Evaluation of the viability of cultured corneal endothelial cells by quantitative electron probe X-ray microanalysis
Abstract
Construction of artificial organs and tissues by tissue engineering is strongly dependent on the availability of viable cells. For that reason, the viability and the physiological status of cells kept in culture must be evaluated before the cells can be used for clinical purposes. In this work, we determined the viability of isolated rabbit corneal endothelial cells by trypan blue staining and quantitative electron probe X-ray microanalysis. Our results showed that the ionic content of potassium in cultured corneal endothelial cells tended to rise initially, but significantly decreased in cells in the fifth (and final) subculture, especially in comparison to cells in the fourth subculture (P < 0.001). However, the concentration of sulfur was higher in the fifth subculture than in the fourth subculture (P < 0.001), with a nonsignificant increase in sodium in the fifth subculture (P = 0.031). These data imply a remarkable decrease in the K/Na ratio from the fourth to the fifth subculture. Our microanalytical results, along with the morphological differences between cells in the last two subcultures, are compatible with an early phase of the preapoptotic process in the fifth subculture, and suggest that cells of the first four subcultures would be better candidates for tissue engineering. J. Cell. Physiol. 211: 692–698, 2007. © 2007 Wiley-Liss, Inc.
As a transparent structure, the cornea is a specialized organ that plays an important role in the transmission of light to the retina. The histological structure of the cornea allows it to serve as a barrier to the outside environment and as a major element in the optical pathway of the eye (Griffith et al., 1999). This organ comprises three major cell layers: the outermost stratified epithelium, the stroma containing keratocytes, and the innermost corneal endothelium. The corneal endothelium is a single layer of specialized epithelium with an important physiological function in the regulation of sodium and potassium concentration in the anterior pole of the eye. This mechanism of regulation is able to control the level of hydration of the cornea (Bonanno, 2003).
The construction of artificial organs by tissue engineering is one of the fields of medical research that has experienced major progress in recent years (Atala, 2000). Different groups have tried to develop an efficient substitute for the cornea with tissue engineering techniques, although the ex vivo culture of human corneal endothelial cells is still a challenge for most of the researchers (Tegtmeyer et al., 2001; Joyce, 2003). In this context, our research group has recently developed an efficient physiological model of the rabbit cornea by tissue engineering (Alaminos et al., 2006). In this regard, it is important to evaluate the viability of cultured cells before they can be used to produce artificial organs and tissues by tissue engineering, since only viable cells are suitable for clinical use. This is important not only in human tissue engineering, but also in the construction of animal models (Tegtmeyer et al., 2001).
Evaluating the viability of the cultured cells, however, is not easy, and different approaches have been proposed. A number of methods focus on the detection of permeability alterations in the cell membrane by trypan blue or propidium iodide staining, or by quantifying lactic dehydrogenase (LDH) in the culture medium (Bouillaguet et al., 2000, Chen and Wagner, 2001). However, most of these techniques are not accurate enough to detect early cell damage, but only identify cell alterations once they have become irreversible. In most cases a positive result with these techniques reveals that the integrity of the cell membrane has been lost. For these reasons, such methods cannot detect cells that are prone to death but do not yet manifest cell membrane alterations.
On the other hand, one of the most sensitive techniques for determining the viability of cultured cells is quantification of the ionic content, especially potassium and sodium (Zierold, 1997; Fernandez-Segura et al., 1999; Roomans, 1999; Roomans, 2002). Indeed, the intracellular concentration of these ions correlates well with the vital status of cells and is an excellent marker of cell physiology and cell viability. Electron probe X-ray microanalysis associated with electron microscopy is the most powerful approach to measure total elemental composition, making it possible to simultaneously determine the concentrations of different elements and the ultrastructure of cells (Buja et al., 1985; Hall, 1988; Somlyo et al., 1989; Krep et al., 1996; Warley, 1997; Vanthanouvong et al., 2003). By using this combined biochemical and morphological technique, our group has previously quantified the ionic content of different cell lines including U937 (Fernandez-Segura et al., 1999; Arrebola et al., 2005), MCF7 (Fernandez-Segura et al., 1997) and K562 cells (Warley et al., 1994), and others have measured the mineral concentrations of different layers of the healthy cornea in situ (Schrage et al., 1993). However, the microanalytical profile of isolated corneal endothelial cells kept in culture has not been described to date. Some authors have used histological and morphological methods, and suggested that cultured corneal cells in the fourth subculture may be the most appropriate for tissue engineering (Zhu and Joyce, 2004). However, the microanalytical composition of cells from different subcultures has not been established to date.
In this work we investigated the local content of several key elements (sodium, magnesium, phosphorus, chlorine, potassium, sulfur, and calcium) in isolated corneal endothelial cells kept in culture. Cell viability in each subculture was assessed with quantitative electron probe X-ray microanalysis, a method that holds considerable potential for the evaluation of cultured cells to determine the most suitable subculture for harvesting corneal cells to be used in tissue engineering.
MATERIALS AND METHODS
Rabbit corneas
Ten rabbit corneas were obtained from five adult New Zealand white rabbits weighting approximately 2 kg. All procedures were carried out in animals killed by lethal intracardiac injection of potassium chloride under general anesthesia. Immediately thereafter, whole corneas were extracted under a surgical microscope and preserved at 4°C in RPMI culture medium supplemented with penicillin, streptomycin, and antimycotics (Sigma-Aldrich, St. Louis, MO ref. #A5955). All corneas were processed within 6 h after extraction.
This research was approved by the institutional experimentation committee, and all animals were treated according to national and international guidelines on animal welfare.
Isolation and culture of corneal endothelial cells
To obtain endothelial cells, the inner side of each cornea was treated with trypsin 0.5 g/L-EDTA 0.2 g/L solution (Gibco BRL Life Technologies, Karlsruhe, Germany) and corneas were incubated for 10 min at 37°C. Then the endothelial cells attached to Descemet's layer of the corneas were mechanically dissected under surgical microscope. All cells were cultured in 25-cm2 tissue culture flasks with Dulbecco's modified Eagle's medium (DMEM) (Sigma-Aldrich) supplemented with 10% fetal calf serum (Sigma-Aldrich), 4 mM L-glutamine, 1% antibiotic solution (Gibco BRL) and different growth factors: insulin (5 mg/ml), triiodothyronine (1.3 ng/ml), cholera toxin (8 ng/ml), and hydrocortisone (0.4 mg/ml) (Alaminos et al., 2006). The medium was changed once every 3 days. Subculture of the corneal endothelial cells was carried out with trypsin 0.5 g/L-EDTA 0.2 g/L solution on subconfluent cell cultures. The cells were kept in culture up to the fifth subculture.
The percentages of dead and live cells were found by staining the trypsinized cells with trypan blue and counting the number of blue cells under light microscope. All counts were done in triplicate, and the mean and standard deviation were calculated for each subculture.
To establish the microanalytical pattern of the cells, we decided to use five passages since it was previously suggested that corneal cells kept in culture could begin to display morphological changes compatible with early senescence starting from passages four or five (Zhu and Joyce, 2004).
Identification of endothelial cells by RT-PCR
To confirm that the cultured cells were of endothelial origin, we examined the culture daily under a phase contrast microscope, and determined the expression level of the genes KRT12 (keratin 12) and COL8 (collagen VIII) by RT-PCR according to previously published protocols (Alaminos et al., 2006). Briefly, total RNA from cultured cells was extracted and purified with the QIAgen, Missisauga, Ontario, Canada RNeasy Mini Kit (ref. #74106). The quality of the RNA was assessed by optical inspection in agarose gels under denaturing conditions. Two micrograms of DNA were reverse transcribed using SuperScript II reverse transcriptase (Life Technologies, Gaithersburg, MD) and amplified using specific primers for KRT12 5′-GAACTGGGACTGCAGATGCTT-3′ (forward) and 5′-TTCAGGCTCTCGATCTGCATC-3′ (reverse) and for COL8 5′-CATGCAGAAAGGACCTGTGG-3′ (forward) and 5′-TCCTGGCTTTCCCATGCCT-3′ (reverse). PCR was carried out for 35 cycles (95°C for 30 sec, 52°C for 30 sec, and 72°C for 30 sec) in a volume of 25 µl with a final concentration of 1.5 mmol/L MgCl2, 0.3 mmol/L deoxynucleotide triphosphate, 0.25 mmol/L of each primer and two units of Taq polymerase (Life Technologies) in 1× reaction buffer. Specific primers for the GAPDH gene (glyceraldehyde-3-phosphate dehydrogenase) were used under the same conditions as in control assays to ensure cDNA quality and loading accuracy. PCR-amplified products were resolved by 2% agarose gel electrophoresis and visualized by ethidium bromide staining.
Electron probe X-ray microanalysis
For X-ray microanalysis, subconfluent endothelial cells were subcultured using trypsin-EDTA on plated gold grids covered with a thin layer of Pioloform (polyvinyl butyral) (Ted Pella, Inc., Redding, CA) and sterilized overnight under UV light. Cells were seeded at a density of 5,000 cells per grid and cultured in DMEM supplemented with 10% serum, antibiotics and growth factors. After 24 h of culture on the gold grids covered with Pioloform, support grids containing the endothelial cells were washed in ice-cold distilled water for 5 sec to remove the extracellular medium. After washing, excess water was drained from the surface and the grids were immediately plunge-frozen in liquid nitrogen (Abraham et al., 1985; Warley, 1994). After cryofixation, the grids were placed in a precooled aluminum specimen holder at liquid nitrogen temperature and freeze-dried at increasing temperatures for 24 h in an E5300 Polaron freezer-drier apparatus equipped with a vacuum rotatory pump system. Freeze-dried gold grids were carbon-coated in a high-vacuum coating system and microanalyzed within 6 h.
Electron probe X-ray microanalysis of the specimens was performed with a Philips XL30 scanning electron microscope (SEM) equipped with an EDAX DX-4 microanalytical system and a solid-state backscattered electron detector. The samples were examined with SEM with a combination of secondary electron (SE) and backscattered electron (BSE) imaging modes.
For X-ray microanalysis, the analytical conditions were: tilt angle 0°, take-off angle 61.34° and working distance 10 mm. The acceleration voltage was 10 kV. All spectra were collected in the spot mode at 10,000× (equivalent to 50 nm spot diameter) for 200 sec live time, and the number of counts per second recorded by the detector was around 500. All determinations were performed on the central area of the cell nucleus. To determine total ion content, we used the peak-to-local-background (P/B) ratio method (Statham and Pawley, 1978; Boekestein et al., 1984; Fernandez-Segura et al., 1997) with reference to standards composed of 20% dextran containing known amounts of inorganic salts (Warley, 1990).
In all, we quantified the ionic content of 60 cultured corneal endothelial cells in each subculture corresponding to three different grids and three different culture flasks from the same subculture.
Morphological study of isolated corneal endothelial cells
Carbon-coated specimens were analyzed with the same Philips XL30 scanning electron microscopy that was used for microanalysis. This methodology allows synchronic morphological observation of the cells at the same time as microanalysis. Then the same cells were coated with gold and inspected with the SEM.
Statistical analysis
To evaluate the statistical significance of the differences between mean values for two consecutive subcultures, we used the nonparametric Mann–Whitney test. Comparisons of several subcultures at a time were carried out with the Kruskal–Wallis test for multiples samples. To compare percentages of dead cells between different subcultures, we used Fisher's exact test. For individual tests, a two-sided P-value less than 0.05 was considered statistically significant. For multiple comparisons, a Bonferroni-adjusted significance level of 0.001 was considered significant because up to 50 statistical tests were used at the same time.
RESULTS
Culture of rabbit corneal endothelial cells
Isolated endothelial cells proliferated rapidly in culture starting from day 5 (mean 5.1 ± 2.3 days), and reached subconfluency around day 9 (8.9 ± 3.3 days). In culture, endothelial cells displayed a variable polygonal shape (Fig. 1) rather than their typical hexagonal shape in the original cornea. In trypsinized cells, trypan blue staining demonstrated that most of the cells in the first subcultures were alive (91 ± 1.5% for the first subculture, 94 ± 2% for the second, 94 ± 0.5% for the third, and 95 ± 1% for the fourth subculture), and that the percentage of live cells was slightly lower in the fifth subculture (92 ± 1.5%). Differences between the different subcultures were not statistically significant (P > 0.05, Fisher's exact test for all comparisons between different subcultures). No morphological differences were found under light microscope between cells belonging to different passages.
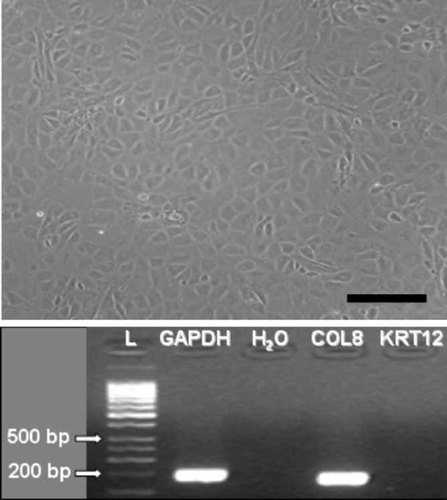
Corneal endothelial cells used in this work. Top panel: Phase contrast micrograph of confluent isolated corneal endothelial cells corresponding to the fourth subculture. Scale bar: 200 µm. Lower panel: Collagen 8 (COL8) and keratin 12 (KRT12) RNA expression in the same cells by reverse transcription PCR. Expression of the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene was used as a control for RNA loading. L: 1-kb ladder used as a molecular weight control for the amplified products.
Cultured cells overexpressed specific genes
As expected, RT-PCR analysis demonstrated that in cultured corneal endothelial cells, there was relative overexpression of the COL8 gene, which encoded for collagen VIII. This gene is selectively expressed by vascular endothelia and corneal endothelial cells, but not by stromal or epithelial cells (Muragaki et al., 1991). However, the KRT12 gene (keratin 12), whose expression is specific for epithelial cells of the cornea (Liu et al., 1999, Wang et al., 2002), was not expressed in cultured corneal endothelial cells (Fig. 1). The housekeeping control gene GAPDH was highly expressed in all cultured corneal cells.
Ionic content of cultured endothelial cells
Analyses of 60 endothelial cells from each subculture showed that cultured corneal endothelial cells were characterized in general by a high K/Na ratios, ranging from 6.94 for the fifth subculture to 10.69 for the third subculture (Table 1). Illustrative examples of spectra corresponding to cells from different subcultures are shown in Figure 2.
| Subculture | Calcium | Chlorine | Magnesium | Phosphorus | Potassium | Sodium | Sulfur |
|---|---|---|---|---|---|---|---|
| 1 | 12.24 ± 1.07 | 85.00 ± 3.52 | 14.89 ± 0.47 | 221.31 ± 5.39 | 288.15 ± 9.24 | 28.97 ± 1.52 | 42.95 ± 1.59 |
| 2 | 11.25 ± 1.19 | 68.29 ± 3.59 | 18.80 ± 0.70 | 305.44 ± 5.58 | 318.92 ± 11.02 | 35.25 ± 1.74 | 55.06 ± 2.32 |
| 3 | 12.34 ± 0.89 | 135.39 ± 4.53 | 21.88 ± 0.68 | 288.70 ± 4.09 | 422.04 ± 11.63 | 39.48 ± 1.68 | 56.61 ± 4.21 |
| 4 | 12.09 ± 1.29 | 104.15 ± 4.30 | 20.64 ± 0.67 | 290.84 ± 3.96 | 403.86 ± 10.87 | 42.37 ± 2.52 | 41.11 ± 1.78 |
| 5 | 13.31 ± 0.90 | 87.41 ± 5.00 | 19.62 ± 0.87 | 302.80 ± 5.48 | 336.41 ± 10.87 | 48.46 ± 2.86 | 58.78 ± 2.17 |
- All concentrations (in mmol/kg dry weight) are expressed as mean ± standard error (SE).
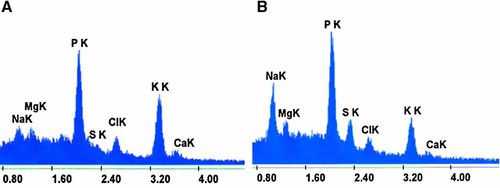
Two examples of microanalytical spectra corresponding to a living cell in the third subculture (A) and to a dying cell in the fifth subculture (B). The peaks correspond to the energy dispersed by electrons located in the k orbitals of sodium (NaK), magnesium (MgK), phosphorus (PK), sulfur (SK), chlorine (ClK), potassium (KK), and calcium (CaK). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
When the microanalytical results were compared among the five subcultures with the Kruskal–Wallis test for multiple samples, significant differences were found for Na, K, Mg, P, Cl, and S (P < 0.001 for each element) but not for Ca (P > 0.01) (Table 2). These data suggest that the concentrations of these elements did not remain constant across subcultures, but varied depending on the passage. One-to-one statistical comparisons between consecutive passages demonstrated a tendency for several elements to increase or decrease in intracellular concentration in later subcultures compared to the earlier passages. For example, the concentration of potassium tended to increase in the first three subcultures, reaching maximum values in the third subculture and decreasing thereafter. As shown in Table 2, the increase between the second and the third subcultures as well as the decrease between the last two subcultures were statistically significant (P < 0.001, Mann–Whitney test). In contrast, the concentration of sodium tended to increase from the first to the last subculture, although the differences were only marginally significant when we compared the first versus the second subculture (P = 0.014), the second versus the third subculture (P = 0.021), and the fourth versus the last subculture (P = 0.031). The data for chlorine showed that the cell concentration of this element tended to decrease from the first to the second passage, significantly increasing in the third subculture and decreasing thereafter. On the other hand, the intracellular levels of sulfur tended to increase with successive passages, but decreased in the fourth subculture, with significant differences between the first two subcultures and between the last two cell passages. Phosphorous and magnesium displayed less dramatic changes across the different subcultures, with significant increases only between the first and the second subcultures. In general, the percentage of cells with a high potassium and chlorine content was higher in the fourth subculture than in the fifth subculture, whereas the percentage of cells with a high sodium and sulfur content was lower (Fig. 3).
| Comparison | Calcium | Chlorine | Magnesium | Phosphorous | Potassium | Sodium | Sulfur |
|---|---|---|---|---|---|---|---|
| 1 vs. 2 | N.S. | P = 0.003 | P < 0.001 | P < 0.001 | N.S. | P = 0.014 | P < 0.001 |
| 2 vs. 3 | N.S. | P < 0.001 | P = 0.004 | P = 0.029 | P < 0.001 | P = 0.021 | N.S. |
| 3 vs. 4 | N.S. | P < 0.001 | N.S. | N.S. | N.S. | N.S. | P = 0.002 |
| 4 vs. 5 | N.S. | P = 0.022 | N.S. | N.S. | P < 0.001 | P = 0.031 | P < 0.001 |
| All five subcultures | N.S. | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 |
- The Mann–Whitney test was used to compare ionic concentrations between the first versus second subculture, second versus third, third versus fourth or fourth versus fifth subculture, and the Kruskal–Wallis test was used to detect global differences among the five subcultures. P values less than 0.001 were considered significant. N.S.: P values >0.05.
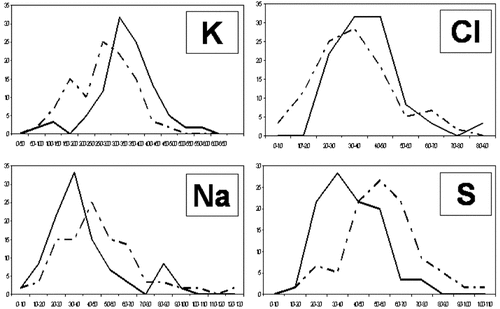
Percentage of cells displaying different intracellular concentrations of sodium, potassium, chlorine, and sulfur in the fourth and fifth subcultures. The dotted lines represent ion concentrations in cells in the fifth subculture; continuous lines correspond to cells in the fourth subculture. For each element the X-axis represents intracellular ion concentration, and the Y-axis represents the percentage of cells with each concentration.
Morphological analysis of isolated corneal endothelial cells
In general, corneal endothelial cells kept in culture tended to display a polygonal shape rather than the typical hexagonal outline of cells in native corneas. As shown in Figure 4, corneal endothelial cells in the first subcultures tended to present morphological similarities to native corneal endothelial cells in vivo, with a polygonal, slightly elongated and generally uniform shape. In contrast, cells in the fifth passage tended to show a more rounded and semispherical silhouette. The cell surface became more heterogeneous in the fifth subculture than in the fourth passage.
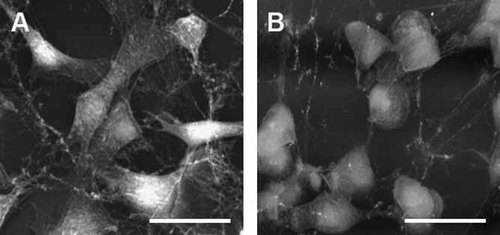
Representative examples of cells in the second passage (A) and the fifth passage (B). Cells were grown on support grids, freeze-dried and carbon-coated before scanning electron microscopy (secondary electron mode). Cells from the first four subcultures were more regular and elongated than cells in the fifth subculture, which were more rounded. Scale bar: 50 µm.
DISCUSSION
Quantitative energy dispersive X-ray microanalysis (EDXA) with electron microscopy is a well established procedure to evaluate the viability and the physiological status of cultured cells, especially when these cells are intended for clinical purposes or the construction of tissue equivalents by tissue engineering (Fernandez-Segura et al., 1999). In contrast with other methods based on the exclusion of dyes such as trypan blue or propidium iodide (Bouillaguet et al., 2000; Chen and Wagner, 2001), EDXA is a highly sensitive technique for determining cell viability. Previous studies by our group have demonstrated a strong correlation between the morphological changes that take place in cells during death and the intracellular levels of different ionic components in human U937 cells (Fernandez-Segura et al., 1999; Arrebola et al., 2005), K562 cells (Warley et al., 1994), and MCF7 cells (Fernandez-Segura et al., 1997).
It is well known that close control of the intracellular levels of different ions is critical for most cell physiological functions. For certain organs including the cornea, tissue levels of hydration must be strictly controlled and regulated (Kostyuk et al., 2002). In the cornea, maintaining transparency of the visual pathway is strongly dependent of the levels of hydration of the corneal stroma. Under normal conditions the corneal stroma shows a strong tendency to swell due to the presence of abundant nondiffusible, negatively charged molecules such as glycosaminoglycans (Hodson, 1997; Fischbarg and Maurice, 2004). However, the water balance is kept under control by an active process of ion transport (especially chlorine, sodium, and potassium) across the corneal endothelium (Maurice, 1984; Bonanno, 2003). Ion transport across the endothelium is thought to involve the active transport of anions from the corneal stroma towards the aqueous humor, followed by the mainly passive diffusion of cations (Bonanno, 2003; Davies et al., 2004). Thus the endothelium is able to counterbalance the continuous leak of fluid into the corneal stroma and avoid the stromal imbibition and edema that would impair vision (Kostyuk et al., 2002; Bonanno, 2003; Davies et al., 2004).
With a view to possible clinical applications, information about the intracellular concentration of anions and cations in corneal endothelial cells is important to determine the vital and functional status of cultured endothelial cells. We therefore suggest that the ionic content of sodium, potassium, chlorine, phosphorous, and other elements should be quantified in corneal endothelial cells intended for clinical use, tissue engineering or in vitro testing of toxics and drugs. This would guarantee the use of only viable and fully functional cells. However, reports of normal elemental concentrations in native corneas or cultured corneal cells are rare, and elements in the corneal endothelium have thus far only been identified in homogenates (Schrage et al., 1993) and ultrathin sections of the normal cornea (Langefeld et al., 1997). Consequently, little is known about the normal ionic content of cultured corneal endothelial cells.
It was previously shown that cell cultures tend to age and lose viability after several passages (Balconi and Dejana, 1986). Some researchers recently reported that corneal endothelial cell cultures displayed evident morphological changes starting from the fourth passage, with a trend for cells to enlarge with increasing passage number (Zhu and Joyce, 2004). According to these authors, morphological changes were associated with cell growth alterations and a high percentage of cell death (Zhu and Joyce, 2004). These findings were associated with increasing heterogeneity in cell shape and increasing numbers of multinucleated cells. Our results are in agreement with these reports, as cells in the fifth subculture contained a slightly higher number of dead cells than early subcultures (5% dead cells in the fourth subculture vs. 8% in the fifth), as determined by trypan blue staining. Although the differences were not dramatic when a classical staining method was used to determine cell mortality, our morphological and microanalytical data suggest that cells in the fifth subculture might have been in an early phase of cell death. In fact, we observed evident morphological alterations in cells in the fifth passage with scanning electron microscopy, whereas cells from earlier subcultures appeared to be morphologically normal.
Energy dispersive X-ray analysis allows both qualitative and quantitative determinations of the ionic elements that play a role in cell viability. This method has been extensively used to identify processes of cell death by necrosis or apoptosis in normal and pathological cells (Hongpaisan and Roomans, 1999; Roomans, 1999, 2002). In this connection, different authors have identified ionic patterns that are highly specific for normal cells, apoptotic cells or necrotic cells. In general, experiments with cells undergoing induced necrosis showed rapid alterations in the intracellular concentration of calcium and, at a latter stage, an increase in the amount of chlorine and a reduction in the intracellular ATP available for different cell functions (Akar et al., 2003; McLaughlin et al., 2004; Salido et al., 2004). However, cells undergoing death by apoptosis showed a different pattern of ionic alterations: an increased concentration of sodium, and the depletion of potassium and chlorine (Fernandez-Segura et al., 1999; Skepper et al., 1999; Salido et al., 2004). Interestingly, although the ionic changes did not necessarily correlate with structural or morphological changes that appeared in the cells, the authors detected a good correlation between ionic changes and structural alterations in a model of induced apoptosis. However, ionic changes which can act as early markers of cell death are detectable in cells before morphological changes appear. Earlier studies have shown that the intracellular concentrations of sodium and potassium are excellent markers of cell viability, and that the K/Na ratio is one of the most powerful parameters of cell damage from a microanalytical standpoint (Roomans, 2001).
In this connection, our results demonstrated that intracellular levels of potassium tended to rise from the first to the second and third subculture, and to decrease thereafter, whereas sodium showed a clear tendency to increase from the first to the last subculture. These data suggest that cells in the last subculture might be less viable than those in the initial passages. In addition, cell levels of both potassium and chlorine decreased in corneal endothelial cells in the fifth subculture, whereas sodium and sulfur tended to increase in these cells. It is important to note that all microanalytical changes were synchronically observed in situ in the same cells that were observed for morphological analysis, and the correlation between morphological changes and ion concentrations was substantial. The substantial decrease of the K/Na ratio that we found in our cells is compatible with the changes described in preapoptotic cells as well as in the necrotic cell death (Fernandez-Segura et al., 1999). In this regard, (Zierold, 1997) demonstrated that the microanalytical changes that happen in cells undergoing necrotic cell death, are generally accompanied by a huge increase in the intracellular concentration of chlorine. In contrast, our results showed that cells at the last subculture tended to show a decrease in the intracellular concentration of chlorine. Different research groups showed that the major early predictor for apoptosis is the early decrease in both potassium and chlorine, reflecting cell shrinkage, and that this decrease in chlorine may be more important than that of potassium and could even act as a signal (Arrebola et al., 2006). Taken together, our microanalytical findings suggest that a number of the corneal endothelial cells in the fifth subculture might have been undergoing an early process of cell death by apoptosis.
On the other hand, the levels of magnesium did not vary between the last two passages in our cultures. Our findings suggest that the amount of ATP might have remained constant in the cells throughout all five subcultures, consistent with earlier research by Buja et al. (1985) and Di Francesco et al. (1998). These authors reported that a decrease in cell magnesium content was usually associated with a decrease in ATP levels under experimental conditions. This result is compatible with changes found in apoptotic and preapoptotic cells. The levels of sulfur, however, increased between the fourth and the fifth subcultures. This suggests that the concentration of sulfated glycosaminoglycans might begin to change during the fifth subculture (Sanchez-Quevedo et al., 1989; Roomans, 2002), although it is also possible that the increase in sulfur at the later stages could reflect shrinkage of the corneal endothelial cells.
As suggested by earlier findings (Zhu and Joyce, 2004), our microanalytical and scanning electron microscopy results imply that although cell survival determined by trypan blue exclusion was high, corneal endothelial cells in the fifth subculture might have undergone a process of preapoptosis. These cells might not be good candidates for the construction of tissues and organs by tissue engineering. We propose that all cells to be used for clinical purposes be previously microanalyzed to determine their vital status and ensure a good rate of survival in vivo.
Acknowledgements
The authors thank MA. Robles for her skillful technical assistance and K. Shashok for checking the use of English in the manuscript.




