Protein kinase D2 potentiates MEK/ERK/RSK signaling, c-Fos accumulation and DNA synthesis induced by bombesin in Swiss 3T3 cells
Abstract
Protein kinase D (PKD) plays an important role in mediating cellular DNA synthesis in response to G protein-coupled receptor (GPCR) agonists but the function of other isoforms of the PKD family has been much less explored. Here, we examined whether PKD2 overexpression in Swiss 3T3 cells facilitates DNA synthesis and the activation of the extracellular regulated protein kinase (ERK) pathway in response to the mitogenic GPCR agonist bombesin. We show that PKD2 overexpression markedly potentiated the ability of this agonist to induce DNA synthesis. Addition of bombesin to Swiss 3T3 cells overexpressing PKD2 also induced a striking increase in the duration of MEK/ERK/RSK activation as compared with cultures of control cells. In contrast, neither DNA synthesis nor the duration of ERK activation in response to epidermal growth factor, which acts via protein kinase C/PKD2-independent pathways, was increased. Furthermore, bombesin promoted a striking accumulation of c-Fos protein in cells overexpressing PKD2. Our study demonstrates that PKD2, like PKD, facilitates mitogenesis and supports the hypothesis that an increase in the duration of the ERK signaling leading to accumulation of immediate gene products is one of the mechanisms by which isoforms of the PKD family enhance re-initiation of DNA synthesis by Gq-coupled receptor activation. J. Cell. Physiol. 211: 781–790, 2007. © 2007 Wiley-Liss, Inc.
Neuropeptides stimulate DNA synthesis and cell proliferation in cultured cells and are implicated as growth factors in a variety of fundamental processes including development, tissue regeneration, and neoplastic transformation in multiple cell types (Rozengurt, 1986, 1998, 2002; Gutkind, 1998; Heasley, 2001). In particular, the growth-promoting neuropeptide bombesin and its mammalian counterpart gastrin-releasing peptide (GRP) bind to a G-protein coupled receptor (GPCR) that promotes Gαq-mediated activation of β isoforms of phospholipase C to produce 2 sec messengers: Ins(1,4,5)P3 that mobilizes Ca2+ from internal stores and diacylglycerol that activates PKC (Exton, 1996; Berridge et al., 2000; Rhee, 2001). There are multiple PKC isoforms (Newton, 1995; Nishizuka, 1995) that is, conventional PKCs (α, β1, β2, and γ), novel PKCs (δ, ϵ, η, and θ) and atypical PKCs (ζ and λ) which have been implicated in the regulation of a wide range of biological responses, including cell proliferation and carcinogenesis (Livneh and Fishman, 1997; Toker, 1998). However, with the development of more selective reagents to directly target single PKC isoforms, distinct and sometimes opposing roles have been shown for different PKCs in cell growth and differentiation (Soloff et al., 2004; Oster and Leitges, 2006). In most cases, the precise downstream targets of specific PKCs remain incompletely defined.
Protein kinase D (PKD; also known initially as PKCµ) is a serine/threonine protein kinase with structural, enzymological and regulatory properties different from the PKC family members (Johannes et al., 1994; Valverde et al., 1994). PKD can be activated in intact cells by multiple stimuli, including bombesin and other GPCR agonists, growth factors and antigen-receptor engagement [see Rozengurt et al. (2005) for review]. In most cases, rapid PKD activation is mediated by PKC-dependent phosphorylation of Ser-744 and Ser-748 within the activation loop of the PKD catalytic domain (Iglesias et al., 1998; Waldron et al., 1999, 2001; Waldron and Rozengurt, 2003; Yuan et al., 2003; Rey et al., 2004). These findings indicate that PKD lies downstream of PKCs in a signal transduction pathway activated by multiple growth-promoting factors (Rozengurt et al., 2005). In accord with a role of PKD in the regulation of cell proliferation, PKD overexpression enhanced the entry into DNA synthesis in response to GPCR agonists in Swiss 3T3 fibroblasts (Zhukova et al., 2001; Sinnett-Smith et al., 2004). Interestingly, our previous results indicated that PKD facilitates GPCR-induced mitogenesis by increasing the duration of the ERK signal (Sinnett-Smith et al., 2004).
PKD is the founding member of a new family of serine/threonine protein kinases, also including PKD2 and PKD3 [Reviewed in Rozengurt et al. (2005)]. Although these kinases share similarities in overall structure, primary sequence, and enzymological properties (Hayashi et al., 1999; Sturany et al., 2001; Rey et al., 2003b), recent studies also revealed interesting differences in their sub-cellular distribution (Rey et al., 2001, 2003a,b), expression in different cell types (Chiu et al., 2006; Oster et al., 2006) and regulation (Mihailovic et al., 2004; Yuan et al., 2006). In this context, it is not known whether the recently identified members of the PKD family also mediate pathways that promote mitogenic signaling in response to GPCR agonists.
The results presented here demonstrate that PKD2 increases the duration of MEK/ERK/RSK signaling, promotes c-Fos accumulation and potentiates DNA synthesis induced by the Gq-coupled receptor agonist bombesin in Swiss 3T3 cells.
MATERIALS AND METHODS
Cell culture
Stock cultures of Swiss 3T3-PKD2.GFP cells, which overexpress PKD2, and control Swiss 3T3-GFP cells were maintained at 37°C in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal bovine serum in a humidified atmosphere containing 10% CO2 and 90% air. For experimental purposes, cells were plated in 100 mm dishes at 6 × 105 cells/dish or 35 mm dishes at 1 × 105 cells/dish and grown in DMEM containing 10% fetal bovine serum for 7–9 days until they became confluent and quiescent (Sinnett-Smith et al., 2004). Phoenix packaging cells (kindly provided by Dr. G. Nolan, Stanford University, Stanford, CA) were cultured in the same media in a humidified atmosphere containing 5% CO2.
Production of retrovirus
In order to generate Swiss 3T3 cells stably overexpressing PKD2, cultures of these cells were infected with retrovirus encoding either wild-type PKD2, in which PKD2 and green fluorescent protein (GFP) were expressed as two separate proteins translated from the same bicistronic mRNA. After infection, cells expressing higher levels of GFP were sorted by FACS, collected and propagated for further studies. Specifically, pMSCVneo retroviral vector was re-engineered to include a single cassette that expresses the murine PKD2 and GFP from the same promoter. MSCV-IRES-GFP plasmid was constructed by substituting the Neo gene in the vector with the IRES-GFP fragment. To generate MSCV-PKD2-IRES-GFP, the fragment of the wild-type PKD2 cDNA spanning from +11 to +2823 of the published GeneBank sequence was amplified by PCR using primers that introduced EcoRI and NotI restriction sites at 5′ and 3′- ends, respectively. The PCR-amplified fragment of PKD2 was introduced into EcoRI/NotI sites of the MSCV-IRES-GFP by ligation, thus producing MSCV-PKDK2-IRES-GFP. The nucleotide sequence of MSCV-PKDK2-IRES-GFP was validated by sequencing.
For retrovirus production, logarithmically growing Phoenix ecotropic cells were transfected with either MSCV-PKD2-IRES-GFP or MSCV-IRES-GFP using Fugene 6 Transfection Reagent as per protocol of the manufacturer. Virus-containing supernatants were collected 48 h after transfection and used immediately. Logarithmically growing Swiss 3T3 cells were incubated with the virus-containing supernatants in the presence of 5 µg/ml polybrene for 5 h. Cells were collected 48–72 h later and GFP positive fractions were FACS-sorted using a Becton Dickinson FACStar PLUS machine. GFP positive cells were propagated, and multiple aliquots were frozen. A fresh batch of tranduced cells was generated every 2 months. Following sorting, GFP positive cells were maintained as described earlier in this section.
Immunoblotting and detection of MEK, ERK, RSK, PKD, and c-Fos
Confluent, quiescent Swiss 3T3-GFP and Swiss 3T3-PKD2.GFP cells were lysed in 2× SDS–polyacrylamide gel electrophoresis sample buffer (20 mM Tris/HCl, pH 6.8, 6% SDS, 2 mM EDTA, 4% 2-mercaptoethanol, 10% glycerol) and boiled for 10 min. After SDS–PAGE, proteins were transferred to Immobilon-P membranes. The transfer was carried out at 100 V, 0.4 A at 4°C for 4 h using a Bio-Rad (Hercules, CA) transfer apparatus. The transfer buffer consisted of 200 mM glycine, 25 mM Tris, 0.01% SDS, and 20% CH3OH. For detection of proteins, membranes were blocked using 5% nonfat dried milk in phosphate buffered saline (pH 7.2) and then incubated for at least 2 h with the desired antibodies diluted in phosphate buffered saline (pH 7.2) containing 3% nonfat dried milk. Bound primary antibodies to immunoreactive bands were visualized by enhanced chemiluminescence (ECL) detection with horseradish peroxidase conjugated anti-mouse, anti-rabbit, or anti-goat antibodies. The increase in expression of human PKD2 over endogenous PKD2 levels was determined with an antisera directed against the C-terminus of both human and mouse PKD2 and PKD (Santa Cruz, Santa Cruz, CA). PKD2 expression was also confirmed by using a human PKD2 specific antibody raised against amino acids 850–865 at a dilution of 1 µg/ml. PKD2 phosphorylation was determined using either the phospho-specific pS876 antibody recognizing PKD2 phosphorylated at Ser-876 (1 µg/ml), or the phospho-specific PKD pS744/748 antibody which recognizes PKD2 phosphorylated at Ser-706 and Ser-710 (1 µg/ml). The other phosphospecific antibodies used were as follows; the phospho-ERK1/2 monoclonal antibody recognizes the ERK-1/2 only when they are phosphorylated on Thr-202 and Tyr-204 (pERK1/2); the phospho MEK1/2 polyclonal antibody recognizes MEK1/2 only when they are phosphorylated on Ser-217 and Ser-221 (pMEK1/2) and the phospho-p90RSK polyclonal antibody is specific to p90RSK only when it is phosphorylated on Thr-574.
Assay of DNA synthesis
Confluent and quiescent cultures of Swiss 3T3-GFP and Swiss 3T3-PKD2.GFP cells were washed twice with DMEM and incubated with DMEM/Waymouth's medium (1:1, v/v) containing [3H]thymidine (0.2 µCi/ml, 1 µM) and various agonists as described in the figure legends. After 40 h of incubation at 37°C, cultures were washed twice with PBS and incubated in 5% trichloroacetic acid at 4°C for 20 min to remove acid-soluble radioactivity, washed with ethanol, and solubilized in 1 ml of 2% Na2CO3, 0.1 M NaOH. The acid-insoluble radioactivity was determined by scintillation counting in 6 ml of Beckman Readysafe.
Materials
pMSCVneo retroviral vector was from Clontech (Palo Alto, CA). Restriction enzymes were purchased from New England Biolabs (Beverly, MA). Fugene 6 Transfection Reagent was obtained from Roche Molecular Biochemicals (Indianopolis, IN). Polybrene was from Aldrich (Milwaukee, WI). U0126 was from Calbiochem (San Diego, CA). EGF and bombesin were obtained from Sigma (St. Louis, MO). [3H]thymidine was from Amersham Pharmacia Biotech (Piscataway, NJ). Protein A-agarose was from Boehringer Mannheim (Indianapolis, IN). The following antibodies were purchased from Cell Signaling Technology (Beverly, MA), phospho-PKD/PKCµ Ser-744/748 antibody which detects the phosphorylated state of the activation loop serines of all members of the PKD family, phospho-p44mapk/p42mapk (pERK1/2), phospho-MEK1/2 and phospho-p90RSK. Anti-PKD2 polyclonal, PKD2 phospho-Ser876 polyclonal and anti-c-Fos polyclonal were from Chemicon, Temecula, CA. All other materials were of the highest grade available.
RESULTS
Establishment of Swiss 3T3 Cells stably expressing PKD2
In order to generate Swiss 3T3 cells stably overexpressing PKD2, cultures of these cells were infected with MSCV retrovirus encoding human PKD2 linked via an internal ribosome entry site (IRES) to GFP. This bicistronic retroviral vector expresses PKD2 and GFP as two separate proteins. GFP was used as a marker for selection of PKD2-positive cells (termed Swiss 3T3-PKD2.GFP cells). Following retroviral infection, cells that expressed higher levels of GFP were sorted by FACS, collected and propagated for further studies (Fig. 1A, left). As a control, parallel cultures of Swiss 3T3 cells were infected with MSCV retrovirus encoding only GFP and then FACS-sorted, collected and propagated to generate Swiss 3T3-GFP cells (Fig. 1A, right).
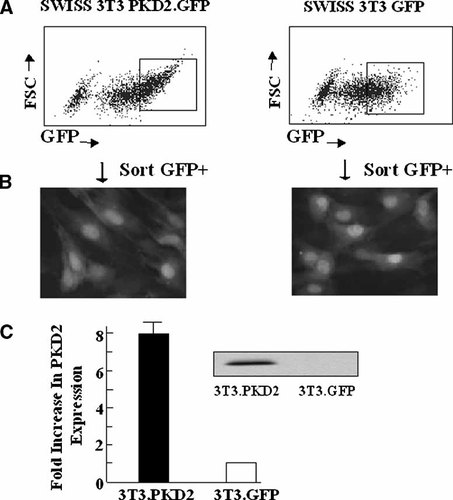
Retrovirus-mediated transfer of PKD into Swiss 3T3 fibroblasts. Swiss 3T3 fibroblasts were infected with MSCV PKD2.GFP or MSCV GFP retroviruses. A: GFP positive cells were FACS sorted 48 h later. The squares indicate the population of cells that were collected and propagated in each case. B: Fluorescent microscopy; representative fields of Swiss 3T3 PKD2.GFP cells and Swiss 3T3 GFP cells that were FACS sorted, collected and propagated. Cells were examined with a Zeiss fluorescence microscope to visualize GFP in both cell populations. C: Confluent and quiescent cultures of Swiss 3T3 PKD2.GFP cells and Swiss 3T3 GFP cells were lysed in 2× SDS–PAGE sample buffer. The samples were analyzed by SDS–PAGE and immunoblotting with PKD2 (Chemicon) antibody as described in “Experimental Procedures”. The autoradiogram shown is representative of 12 independent experiments. The bars (Swiss 3T3 PKD2.GFP cells solid and Swiss 3T3 GFP cells open) represent the optical density obtained by scanning densitometry. These results represent the mean ± SE of 12 independent experiments using populations of Swiss 3T3 PKD2.GFP cells and Swiss 3T3 GFP cells generated in three separate infections and FACS sorting.
In order to verify the increase in expression of PKD2 in the FACS-sorted cells, lysates of these cells were analyzed by SDS–PAGE and Western blotting, using an antibody directed against the COOH-terminal region of this enzyme. As shown in Figure 1B, lysates of Swiss 3T3-PKD2.GFP cells exhibited a marked increase (7.8 ± 1.2; n = 12) in the expression of the immunoreactive 105 kDa band, which corresponds to PKD2, as compared with Swiss 3T3-GFP cells. A prominent PKD2 band was also obtained when lysates from Swiss 3T3-PKD2.GFP cells were analyzed by Western blotting using a different antibody directed against human PKD2, which detects poorly the murine PKD2 (Fig. 1B). As expected, we did not detect any immunoreactive band in Western blots of lysates from Swiss 3T3-GFP cells using the antibody that recognizes human PKD2 (Fig. 1B). Thus, using high efficiency retroviral-mediated transfection we generated Swiss 3T3 cells that over-express PKD2.
Bombesin stimulates PKD2 activation in Swiss 3T3-PKD2.GFP cells
To determine whether bombesin can induce PKD2 activation in intact Swiss 3T3-PKD2.GFP cells, cultures of these cells were treated with this neuropeptide for increasing times and lysed. PKD2 phosphorylation at Ser-706 and Ser-710, located in the activation loop of the kinase catalytic domain, was determined by Western blotting using a specific antibody that detects the phosphorylated state of these residues. As shown in Figure 2A, PKD2 from un-stimulated Swiss 3T3-PKD2.GFP cells had very low level of activation loop phosphorylation, indicating that overexpression did not lead to constitutive activation loop phosphorylation. Stimulation of these cells with bombesin induced a striking time-dependent increase in PKD phosphorylation at Ser-706 and Ser-710 that was evident as early as 2.5 min after bombesin stimulation and remained elevated for at least 4 h.
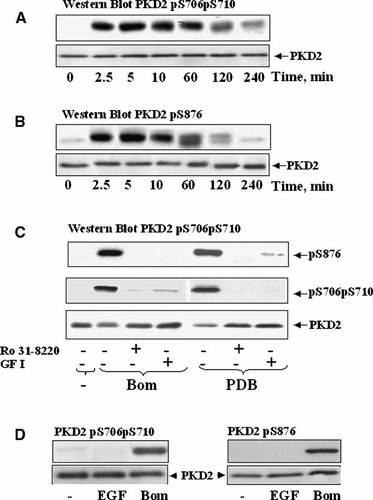
Bombesin and PDB stimulate PKD2 phosphorylation at Ser-876, Ser-706, and Ser-710 in Swiss 3T3 PKD2.GFP cells through a PKC-dependent pathway. A and B: Kinetics of PKD2 Ser-706/Ser-710 and Ser-876 phosphorylation induced by bombesin in Swiss 3T3 PKD2.GFP cells. Confluent and quiescent Swiss 3T3 PKD2.GFP cells were washed twice with DMEM and incubated for various times at 37°C with 10 nM bombesin and then lysed in 2× SDS–PAGE sample buffer. C: Pharmacological inhibition of PKCs during cell stimulation inhibits PKD2 Ser-876 and Ser-706/Ser-710 phosphorylation in Swiss 3T3 PKD2.GFP cells. Confluent and quiescent Swiss 3T3 PKD2.GFP cells were washed twice with DMEM and incubated without (−) or with (+) the PKC inhibitors GF1 at 3.5 µM, (GFI), or Ro 81-3220 at 2.5 µM for 1 h. After this preincubation period, cells were either left unstimulated or stimulated with 10 nM bombesin (Bom) or 100 nM PDB for 10 min at 37°C, then lysed in 2× SDS–PAGE sample buffer. D: EGF does not stimulate Ser-876 and Ser-706/Ser-710 phosphorylation in Swiss 3T3 PKD2.GFP cells. Confluent and quiescent Swiss 3T3 PKD2.GFP cells were washed twice with DMEM and incubated either with 5 ng/ml of EGF or 10 nM bombesin (Bom) for 10 min at 37°C. The cultures were then lysed in 2× SDS–PAGE sample buffer. All other experimental details were as described in “Materials and Methods.” In all cases, the samples were analyzed by SDS–PAGE and immunoblotting with either an antibody which recognizes phosphorylation of Ser-706/Ser-710 located in the activation loop of PKD2 (pS706pS710) or an antibody that recognizes the autophosphorylation site, Ser-876 (pS876), as indicated. The autoluminogram shown is representative of at least three independent experiments. The membrane was further analyzed by Western blotting using PKD2 Ab to verify equal loading. All other experimental details were as described in “Materials and Methods.”
An antiserum specifically recognizing the phosphorylated state of Ser-876, a PKD2 C-terminal residue, serves to monitor in vivo autophosphorylation at this site by active PKD2. This antibody detects conversion of PKD2 to an active form within intact cells. As shown in Figure 2B, bombesin stimulation induced a dramatic increase in the phosphorylation at Ser-876, in a time-dependent manner. The kinetics of PKD autophosphorylation at Ser-876 was in excellent agreement with that of Ser-706 and Ser-710, at the activation loop of the enzyme.
Next, we examined whether PKD2 phosphorylation induced by bombesin in Swiss 3T3-PKD2.GFP cells occurs through a PKC-dependent pathway. Cultures of these retrovirally transfected cells were treated with the selective PKC inhibitors Ro 31-8220 or GF I (also known as bisindolylmaleimide I) before bombesin stimulation. As illustrated in Figure 2C, the increase in PKD2 phosphorylation at either Ser-706/Ser-710 or Ser-876 induced by bombesin was strikingly decreased by treatment with Ro 31-8220 or GF I. In contrast to the results obtained with bombesin, EGF, which does not produce any substantial PKC activation in Swiss 3T3 cells, did not induce a significant increase in the phosphorylation of PKD2 at either Ser-706/710 or Ser-876 (Fig. 2D). These results indicate that bombesin activates PKD2 phosphorylation at Ser-706, 710, and Ser-876 in Swiss 3T3-PKD2.GFP cells through a PKC-dependent pathway.
Over-expression of PKD2 potentiates mitogenesis induced by bombesin
Bombesin and EGF are potent mitogens for Swiss 3T3 cells through PKC-dependent and independent signaling pathways, respectively (Rozengurt, 1986, 1991, 1998). Since the results shown in Figure 2 indicate that PKD2 is a downstream target of PKCs, we hypothesized that PKD2 could play a role in mediating PKC-dependent mitogenesis. To test this hypothesis, we examined whether PKD2 overexpression selectively potentiates the proliferative response to bombesin in Swiss 3T3 cells.
Quiescent cultures of either Swiss 3T3-PKD2.GFP or Swiss 3T3-GFP cells were transferred to media containing bombesin at 1 or 10 nM or EGF. Incorporation of [3H] thymidine into DNA was measured after 40 h of incubation. Stimulation of confluent Swiss 3T3 cells over-expressing PKD2 with either 1 or 10 nM bombesin induced a striking increase in cellular DNA synthesis as compared to cultures of Swiss 3T3-GFP cells (Fig. 3). In contrast, DNA synthesis induced by EGF, which does not stimulate PKD2 activation (Fig. 2D), was not enhanced in Swiss 3T3 cells over-expressing PKD2. These results indicate that PKD2 selectively potentiates GPCR/PKC-mediated mitogenesis in Swiss 3T3 cells.
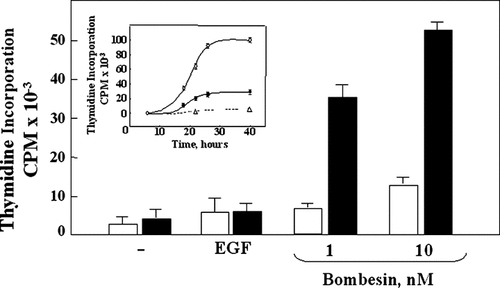
PKD2 overexpression selectively potentiates DNA synthesis induced by bombesin, in Swiss 3T3 cells. Confluent and quiescent cultures of Swiss 3T3 PKD2.GFP cells (solid bars) and Swiss 3T3 GFP cells (open bars) were washed and incubated at 37°C in 2 ml of DMEM/Waymouth's medium containing [3H]thymidine with either 1 or 10 nM bombesin or 5 ng/ml EGF, as indicated. After 40 h, DNA synthesis was assessed by measuring the [3H]thymidine incorporated into acid-precipitable material. Results are expressed in cpm mean ± SE (n = 6). Inset, Confluent and quiescent cultures of Swiss 3T3 PKD2.GFP cells (●) and Swiss 3T3 GFP cells (○) were washed and incubated at 37°C in 2 ml of DMEM/Waymouth's medium containing [3H]thymidine either without (▵) or with 10 nM bombesin (●, ○) for various times as indicated. DNA synthesis was assessed by measuring the [3H]thymidine incorporated into acid-precipitable material; Results are expressed in cpm mean ± SE (n = 3).
Protein kinase D2 overexpression increases the duration of ERK signaling induced by bombesin
The magnitude and duration of the ERK pathway activation plays a pivotal role in the transmission of mitogenic signals. In order to determine the mechanism(s) by which PKD2 overexpression potentiates the mitogenic activity of bombesin, we examined the kinetics of ERK1/2 activation in bombesin-stimulated Swiss 3T3-PKD2.GFP cells and control Swiss 3T3-GFP cells. Quiescent monolayers of Swiss 3T3-PKD2.GFP cells and control Swiss 3T3-GFP cells were challenged with 10 nM bombesin at 37°C for various times and lysed. The active forms of ERK-1 and ERK-2 in the extracts were detected by Western blotting using an antibody that recognizes the phosphorylated forms of these enzymes. As shown in Figure 4A, ERK phosphorylation (activation) in both Swiss 3T3-PKD2.GFP cells and Swiss 3T3-GFP cells increased dramatically within 5 min of bombesin stimulation. In Swiss 3T3-GFP cells, ERK phosphorylation returned to baseline levels by 120 min. In striking contrast, bombesin-induced ERK phosphorylation in Swiss 3T3 cells overexpressing PKD2 is prolonged as compared with either Swiss 3T3-GFP cells (Fig. 4A) or with Swiss 3T3-PKD2.GFP stimulated with EGF (Fig. 4B).
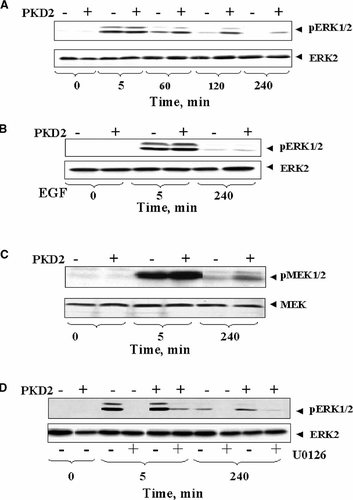
PKD2 overexpression selectively prolongs ERK1/2 and MEK activation induced by bombesin in Swiss 3T3 cells. A: Kinetics of ERK1/2 activation induced by bombesin. Confluent and quiescent cultures of Swiss 3T3-PKD2.GFP cells (+) and Swiss 3T3-GFP cells (−) were washed and incubated at 37°C in 2 ml DMEM containing 10 nM bombesin for various times as indicated and then lysed with 2× SDS–PAGE sample buffer. B: ERK1/2 activation in response to EGF. Confluent and quiescent cultures of Swiss 3T3-PKD2.GFP cells (+) and Swiss 3T3-GFP cells (−) were washed and incubated at 37°C in 2 ml DMEM containing 5 ng/ml EGF (EGF) for 5 or 240 min, as indicated, and then lysed with 2× SDS–PAGE sample buffer. The samples were analyzed by SDS–PAGE and immunoblotting with phospho-ERK antibody (pERK1/2). The autoluminograms shown are representative of four independent experiments. The membrane was further analyzed by Western blotting using ERK2 Ab (ERK2) to verify equal loading. C: PKD2 overexpression prolongs MEK activation induced by bombesin. Confluent and quiescent cultures of Swiss 3T3-PKD2.GFP cells (+) and Swiss 3T3-GFP cells (−) were washed and incubated at 37°C in 2 ml DMEM containing 10 nM bombesin for various times as indicated and then lysed with 2× SDS–PAGE sample buffer. The samples were analyzed by SDS–PAGE and immunoblotting with an antibody that detects the phosphorylated state of MEK1/2 only when they are phosphorylated on Ser-217/221 (p-MEK). The membranes were then stripped and further analyzed by Western blotting using MEK antibody (MEK) to verify equal loading. Shown are representative autoluminograms; similar results were obtained in three independent experiments. D: Bombesin induced ERK1/2 activation is inhibited by the MEK inhibitor U1026. Confluent and quiescent cultures of Swiss 3T3-PKD2.GFP cells (+) and Swiss 3T3-GFP cells (−) were washed and incubated at 37°C in 2 ml DMEM either in absence (−) or presence (+) of 10 µM U0126 for 1 h. The cultures were then stimulated with 10 nM bombesin for either 5 or 240 min as indicated. The cultures were then lysed with 2× SDS–PAGE sample buffer and the samples analyzed by SDS–PAGE and immunoblotting with phospho-ERK1/2 antibody (p-ERK1/2). Shown are representative autoluminograms; similar results were obtained in two independent experiments.
We next determined whether PKD2 overexpression also prolongs the activation of MEK induced by bombesin. Activation of MEK1 and MEK2 occurs through phosphorylation of Ser-217 and Ser-221, located in the kinase activation loop (Alessi et al., 1994). Consequently, MEK activation in response to 10 nM bombesin added for 5 or 240 min was evaluated by Western blotting using an antibody that detects the phosphorylated forms of MEK. As shown in Figure 4C, bombesin stimulation for 5 min induced a striking increase in MEK phosphorylation in both Swiss 3T3-PKD2.GFP and Swiss 3T3-GFP cells. After 240 min of incubation with bombesin, MEK from Swiss 3T3-PKD2-GFP cells was markedly phosphorylated as compared with that from Swiss 3T3-GFP cells, indicating that the duration of MEK activation in response to bombesin is also increased in cells overexpressing PKD2.
To substantiate that PKD2 overexpression increases the duration of ERK activation via MEK, cultures of Swiss 3T3-GFP or Swiss 3T3-PKD2.GFP cells were treated with the selective inhibitor of MEK activation U0126 (Favata et al., 1998). The results shown in Figure 4D demonstrate that exposure of these cells to U0126 prevented ERK-1 and ERK-2 activation in response to bombesin stimulation for either 5 or 240 min. Collectively, the results shown in Figure 4 indicate that PKD2 prolongs ERK signaling by increasing the duration of MEK activation.
PKD2 overexpression increases RSK signaling and enhances c-Fos accumulation in response to bombesin
The 90 kDa Ribosomal S6 Kinases (RSK1-3) are serine/threonine protein kinases that are activated via ERK-mediated phosphorylation (Zhao et al., 1996; Frodin and Gammeltoft, 1999). If PKD2 overexpression increases the duration of ERK catalytic activity within cells in response to GPCR agonists, we would expect a prolongation of the activation/phosphorylation of RSK, a well-defined downstream target of ERK. To test this possibility, we determined the effect of bombesin on RSK activation in Swiss 3T3-PKD2.GFP cells and Swiss 3T3-GFP cells, as revealed by immunoblotting with an antibody that detects phosphorylated Ser-574, a site important for RSK activation (Dalby et al., 1998).
As shown in Figure 5 (upper part), bombesin stimulation for 5 min with bombesin induced a striking increase in RSK phosphorylation in both Swiss 3T3-PKD2.GFP cells and Swiss 3T3-GFP cells, in agreement with the ability of this agonist to induce rapid ERK activation in these cells. After 240 min of incubation with bombesin (but not with EGF), the phosphorylation of RSK from Swiss 3T3-PKD2.GFP cells was markedly increased as compared with that from Swiss 3T3-GFP cells, which is entirely consistent with the increased duration of ERK signaling induced by bombesin in PKD2 overexpressing cells.
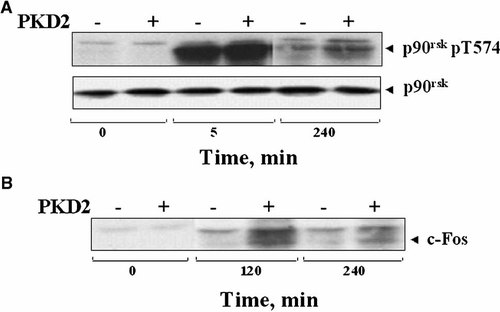
PKD2 overexpression increases the duration of RSK activation and accumulation of c-Fos protein in response to bombesin in Swiss 3T3 cells. A: PKD2 overexpression increases the duration of RSK signaling induced by bombesin. Confluent and quiescent cultures of Swiss 3T3-PKD2.GFP cells (+) and Swiss 3T3-GFP cells (−) were washed and incubated at 37°C in 2 ml DMEM with 10 nM bombesin (Bom), for either 5 or 240 min as indicated. The cultures were then lysed with 2× SDS–PAGE sample buffer and the samples analyzed by SDS–PAGE and immunoblotting with an antibody that detects the phosphorylated state of p90rsk at Thr-574 (p90RSKpT574). Shown here are representative autoluminograms, similar results were obtained in three independent experiments. B: PKD2 overexpression enhances the accumulation of c-Fos protein in Swiss 3T3 cells. Confluent and quiescent cultures of Swiss 3T3-PKD2.GFP cells (+) and Swiss 3T3-GFP cells (−) were washed and incubated at 37°C in 2 ml DMEM in the absence or presence of 10 nM bombesin for 120 or 240 min and then lysed with 2× SDS–PAGE sample buffer. The samples were analyzed by SDS–PAGE and immunoblotting with an antibody that detects c-Fos, the product of the c-fos proto-oncogene (c-Fos). Shown here are representative autoluminograms, similar results were obtained in three independent experiments.
Immediate early gene products, including members of the c-fos proto-oncogene family, function as molecular sensors of ERK1/2 signal duration (Murphy et al., 2002). When ERK activation is transient, its activity declines before the c-Fos protein accumulates, and c-Fos is degraded rapidly. However, when ERK signaling is sustained, c-Fos is phosphorylated by ERK and RSK at serines 362 and 374 (Chen et al., 1993, 1996). These carboxy-terminal phosphorylations stabilize c-Fos (Chen et al., 1993; Okazaki and Sagata, 1995) and promote its cellular accumulation. We hypothesized that sustained bombesin-induced ERK1/2 and RSK signaling in PKD2 overexpressing Swiss 3T3 cells leads to c-Fos protein accumulation in these cells.
In order to test this hypothesis, cultures of Swiss 3T3.PKD2-GFP and Swiss 3T3.GFP cells were treated with bombesin for 120 and 240 min, lysed and the levels of c-Fos protein were determined in cell extracts by Western blot analysis. As shown in Figure 5 (lower part), bombesin stimulation of Swiss 3T3.PKD2-GFP caused a striking accumulation of c-Fos protein at either 120 or 240 min, as compared with that induced by bombesin in Swiss 3T3.GFP cells.
Requirement of ERK activation for the potentiation of mitogenic activity of bombesin in PKD2 overexpressing cells
To determine whether the potentiation of the mitogenic activity of bombesin in PKD2 overexpressing cells is mediated by an increase in the duration of MEK/ERK/RSK signaling, cultures of Swiss 3T3-PKD2.GFP and Swiss 3T3-GFP cells were treated with the selective MEK inhibitor U0126. As illustrated in Figure 6, and in agreement with results shown in Figure 3, stimulation with 10 nM bombesin of Swiss 3T3 cells overexpressing PKD2 induced a striking increase in the level of [3H]thymidine incorporation into DNA, as compared with that produced by bombesin in cultures of Swiss 3T3-GFP cells. Addition of U0126 together with bombesin completely blocked the enhancement of DNA synthesis seen in Swiss 3T3-PKD2.GFP cells stimulated with this agonist.
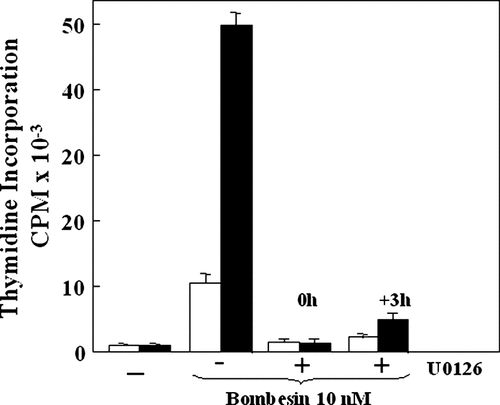
The potentiation of DNA synthesis induced by bombesin in PKD2 overexpressing cells requires increased duration of ERK activation. Confluent and quiescent cultures of Swiss 3T3-PKD2.GFP cells (solid bars) and Swiss 3T3-GFP cells (open bars) were washed and incubated at 37°C in 2 ml of DMEM/Waymouth's medium containing [3H]thymidine and 10 nM bombesin. To some of these cultures, 5 µM U0126 was added at the same time as the addition of bombesin (0 h) and to other cultures 5 µM U0126 was added 3 h after the addition of bombesin (+3 h). After 40 h of incubation, DNA synthesis was assessed by measuring the [3H]thymidine incorporated into acid-precipitable material; Results are expressed in cpm mean ± SE (n = 3).
To demonstrate that the sustained phase of ERK activation was required to mediate the enhancement of agonist-induced DNA synthesis in PKD2-overexpressing cells, ERK activity was inhibited by adding 5 µM U0126 to cultures that have been stimulated with bombesin for 3 h. Addition of U0126 3 h after bombesin stimulation also prevented the increase in DNA synthesis produced in cells overexpressing PKD2 (Fig. 6). These results imply that the sustained phase of ERK signaling is critical for the enhancement of agonist-induced DNA synthesis in PKD2-overexpressing cells.
DISCUSSION
PKD is the founding member of a new family of serine/threonine protein kinases, including PKD, PKD2 and PKD3 [Reviewed in Rozengurt et al. (2005)]. These kinases share similarities in overall structure, primary sequence, and enzymological properties (Hayashi et al., 1999; Sturany et al., 2001; Rey et al., 2003b) but interesting differences in their expression in different cell types and in their sub-cellular distribution are also emerging. Recently, we reported that PKD overexpression enhances the proliferative response to GPCR agonists in Swiss 3T3 cells (Zhukova et al., 2001; Sinnett-Smith et al., 2004). These results indicated that PKD mediates cellular DNA synthesis in response to GPCR agonists but the role of the other isoforms of the PKD family in mitogenic signaling remained largely undefined.
As a first step to examine the contribution of PKD2 to GPCR-induced mitogenesis, we generated Swiss 3T3 cells that overexpress this PKD isoform and determined whether PKD2 enhances mitogenic signaling in response to bombesin/GRP receptor activation. PKD2 overexpressed in Swiss 3T3 cells was activated rapidly by treatment of intact cells with bombesin, as judged by increased phosphorylation at multiple sites, including Ser-706 and Ser-710 in the kinase activation loop and Ser-876, an autophosphorylation site located in the COOH-terminal region of the molecule. PKD2 activation was prevented by exposure of the cells to PKC-specific inhibitors. These findings demonstrate that PKD2 overexpressed in Swiss 3T3 cells retains signal-dependent regulation of kinase catalytic activity.
One of the salient features of the results presented here is that PKD2 overexpression selectively potentiated the stimulation of DNA synthesis induced by bombesin in Swiss 3T3 cells. In contrast, stimulation of DNA synthesis in response to EGF, which does not elicit any detectable PKD2 activation, was not enhanced. These results indicate that overexpression of PKD2 selectively facilitates PKC-dependent mitogenesis in Swiss 3T3 cells and thus, suggest that PKD2, like PKD, plays a role in mediating agonist-induced cell proliferation.
A substantial body of evidence indicates that Gq-coupled receptor activation leads to the stimulation of the ERKs which are implicated in the regulation of such fundamental cellular processes as proliferation, differentiation, survival and apoptosis (Treisman, 1996; Khokhlatchev et al., 1998). It is increasingly recognized that the duration of ERK pathway activation is critical for determining specific biological outcomes (Marshall, 1995; Murphy et al., 2002; Pouyssegur and Lenormand, 2003; Whitehurst et al., 2004). For example, sustained activation of ERK is associated with re-initiation of DNA replication in fibroblasts whereas transient ERK activation is not sufficient to promote mitogenesis in these cells. As a first step to elucidate the mechanism(s) by which PKD2 potentiates GPCR-induced mitogenesis and to identify signaling pathways that regulate the duration of ERK activation, we examined whether PKD2 overexpression influences the magnitude and/or duration of GPCR-promoted ERK pathway activation.
Our results show that MEK/ERK/RSK pathway activation induced by bombesin is prolonged markedly in Swiss 3T3 cells overexpressing PKD2. In contrast, we demonstrate that the duration of ERK activation induced by EGF in Swiss 3T3-PKD2.GFP cells was identical to that generated in Swiss 3T3-GFP cells in response to this growth factor. This result is consistent with the fact that EGF does not induce any significant increase in the activity of PKD2 in Swiss 3T3 cells but stimulates ERK1/2 activation trough a Ras-dependent, PKC-independent pathway in these cells (Santiskulvong et al., 2001). These results indicate that PKD2 selectively prolongs MEK/ERK/RSK signaling in response to bombesin receptor stimulation in Swiss 3T3 cells.
The immediate early gene products (e.g., c-fos) have been proposed to function as molecular sensors of ERK1/2 signal duration (Murphy et al., 2002). When ERK activation is transient, its activity declines before the c-Fos protein accumulates, and unphosphorylated c-Fos is degraded rapidly. However, when ERK signaling is sustained, ERK and RSK phosphorylate c-Fos protein at serines 362 and 374 (Chen et al., 1993, 1996) leading to its stabilization (Chen et al., 1993; Okazaki and Sagata, 1995; Murphy et al., 2002). Interestingly, the transcription factors of the c-Fos family have been proposed to function as a link between early signaling events and subsequent cell cycle progression (Brown et al., 1998).
Consequently, we determined whether sustained bombesin-induced ERK1/2 and RSK signaling in PKD2 overexpressing Swiss 3T3 cells increases c-Fos protein accumulation in these cells. In line with this possibility, we found a dramatic increase in the level of c-Fos protein in response to bombesin in Swiss 3T3-PKD2.GFP but not in control Swiss 3T3 cells. These results indicate that sustained bombesin-induced ERK1/2 and RSK signaling in PKD2 overexpressing Swiss 3T3 cells leads to c-Fos protein accumulation in these cells. Given that our results demonstrated that PKD2 overexpression potentiates the mitogenic activity of bombesin, we hypothesize that the increase in the duration of MEK/ERK/RSK signaling leading to the accumulation of immediate gene products plays a critical role in the mechanism by which PKDs enhance the mitogenic effects of these agonists.
In conclusion, our results demonstrate that PKD2, like PKD, facilitates DNA synthesis, increases the duration of the MEK/ERK/RSK pathway and promotes the accumulation of c-Fos protein in response to GPCR agonists. Thus, functional overlap in mitogenic signaling appears to occur within the PKD gene family. Our study supports the hypothesis that an increase in the duration of the ERK signaling leading to accumulation of immediate gene products is, at least, one of the mechanisms by which isoforms of the PKD family selectively enhance re-initiation of DNA synthesis by Gq-coupled receptor activation.
Acknowledgements
This work was supported by National Health Institute Grants DK 55003, DK56930 and DK41301. OR was supported by a NCI Mentored Career Development Award KO1CA097956.




