETA and/or ETB receptors, as evaluated by calcium imaging and scanning electron microscopy. These observations demonstrate that engagement of ET receptors is capable of inducing potentiation of the contractile phenotype and functional hypertrophy of PSMC. J. Cell. Physiol. 212: 264–273, 2007. © 2007 Wiley-Liss, Inc.
Smooth muscle cells (SMCs), unique among contractile cell types, display a multifunctional capacity for contraction, migration, proliferation, synthesis and secretion of extracellular matrix components, growth factors and cytokines. These cells can exhibit a wide range of different phenotypes at different stages of development and, even in adult organisms, are not irreversibly differentiated. In fact, unlike skeletal and cardiac muscle, that are terminally differentiated, SMC within adult animals retain a high degree of plasticity and can reversibly undergo major changes in phenotype in response to changes in local environmental signals that control normal phenotype (Schwartz et al., 1990; Owens, 1995; Owens et al., 2004). This characteristic plasticity exhibited by the fully mature SMC is an inherent property of the differentiated phenotype of the SMC and uniquely equip these contractile cells with the potential to regulate lumen diameter of hollow organs both transiently, via reversible contraction, and chronically, via structural remodeling due to fibrosis and muscle hypertrophy (Heagerty et al., 1993). The rather profound and reversible changes in phenotype SMCs can undergo in response to changes in local environmental cues are evident also in vitro. When isolated in culture, SMCs respond to serum resuming proliferation and undergoing a characteristic switch from contractile to synthetic phenotype, in which loss of myofilament components is accompanied by an increase in synthesis and secretion of macromolecules (Halayko and Solway, 2001). Phenotypic switching from a differentiated to a proliferative phenotype and vice versa (“maturation”) (Chamley-Campbell et al., 1979) is of particular interest as involved in basic aspects of relevant pathologies of the vascular system (Ross, 1993; Thyberg, 1998). Given the high degree of plasticity exhibited by the SMC, an interesting issue is represented by the mechanisms through which the contractile differentiated state is maintained. Unfortunately, the pathogenesis of the hypertrophic status is not yet fully understood. The heterogeneous composition of the cell population, which may result in uncontrolled selection of cellular subtypes in serum-grown cultures, and the instability of the contractile differentiated phenotype in vitro are commonly reported as factors contributing to methodological difficulties in this field of study. Therefore, given SMC plasticity, it is important to use a more stable experimental model in which interconversion between contractile and dedifferentiated phenotype can be studied in controlled conditions.
We have previously reported that peritubular smooth muscle cells (PSMC), responsible for seminiferous tubule contractility, are a favorable model for in vitro studies of SMC phenotype control because they can be cultured in simple medium under totally controlled conditions, maintaining both quiescence and traits of the contractile phenotype (Filippini et al., 1993). PSMC, which surround the seminiferous epithelium forming a single contractile layer at the border with the interstitium, are targets for endothelin (ET-1) (Filippini et al., 1993; Tripiciano et al., 1996, 1997).
ET-1 is a vasoactive peptide, originally isolated from porcine aortic endothelial cell-conditioned medium, that acts in a paracrine fashion on different vascular and nonvascular tissues (Yanagisawa et al., 1988; Sakurai et al., 1991; Wagner et al., 1992; Goldie and Henry, 1999) through two distinct receptor types (Arai et al., 1990; Sakurai et al., 1990). ET-1 is a powerful vasoconstrictor (Yanagisawa et al., 1988), has mitogenic (Hirata et al., 1989) and antiapoptotic properties (McWhinnie et al., 2006; Zhao et al., 2006), induces inflammatory responses (Touyz and Schiffrin, 2003) and cardiovascular hypertrophy (Sugden, 2003; Bakker et al., 2004). These effects contribute to altered vascular reactivity and to vascular remodeling, important processes in blood pressure elevation and cardiovascular diseases associated with hypertension (Schiffrin, 2005). Moreover, in a very recent report ET was shown to induce hypertrophy and reduce apoptosis of airway SMCs (McWhinnie et al., 2006), possibly contributing to bronchial remodeling found in severe asthma (Benayoun et al., 2003).
Endothelin-mediated spatio-temporal control of peritubular contractility is regulated by the cyclic expression, in adjacent Sertoli cells, of endothelin-converting enzyme (ECE) (Tripiciano et al., 1999). We have provided original evidence that endothelin is capable of stimulating tubular contractility directly and specifically acting on PSMC (Filippini et al., 1993; Tripiciano et al., 1996) through a double control mechanism. In particular, (1) single PSMC co-express both kinds of ET receptors (ETA-R and ETB-R); (2) both ETA-R and ETB-R mediate endothelin-induced PSMC contraction; (3) ETA-R induces PLC activation resulting in InsP3 mediated calcium mobilization; (4) ETB-R mediates intracellular Ca2+ mobilization (from a thapsigargin sensitive pool) through a cADPR pathway, independent from InsP3, in turn activating the type 2 ryanodine receptor (Tripiciano et al., 1997; Barone et al., 2002).
The present study was aimed to identifying the possible hypertrophic effect of prolonged exposure to ET-1 on PSMC, an experimental model in which a well-characterized contractile phenotype is maintained in culture in totally controlled conditions. Here we show that the response of PSMC to ET-1, through either ETA-R or ETB-R, consists in increase in size, potentiation of contractile apparatus and increase in SMC specific markers, without DNA duplication. In addition, we describe endothelin-dependent Ca2+ signaling and contraction in hypertrophic PSMC.
Methods
Animals
The animals used were 18- to 20-day-old Wistar rats (Charles River Laboratories, Inc., Como, Italy). Animals were kept in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were killed by CO2 asphyxia. Eight to ten rats were used for each experiment.
Cell isolation, culture, and treatments
Purified PSMC were prepared as previously described (Filippini et al., 1993). Briefly, the peritubulum was detached from trypsin-dispersed seminiferous tubules by collagenase digestion and subjected to centrifugation at 40g. The pellet was further digested in trypsin and EDTA to a single cell suspension and fractionated on a discontinuous Percoll density gradient. Collagenase A from Clostridium histolyticum and deoxyribonuclease I were obtained from Roche Molecular Biochemicals (Mannheim, Germany), trypsin was purchased from Difco Laboratories (Detroit, MI) and Percoll was obtained from Pharmacia Biotech (Uppsala, Sweden). The cells were routinely seeded on Falcon plastic dishes (Becton Dickinson and Co., Franklin Lakes, NJ), except where otherwise specified, at a concentration of 50 × 103 cells/cm2 and were cultured under serum free conditions at 37°C in humidified atmosphere of 5% CO2 and 95% air in Eagle's MEM (Life Technologies, Inc., Grand Island, NY). Cell purity, assessed for each preparation by alkaline phosphatase cytochemistry (3), was never below 95%. Treatments were with 10−9 M ET-1, or 10−7 M IRL 1620, or 10−6 M BQ788 plus 10−9 M ET-1 (in this case ETB-R antagonist was administered twenty minutes before ET-1) all from Peninsula Laboratories Europe LTD (Merseyside, England). The first stimulation was 3 days after plating in plain medium and the second stimulation followed 48 h later, when the cells received fresh medium.
Amino acid incorporation
Both cells stimulated with ET-1 for 24–96 h and unstimulated cells were labeled with 2 µCi/ml [35S]-methionine, specific activity 1,175 Ci/mmol (Perkin Elmer Life Sciences, Inc., Boston, MA), for 2 h in MEM. Cells were lysed in 50 mM Tris containing 100 mM EDTA, 100 mM NaCl, and 0.1% SDS; the lysate was subjected to trichloroacetic acid precipitation and the radioactivity incorporated into proteins was measured by liquid scintillation spectrometry.
Proliferation assays
To evaluate a possible increase in cell number after ET-1 treatment [3H]-thymidine incorporation analysis was performed. Cells were cultured in 12-multiwell plates (150,000 cells/well) and exposed to 10−9 M ET-1 or 10% FCS (Life Technologies, Inc.) for 1–3 days. The cells were labeled with 1 µCi/ml [methyl-3H]-thymidine (SA, 20 Ci/mmol; NEN Life Science Products, Inc., Boston, MA) for additional 18 h in the same culture conditions, then mechanically lysed and harvested onto fiber glass filters by means of a Skatron cell collector. The radioactivity incorporated into DNA was evaluated by liquid scintillation spectrometry.
Flow cytometry
Cells stimulated with ET-1 for 24–96 h and parallel control samples were detached by 15-min incubation in 0.02% EDTA followed by 3-min treatment in 0.05% trypsin-0.02% EDTA and finally resuspended in 0.1% BSA in HBSS. Changes in cell size [forward scatter (FS)] and complexity [side scatter (SS)] were studied by means of a Coulter EPICS XL cytometer (Beckman Coulter, Inc., Fullerton, CA). Cells were gated using FS versus SS to exclude cell debris and occasional aggregates. For each condition, 5,000 events were acquired in the linear mode.
Immunoblotting
The cells were lysed and scraped in a commercial cell lysis buffer (New England Biolabs, Inc., Beverly, MA) containing 20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, and 1 mg/ml leupeptin to which 2% SDS and 1 mM phenylmethylsulfonylfluoride were added. The protein concentration of each sample was determined using the microbicichoninic acid method (Pierce Chemical Co., Rockford, IL). Equal amounts of proteins were separated on an SDS–polyacrylamide gel in reducing conditions and transferred onto a nitrocellulose membrane (Hybond C, Amersham Pharmacia Biotech, Aylesbury, UK). The membrane was saturated with 5% nonfat dry milk and 0.05% Tween-20 and probed with 1:2,500 monoclonal anti-smooth muscle-α-actin (SM-α-actin) (clone 1A4, Sigma, St. Louis, MO), 1:400 monoclonal anti-desmin (clone DE-U-10, Sigma), 1:100 polyclonal anti-caldesmon (Upstate Biotechnology, Lake Placid, NY), 1:5,000 monoclonal anti-calponin (clone hCP, Sigma), 1:100 polyclonal anti-p38 (Santa Cruz Biotechnology, Inc., CA). The secondary antibodies used were horseradish peroxidase-conjugated anti-mouse antiserum (Bio-Rad Laboratories, Hercules, CA) and anti-rabbit antiserum (Amersham Pharmacia Biotech). Detection was performed using the chemiluminescence system (ECL, Amersham Pharmacia Biotech).
Immunofluorescence
Cells for immunofluorescence were fixed in cold ethanol/acetone (1:1, vol/vol) for 10 min and pretreated with 2% BSA in PBS before incubation with monoclonal anti-SM-α-actin (1:300; clone 1A4, Sigma), or anti-desmin (1:20; clone DE-U-10, Sigma); the secondary antibody used was in both cases anti-mouse fluorescein isothiocyanate-conjugated secondary antibody (also from Sigma). The specifity of immunolabeling was verified in control samples prepared with the primary antibody omitted.
Calcium imaging
[Ca2+]i was measured by dual wavelength fluorescence in single cells loaded with the Ca2+-sensitive indicator Fura-2. PSMCs were plated on glass coverslips in serum free medium (MEM) and cultured in control or ET-1 stimulated conditions as described above. Cells to be analyzed were incubated in MEM containing 3 mM fura-2-AM for 1 h at 37°C. The cells were then rinsed with Krebs–Henseleit–HEPES (KHH) buffer (140 mM Na+, 5.3 mM K+, 132.4 mM Cl−, 0.98 mM PO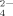 , 1.25 mM Ca2+, 0.81 mM Mg2+, 5.5 mM glucose, and 20 mM HEPES) supplemented with 0.2% fatty acid free BSA. The coverslips were placed into a culture chamber, kept at 37°C constant temperature, on the stage of an inverted fluorescence microscope (TE2000E Nikon, Florence, Italy), connected to a cooled charge-coupled-device 512B Cascade camera (Princeton Instruments, Monmounth Junction, NJ). Samples were illuminated alternately at 340 and 380 nm using a random access monochromator (Photon Technology International, Birmingham, NJ) and emission was detected using a 510 nm emission filter. Images were acquired (1 ratio image/s) using Metafluor software (Universal Imaging Corporation, West Chester, PA). Calibration of the signal was obtained at the end of each experiment by maximally increasing intracellular Ca2+-dependent fura-2 fluorescence with 5 µM ionomycin, followed by recording minimal fluorescence in Ca2+-free medium. [Ca2+]i was calculated according to previously described formulas (Grynkiewicz et al., 1985).
, 1.25 mM Ca2+, 0.81 mM Mg2+, 5.5 mM glucose, and 20 mM HEPES) supplemented with 0.2% fatty acid free BSA. The coverslips were placed into a culture chamber, kept at 37°C constant temperature, on the stage of an inverted fluorescence microscope (TE2000E Nikon, Florence, Italy), connected to a cooled charge-coupled-device 512B Cascade camera (Princeton Instruments, Monmounth Junction, NJ). Samples were illuminated alternately at 340 and 380 nm using a random access monochromator (Photon Technology International, Birmingham, NJ) and emission was detected using a 510 nm emission filter. Images were acquired (1 ratio image/s) using Metafluor software (Universal Imaging Corporation, West Chester, PA). Calibration of the signal was obtained at the end of each experiment by maximally increasing intracellular Ca2+-dependent fura-2 fluorescence with 5 µM ionomycin, followed by recording minimal fluorescence in Ca2+-free medium. [Ca2+]i was calculated according to previously described formulas (Grynkiewicz et al., 1985).
Transmission and scanning electron microscopy
The ultrastructural modifications induced in PSMC cytoplasm by 4-day ET-1 treatment were studied after cells had been detached by 10 min incubation in 0.02% EDTA, followed by 3 min digestion in 0.05% trypsin-0.02% EDTA (HyClone Laboratories, Inc., Cramlington, NE). The samples were fixed in 2.5% buffered glutaraldehyde, postfixed in OsO4, dehydrated in ethanol, embedded in epoxy resin. Ultrathin sections were conventionally contrasted in uranyl acetate and lead hydroxide and viewed in a Hitachi 7000 transmission electron microscope. Cell contractile response to acute stimulation was studied by scanning electron microscopy; cells, grown on plastic coverslips, were fixed in 2.5 buffered glutaraldehyde after 5 min stimulation, dehydrated and critical point dried in ethanol, then coated with gold and viewed in a Hitachi S-570 scanning electron microscope.
Statistical analysis
Data are presented as the mean ± SE of results from at least three independent experiments. Student's t-test was used for statistical comparison between means where applicable.
Results
Endothelin increases protein synthesis without inducing proliferation
To check whether prolonged ET-1 treatment induces an increase in protein synthesis, we performed an [35S]-methionine incorporation assay. The time course of [35S]-methionine incorporation was evaluated in 2 h pulse-labeling experiments on cells plated in 6-multiwell plates (3 × 105 cells/well). As shown in Figure 1A, ET-1 stimulated protein synthesis in 4 days continued treatment.
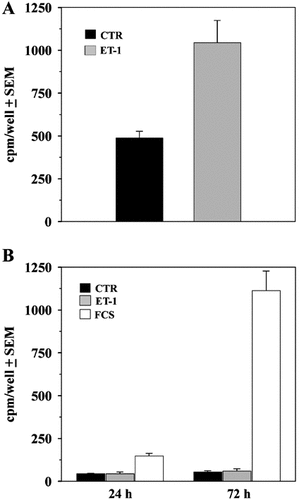
Protein synthesis but not DNA duplication is stimulated by ET-1. A: Incorporation of [35S]-methionine was evaluated in a 2-h pulse after culturing cells in the presence or absence of ET-1 for 4 days. B: After 1- to 3-day exposure to ET-1 or 10% FCS, cells were labeled with [3H]-thymidine for additional 18 h in the same culture conditions. Increasing precursor incorporation is apparent in samples treated with FCS, but not in those treated with ET-1. The data are representative of three independent experiments.
In order to rule out the possibility that the observed increase in total protein synthesis might result from cell proliferation, proliferation analysis was performed by measuring [3H]-thymidine incorporation after pulse-labeling at two different experimental times, 24 and 72 h. The conditions were: in plain medium, in the continuous presence of ET-1, and in medium supplemented with 10% FCS. The pattern of [3H]-thymidine incorporation showed that 3-day treatment with endothelin failed to induce significant DNA duplication, whereas serum stimulated the expected increase in precursor incorporation, indicating that the cells are capable of proliferative response (Fig. 1B).
Increase in cell size following long-term endothelin treatment
To evaluate a possible increase in cell size in response to ET-1, cells were cultured for 1–4 days with or without ET-1 and then enzymatically detached and subjected to cytofluorimetric analysis. After 4-day treatment the mean value of FS, an index of cell size, increased in response to the contractile agonist. A parallel increase in SS was observed, indicating that ET-1 induced higher cellular complexity possibly due to an increase in the contractile machinery (Fig. 2).
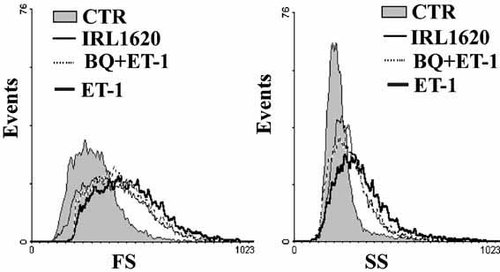
Increase in cell size and complexity induced by prolonged engagement of ET receptors. Cultures were treated for 4 days with either ET-1 or agonists selectively stimulating ETA-R (with BQ 788 + ET1) or ETB-R (with IRL 1620). Control and stimulated cells were detached and subjected to cytofluorimetric analysis of forward scatter (FS) and side scatter (SS). The increase in FS and SS observed in the three experimental conditions demonstrates an increase, respectively, in cell size and complexity. The data are representative of at least three independent experiments and were obtained by analyzing 5,000 cells (events) for each condition in each experiment.
The same analysis of the hypertrophic response of PSMC to ET-1 chronic stimulation was performed using IRL1620 or BQ788 plus ET-1 in order to separately stimulate, respectively, either ETB-R or ETA-R. IRL1620 is a specific stimulator of ETB-R, while BQ788 is an inhibitor of the same receptor and was used in addition of ET-1 to stimulate only ETA-R. As shown in Figure 2, the separate and selective stimulation of ETA and ETB receptors was found to be able to induce a hypertrophic response comparable to ET-1.
The effects of long-term ET-1 treatment on the ultrastructural characteristics of the PSMC phenotype were examined in enzymatically detached cells, so that a significant amount of cell body could be analyzed in each section. Both after 4-day treatment with the factor and in control conditions, a characteristic feature of the cell cytoplasm was the presence of extended organelle-free areas occupied by filament bundles and bordered by dense bodies, which are typical of SMCs (Fig. 3).
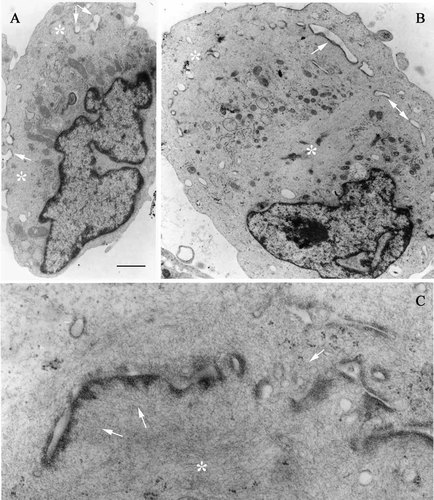
Cell ultrastructure following 4-day stimulation with ET-1. A,B: Low magnification electron micrographs of control (A) and ET-1 treated (B) cells. C: Detail of ET-1 treated cell cytoplasm. The cytoplasm of the hypertrophic cells (B,C) appears rich in areas filled with filamentous structures (asterisks) and dense bodies (arrows). At higher magnification (C) filaments appear to connect to dense anchoring bodies. Cells were fixed and processed for transmission electron microscopy after enzymatic detachment from the culture dish. Bar: A, B: 1 µm; C: 0.25 µm.
Endothelin-induced up-regulation of contractile markers
In order to verify whether the observed increase in size and in protein synthesis was accompanied by the potentiation of contractile phenotype, we have analyzed the synthesis of four specific markers, α-SM isoactin and desmin, as well as calponin and caldesmon, two actin-binding proteins involved in the formation and activation of the acto-myosinic complex (Gusev, 2001). The content in each marker was evaluated in Western blot using total p38 for normalization, a parameter which is more suitable than usual cytoskeletal markers in cells undergoing hypertrophy. The results obtained (Fig. 4) indicate that ET-1 induced an increase in the content of each specific differentiation marker examined, most apparent for calponin. Western blot analysis of samples stimulated through either ETA or ETB receptors showed that both receptors contribute to the increased synthesis of specific smooth muscle contractile markers.
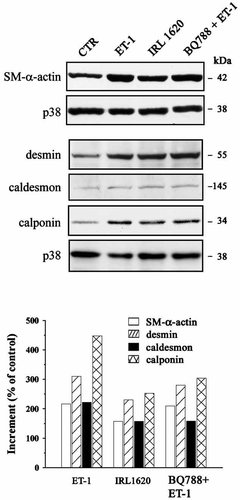
Increase in smooth muscle contractile markers after prolonged ET-1 stimulation. Immunoblot determination of SM-α-actin, desmin, caldesmon, and calponin in cells chronically stimulated with ET-1. Selective stimulation of either ETA-R or ETB-R induces an increase in the synthesis of specific SM-contractile markers. For each sample, densitometric arbitrary units were normalized for the respective p38 values. These values are expressed in the histogram as percent increase over the control. The data shown are representative of at least three independent experiments.
Chronic treatment with ET-1 induces morphological changes of PSMC
The morphological response to ET-1 was studied both immediately after stimulation and after 1–4 days continued treatment. Following immediate and powerful contraction, prolonged treatment of PSMC with 10−9 M ET-1 induces a gradual morphological change well apparent in phase contrast light microscopy. After 4 days in continued presence of the agonist, most cells assume an oriented and more extended shape. We have studied the cytoskeletal remodeling which parallels the morphological changes resulting from chronic treatment with ET-1. Immunofluorescence analysis (Fig. 5) shows that in the control cells α-isoactin and desmin filaments are characteristically slender and radially oriented, reflecting the unoriented shape of the PSMC, a pattern which remains unchanged for at least 6 days. In ET-1 treated samples, conspicuous SM α-actin-positive stress fibers and desmin filaments appear after 4-day continuous treatment.
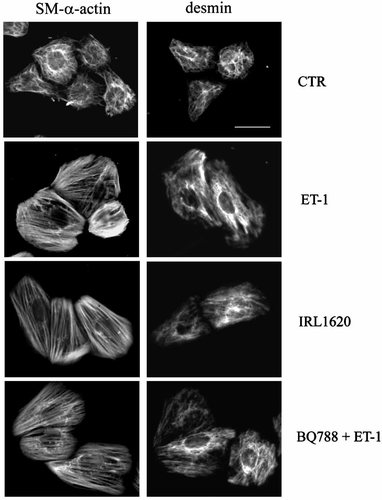
Long-term stimulation of endothelin receptors results in potentiation of PSMC contractile phenotype. Immunofluorescence images showing the distribution of SM-α-actin and desmin in control and hypertrophic PSMC. In cells cultured in plain medium (CTR) both markers appear distributed along radial filaments, and the pattern of fluorescence reveals the unoriented cell shape. In response to 4-day treatment with ET-1, SM-α-actin filaments reorganize in strongly labeled stress fibers. The distribution of both markers shows that the cells have acquired an elongate shape. A comparable response is observed after 4-day selective stimulation of either ETA-R (with BQ788 + ET-1) or of ETB-R (with IRL1620). Bar = 30 µm.
A comparable pattern was observed after treating PSMC with 10−7 M IRL1620, or with 10−9 M ET-1 in addition to 10−6 M BQ788, indicating that either receptors are able to mediate a comparable response to the agonist (Fig. 5).
Calcium signaling response to acute stimulation of ETRs is functionally increased in hypertrophic cells
The increase in contractile proteins suggests the possibility that chronic ET treatment results in functional hypertrophy. This hypothesis was tested in cells chronically pretreated with ET-1 and then subjected to acute stimulation of ET receptors after a brief washout. ETRs-mediated intracellular calcium release was analyzed in fura-2-loaded control and hypertrophic cells. Calcium imaging (Fig. 6) showed that induction of Ca2+ mobilization by either combined or selective ETRs stimulation, though varying in degree in individual cells, was in all cases significantly higher in cells previously subjected to ET-1 chronic treatment. Particularly high responses were found to result from stimulation through ETA receptor.
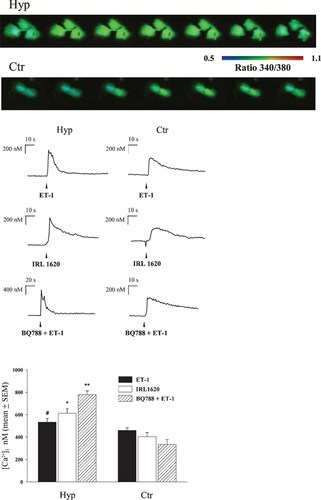
Calcium signaling through ET-receptors is enhanced in hypertrophic PSMC. Upper part: series of pseudocolor images of fura-2 fluorescence ratio (F340/F380) sequentially recorded during ET-1 stimulation of cells previously subjected to 4-day ET-1 chronic treatment and of control cells. Lower part: representative single cell recordings of Δ[Ca2+]i against time, in response to either combined or selective stimulation of ET receptors in hypertrophic (left) and control (right) PSMCs. Bar chart shows the size of Δ[Ca2+]i values in hypertrophic (Hyp) and control (Ctr) cells following selective or combined ETA-ETB receptor stimulation. The data represent the mean ± SE of 4–7 determinations obtained from three independent experiments. Symbols denote: *P < 0.002, **P < 0.0002, #P < 0.03 when compared with respective controls.
The changes in cell shape elicited by these acute treatments were visualized by means of scanning electron microscopy. As shown in Figure 7, stimulation with ET-1, as well as selective engagement of either ETA or ETB receptors, induced apparent cell contraction as indicated by retraction of cell contours and lifting of central area.
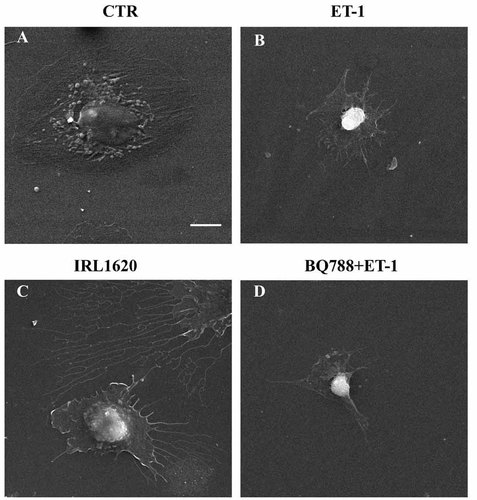
Contractile response of hypertrophic cells to acute stimulation of ET receptors. Scanning electron micrographs of PSMCs exposed to ET-1 for 4 days and subsequently subjected to acute combined or selective stimulation of ET receptors. A: plain medium, (B) ET-1; (C) BQ788 + ET-1 (ETA-R selective); (D) IRL1620 (ETB-R selective). Contraction of the hypertrophic cells in response to acute stimulation is apparent from lifting of central area and formation of highly indented cell contours. Bar = 5 µm.
Discussion
Our data on freshly isolated PSMC in mitogen free primary cultures add new information on the ability of ET-1 to potentiate the differentiated phenotype of SMC, showing that continued selective or combined stimulation of ET receptors results in increased cell size and expression of a wide range of SM specific markers. Moreover, we provide functional data demonstrating that hypertrophic PSMC are capable of contractility and of calcium hyper-responsiveness to both selective and combined stimulation of ET receptors. When referred to smooth muscle tissue, the term hypertrophy is often used to indicate an increase in volume of the overall tissue, while at a close analysis the majority of reports show proliferative responses, that is, cell hyperplasia rather than cell hypertrophy. From the point of view of cellular phenotype control, tissue hypertrophy and cellular hypertrophy may be envisaged as opposite responses, the first involving loss of differentiated traits and of quiescence, the latter based on potentiation of specific contractile traits (Berk, 2001). Our experiments show that PSMC in primary culture and in absence of serum do not proliferate in response to ET-1, although this cell type is capable of proliferative response if stimulated by serum. While maintaining conditions of quiescence in plain controlled medium, PSMC respond to ET-1 with increased protein synthesis activity. In itself this datum is suggestive of a hypertrophic response, but as a single datum increased amino acid incorporation might result from a dedifferentiation switch to a synthetic phenotype. In this case the cells actively synthesize and secrete matrix macromolecules, strongly reducing the synthesis of components of their contractile machinery (Halayko and Solway, 2001). A number of experiments prove that is not the case for the response of PSMC to ET-1. The ultrastructure of the cytoplasm shows no significant endoplasmic reticulum or Golgi development, while typical traits of contractile phenotype are well apparent, such as extended areas entirely occupied by filament bundles connecting to typical submembrane densities. Western blot analysis proved the potentiation of the contractile phenotype induced by long-term ET-1 treatment, showing an increase in all the differentiation markers tested, that is, α-SM actin, desmin, caldesmon and calponin, while a marker of extracellular matrix (fibronectin) was only slightly increased in ET-1 treated cultures, and equal to controls in IRL1620 treated samples (not shown). The increased expression of contractile markers is accompanied by a change in cell shape and redistribution of α-SM actin-containing filaments, which reorganize in well apparent parallel bundles resembling typical stress fibers, resulting in a more oriented cell shape. The tendency of α-SM actin-containing filaments to form well apparent bundles in conditions in which the production of α-SM actin is increased has been previously described in PSMC induced to hypertrophy by continued exposure to PDGF-BB (Chiarenza et al., 2000).
The whole of these data show that ET-1 induces up-regulation of contractile phenotype in PSMC, similarly to what described for VSMC stimulated with other agonists of contraction, that is, Ang II (Geisterfer et al., 1988; Berk et al., 1989) and AVP (Geisterfer and Owens, 1989) and for ET itself in VSMC (Chua et al., 1992; Andrawis et al., 1996) and in airway SMC (McWhinnie et al., 2006). Moreover, consistent with its action on airway SMC and myocardiocytes (Sugden, 2003; McWhinnie et al., 2006), ET-1 treatment resulted in increased cell size, as observed in VSMC exposed to Ang II (Okamoto et al., 2004).
PSMC are known to express two distinct types of ET receptors both mediating contractile responses to the agonist (Tripiciano et al., 1997), though with only partially coincident signal transduction pathways (Tripiciano et al., 1997; Barone et al., 2002). In our experiments, the involvement of each specific type of receptor was tested by means of selective ETB-R agonist (IRL 1620) (Takai et al., 1992) and antagonist (Ishikawa et al., 1994). These experiments showed that selective engagement of either ETA-R or ETB-R results in increase in cell size and in phenotype potentiation. This finding is in line with the ability of both receptor types to elicit calcium mediated acute PSMC contraction (Tripiciano et al., 1997) and is, to our knowledge, the first report showing that selective stimulation of either ETA-R or ETB-R results in SMC hypertrophy. So far, the only report partially addressing this issue in serum starved airway SMC suggests that combined ET-Rs stimulation is required to elicit a mild increase in protein synthesis (McWhinnie et al., 2006). Definition of the specific involvement of ETA/ETB receptors in SMC from different organs might prove relevant for therapeutic targeting of ET system local inhibition.
An interesting question in agonist-induced SMC hypertrophy is whether the ability of the cell to respond to contractile stimuli is maintained, lost or modified. Despite its potential relevance, this issue has hardly been approached directly at the cellular level. Our calcium imaging experiments show that selective or combined acute stimulation of ETRs in hypertrophic PSMC results in Ca2+ hyper-responsiveness. In scanning electron microscopy, the hypertrophied cells appear very flat and display regular outline. When subjected to selective or combined ETR stimulation, the cells undergo rapid morphological changes strongly indicating an acute contractile response. These data show that hypertrophic PSMC are functional.
A number of studies have dealt with the functionality of SMC in different models of tissue hypertrophy, mainly in relation to remodeling of blood vessels. Recently, the functionality of experimentally hypertrophied vessels has been studied in a transgenic mouse overexpressing ET-1 specifically in the endothelium (Amiri et al., 2004). These vessels were found to undergo alterations in ET-induced contractile response and increased production of ETB receptors. Recently, the long-term effect of ET-1 infusion has been studied in organoid cultures of small arteries (Bakker et al., 2004), resulting in vasoconstriction and eutrophic (instead of hypertrophic) tissue remodeling. The ability of the organoid to contract in response to ET-1 was found to be maintained during the entire experimental time (3 days), although somewhat decreasing. Unfortunately, these articles do not report on possible changes in cell size, differentiation traits and functionality induced by ET in these specific experimental models. While the present manuscript was in preparation an interesting report appeared (Dao et al., 2006) showing in the rat that 4-week intraperitoneal administration of ET-1 results in either hypertrophy or hyperplasia of the small mesenteric artery, depending on the dose of the agonist (respectively low or high). In our experiments ET-1 stimulation, either at low or at high concentrations (10−10 M to 10−7 M), was never observed to result in cell proliferation (not shown); interestingly, PSMC are stationary in vitro, although capable of proliferating in response to serum but, when stimulated by PDGF-BB, instead of proliferating undergo phenotypic potentiation and cell hypertrophy (Chiarenza et al., 2000), a quite unorthodox response to the growth factor (Owens, 1995).
The whole of the present data combine biochemical, morphological, and functional evidence giving new insights into the long-term response of SMCs to endothelin. Mechanical plasticity of SMC is a functional characteristic necessary to adapt mechanical forces on muscle surrounding hollow organs which are chronically and variably contracted. On the other hand, SMC plasticity and hypertrophic response represent a Janus-faced issue that may imply maladaptive or compensatory reactions depending on a large number of physiopathological stimuli. In fact, alterations of the differentiated phenotype and of contractile functions, as well as cell hypertrophy, have been linked to several diseases, including hypertension-induced vascular remodeling, neointimal thickening associated with the pathogenesis of atherosclerosis and postangioplasty restenosis, and to the insurgence of asthma. Our experimental model of hypertrophied SMCs is a novel tool in which the specific intracellular pathways, leading either to hypertrophy or to hyperplasia of SMC, could be further investigated. Such detailed dissection of hypertrophic signaling might eventually make it possible to specifically exploit therapeutical aspects of hypertrophy while inhibiting others.
Acknowledgements
Research supported by grants from Italian Ministero dell'Università e della Ricerca and from Agenzia Spaziale Italiana (ASI).




