Dual transcriptional control of claudin-11 via an overlapping GATA/NF-Y motif: Positive regulation through the interaction of GATA, NF-YA, and CREB and negative regulation through the interaction of Smad, HDAC1, and mSin3A
Abstract
The expression of claudin-11, a key integral tight junction protein, is tightly regulated to ensure that the integrity of the seminiferous epithelium could be maintained during the translocation of spermatocytes at the blood–testis barrier at stages VIII–IX. In this study, we elucidate how the overlapping GATA/NF-Y motif within the core promoter of claudin-11 gene is modulated by differential binding of various transcription factors, resulting in dual transcriptional control. Using electrophoretic mobility shift assay (EMSA) and chromatin immunoprecipitation (ChIP) assay, we confirmed that GATA, nuclear factor YA (NF-YA), and cAMP response element-binding protein (CREB) form a complex in vivo and bind to the GATA/NF-Y region to promote claudin-11 gene transcription. Such gene activations were significantly reduced in the presence of siRNA specific to these transcription factors. GATA and CREB transactivation could be further modulated by the presence of Smad3 and Smad4 proteins. Binding of Smad proteins at the GATA/NF-Y motif could repress the GATA and CREB transactivation of claudin-11 gene. Such repression which required the recruitment and physical interactions of histone deacetylase 1 and its co-repressor, mSin3A with Smad proteins, was abolished by treatment with Trichostatin A, thus suggesting the involvement of histone deacetylation at the site of the promoter region. It is believed that cyclic changes in the ratio of positive regulators (GATA, NF-Y, and CREB) to negative regulators (Smads) in the seminiferous epithelium during the spermatogenic cycle might provide a precise control in claudin-11 gene transcription. J. Cell. Physiol. 211: 638–648, 2007. © 2007 Wiley-Liss, Inc.
Spermatogenesis is a process by which spermatogonia develop into mature spermatids in the seminiferous epithelium (for reviews, see de Kretser and Kerr, 1988; Cheng and Mruk, 2002; Lui et al., 2003). For this event to proceed, pre-leptotene and leptotene spermatocytes must traverse the blood–testis barrier (BTB) at late stages VIII and early IX of the epithelial cycle and enter the adluminal compartment of the seminiferous epithelium for further development (Russell, 1977). This passage is associated with extensive restructuring of the BTB which is constituted largely by inter-Sertoli tight junctions (TJ) (for reviews, see Cheng and Mruk, 2002; Lui et al., 2003).
Claudin-11 (also named as oligodendrocyte-specific protein, OSP) is a TJ integral protein found in testis and CNS myelin (Morita et al., 1999). Similar to occludin which is a known TJ protein found in most tissues (Lui and Lee, 2005), claudin-11 also consists of four transmembrane domains with both NH2 and COOH termini present in the cytoplasm. Claudin-11 is an important TJ building block that constitutes TJ strands between Sertoli cells in the seminiferous epithelium, as similar to occludin null mice, male claudin-11 null mice are sterile (Gow et al., 1999). The seminiferous tubules from knockout mice are abnormally narrow and spermatozoa are not found. The disruption of the BTB results in the interruption of the germ cell differentiation, which leads to germ cell death and sloughing (Gow et al., 1999). These studies have clearly demonstrated that claudin-11 at the BTB plays a crucial role in segregating spermatogonia and pre-leptotene spermatocytes at the basal compartment for development.
The precise control of junction dynamics in the testis could be achieved by transcriptional regulation of junction proteins (Lui and Lee, 2006a; Lui et al., 2006b). Detailed analysis of occludin promoter has shown that tumor necrosis factor α (TNF-α) modulates occludin gene expression through the suppression of the promoter activity by a number of cis-acting motifs, such as CCAAT enhancer-binding protein β (C/EBPβ or NF-IL6) and nuclear factor κB (NFκB), which were identified within the promoter sequence and pertinent to TNF-α-mediated gene transcription (Mankertz et al., 2000). However, the machinery for claudin-11 gene transcription remains entirely unknown. To address this issue, we have mapped the core promoter region and identified its trans-acting factors involved in claudin-11 gene transcription. Within the core promoter region, we have found that a GATA/NF-Y motif can either activate or suppress the promoter activity via the interaction with different transcription factors. Transcription factors including GATA, NF-YA, and CREB family interact with each other at the GATA/NF-Y motif, leading to the activation of gene transcription; while the binding of Smad3 and Smad4 onto this GATA/NF-Y motif recruits histone deacetylase (HDAC1) and co-repressor, mSin3A, resulting in gene suppression. The ratio of the positive/negative regulators at the GATA/NF-Y motif is a crucial factor to determine the fate of claudin-11 gene transcription in Sertoli cells.
MATERIALS AND METHODS
Cells and cell culture
Mouse TM4 cell was obtained from American Type Culture Collection (Manassas, VA). Cells were cultured in DMEM (Invitrogen, Carlsbad, CA) containing 10% FBS. Cultures were maintained at 37°C in humidified atmosphere with 5% CO2 in air and medium was replaced every 3 days. Cells were passaged using trypsin/EDTA (5% trypsin in 0.53 mM EDTA) or harvested for RNA and protein extraction.
Preparation of claudin-11 promoter-luciferase constructs and site-directed mutagenesis
The 5′-flanking region of the claudin-11 gene was generated by the mouse GenomeWalker Kit (Clontech, Palo Alto, CA) using primer #881 and adapter primer 1 for the first PCR, followed by a second PCR using nested primer #922 and adapter primer 2 (Table 1). The accession number for claudin-11 gene is NM_008770. Various 5′-deleted regions generated by PCR were cloned into the promoterless pGL-3 Basic vector (Promega Corp., Madison, WI). Mutant plasmids were generated by a three-step PCR mutagenesis (Lui et al., 2006b) using mutagenic primers (Table 1). All plasmids were prepared by Plasmid Midi Kit (Qiagen, Chatsworth, CA) and confirmed by sequencing analysis.
| Primer name | Location | Orientation | Sequence (5′ → 3′) | Purpose |
|---|---|---|---|---|
| 881 | 26/46 | AS | CGAAGATCTTGACGAAACCCA | Genome walking |
| 882 | −151/−136 | S | CGACGCGTGCTTGCAGCCGCT | Deletion |
| 883 | −367/−352 | S | CCACGCGTGGGGCAGCCTG | Deletion |
| 884 | −494/−475 | S | AGACGCGTTCTCTAGGAGTG | Deletion |
| 885 | −713/−698 | S | CGACGCGTGTGCCAGCGCAG | Deletion |
| 886 | −803/−788 | S | CGACGCGTATCAGTCTAACA | Deletion |
| 887 | −900/−886 | S | CGACGCGTCCTGCTCTAT | Deletion |
| 896 | −825/−812 | S | CGACGCGTCTAGCAAACTCA | Deletion |
| 922 | −15/+1 | AS | GAAGATCTGGTGGCCGCAGCGAC | Genome walking |
| 1020 | 581–600 | S | TGGTTCCAGCTCGCCAACGC | cDNA amplification |
| 1021 | 1062/1040 | AS | CTAAGCCGAAAGGGAGAAGAGAG | cDNA amplification |
| 1120 | −382/356 | S | GACCGTGTGTCACCAGCCGTGGGGCAG | ChIP assay |
| 1121 | −9/+23 | AS | ACCTGAAGGCAAGTGGCTACCATGGTGGCCG | ChIP assay |
| MP-B | S | GGAGTACTAACCCTGGCCTAGCAAAATAGGCTGTCCC | Mutagenic universal primer | |
| MP-C | AS | CTTTATGTTTTTGGCGTCTTCCA | Mutagenic universal primer | |
| MP-D | S | GGAGTACTAACCCTGGC | Mutagenic universal primer | |
| 944 GATA-b | −255/−271 | AS | GCAAGCGgaAtTCCGCGA | Site-directed mutagenesis |
| 928 GATA-c | −286/−302 | AS | AGCCGGCgAtTCGCAAG | Site-directed mutagenesis |
| 945 MZF1-d | −337/−354 | AS | CTGAGGAatTcCAGCAGG | Site-directed mutagenesis |
| 978 GS-GATA-b-S | −250/−277 | S | GGGCTGTCGGATTGGCGCTTGCCAGC | Gel shift |
| 980 GS-GATA-c-S | −282/−307 | S | CCTGGCTTGCGATTGGCCGGCTCGTC | Gel shift |
- S, sense; AS, anti-sense, italic bases indicate nucleotide mutation.
Transient transfection and reporter gene assay
Cells (1 × 105) were seeded onto a 6-well culture plate a day before transfection. Luciferase constructs (1 µg) were co-transfected with pCMV-β-gal (0.5 µg) using GeneJuice® Transfection Reagent (Novagen, Madison, WI) in serum-containing media. Cells were cultured for 48 h before harvest. Luminescence was measured by a Lumat LB 9507 luminometer (EG&G, Berthold, Germany). β-Galactosidase activity was measured by a β-galactosidase enzyme assay system (Promega Corp.) and used to normalize transfection efficiency. Promoter activity was calculated as luciferase activity/β-galactosidase activity.
Silencing of transcription factors by siRNA
The Smart-pool siRNA for silencing CREB (Cat. no. M-040959-00), GATA-1 (Cat no. L-045656-00), and NF-YA (Cat no. D-001810-01) were purchased from Dharmacon (Lafayette, CO). Transfection of the specific siRNA (50 nM) was performed using RiboJuice™ siRNA transfection (Novagen) per the manufacturer's instructions. As a non-specific siRNA control, scrambled siRNA (Dharmacon) was used. To determine the efficiency of siRNA transfection, immunoblot analyses were performed to detect expression level of transcription factors at 48 h post-transfection. To determine the effect of silencing specific transcription factors on claudin-11 gene transcription, co-transfection of specific siRNA and p(−367/−1)Luc construct was performed using RiboJuice and GeneJuice Transfection Reagents as per the manufacturer's instructions.
Electrophoretic mobility shift assay (EMSA)
Oligonucleotides containing the putative GATA-b/NF-Y and GATA-c/NF-Y motifs were annealed to form double-stranded DNA (Table 1). Probes were end-labeled with [γ-32P]-ATP and separated from unincorporated nucleotides via the Microspin G-25 columns (Amersham Biosciences, Piscataway, NJ). Nuclear extracts were prepared as previously described (Lui et al., 2006b). Briefly, cells were washed with phosphate-buffer saline and lysed with nuclear lysis buffer [20 mM HEPES (pH 7.6), 20% Glycerol, 10 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.1% Trition X-100]. Cells were pelleted by centrifugation, resuspended in nuclear extract buffer [20 mM HEPES (pH 7.6), 20% Glycerol, 500 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.1% Trition X-100] and incubated at 4°C at a rocking platform for 1 h. Lysates were subjected to centrifugation and the supernatant was used as the nuclear extract. EMSA was performed in a 20 µl reaction mixture containing 20 mM HEPES (pH 7.5), 50 mM NaCl, 1.5 mM MgCl2, 1 mM DTT, 1 mM EDTA, 10% glycerol, 1 µg poly (dI:dC), 50 fmol radiolabeled probe (50,000 cpm), and nuclear extract. For competitive assay, competitor oligonucleotides were added simultaneously with the radiolabeled probes. For supershift assay, nuclear extracts were pre-incubated with antibodies or rabbit serum for 30 min. Rabbit polyclonal antibodies against transcription factors (GATA-1, -4, -6, NF-YA, CREB, E2F1, and p53) were from Santa Cruz Biotechnology (Santa Cruz, CA) and anti-Smad3 antibody was from Zymed (South San Francisco, CA). The binding reaction was carried out at room temperature for 15 min and the reaction products were separated on a 6% polyacrylamide gel. The gels were dried and then exposed to X-ray film (Eastman Kodak Co., Rochester, NY) at −70°C overnight.
Chromatin immunoprecipitation (ChIP) assay
The ChIP assay was performed as previously described (Lui et al., 2006b). In brief, macromolecules in exponentially growing cells were cross-linked with 1% formaldehyde. Cells were resuspended in lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris, pH 8.1, 1 mM PMSF, and protease inhibitors) and sonicated by Sonifier 450 (Branson, Danbury, CT). Lysates were cleared by centrifugation and diluted in IP buffer (0.1% SDS, 1% Triton X-100, 0.1% sodium deoxychloate, 140 mM NaCl, 1 mM PMSF, and protease inhibitors). Ten micrograms of antibody and 20 µl of protein A/G Agarose were added to the pre-cleared chromatin solution for overnight incubation at 4°C. Beads were washed with washing buffer (0.1% Triton X-100, 20 mM Tris, pH 8.0, 150 mM NaCl, 2 mM EDTA) and eluted in the elution buffer (1% SDS and 0.1 M NaHCO3). The solution was heated at 65°C for 4 h followed by proteinase K digestion and phenol/chloroform extraction. The extracted DNA was used for PCR using the primer pairs #1120/#1121 for promoter region and #1020/#1021 for open reading frame. PCR was performed in their exponential phases (25 cycles).
Immunoprecipitation
About 300 µg of TM4 nuclear extract was diluted in IP buffer and incubated with different antibodies at 4°C overnight. Antibodies against HDAC1, mSin3A, and Smad4 were from Santa Cruz Biotechnology (Santa Cruz) and anti-acetyl histone H3 and H4 antibodies were from Upstate Biotechnology (Charlottesville, VA). Twenty microliters of protein A/G PLUS-agarose was added and incubated for 4 h at 4°C. Samples were centrifuged at 1,000g for 5 min to pellet the beads. Supernatants were discarded and immuno-complexes were washed four times with IP buffer. After washing, the immuno-complexes were resuspended in 25 µl of SDS sample buffer (0.125 M Tris, pH 6.8, 22°C containing 1% SDS (w/v), 1.6% β-mercaptoethanol (v/v), 20% glycerol) and boiled for 10 min. Beads were pelleted by centrifugation and supernatants were collected and resolved by SDS–PAGE using 10% T polyacrylamide gel under reducing condition. Immunoblots were then incubated with anti-CREB or anti-Smad4 antibodies for subsequent immunoblotting analysis.
RT-PCR
Total RNA was isolated from cells by TRIZOL reagent (Invitrogen). RT-PCR was performed essentially as previously described (Lui et al., 2006b). Two micrograms of total RNA was reverse-transcribed into complementary DNA in a total of 25 µl reaction. Two microliters of this RT product was used as template for RT-PCR with claudin-11 primers: #1020 and #1021 (Table 1) with cycling parameters as follows: denaturation at 94°C for 1 min, annealing at 56°C for 1 min, extension at 72°C for 1 min, for a total of 25 cycles, followed by a final extension at 72°C for 10 min. The authenticity of the PCR product was confirmed by nucleotide sequencing.
Data analysis
For all transfection assays, data were shown as mean ± SD of duplicate assays in three independent experiments. For EMSAs, all studies were repeated three times and consistent results were obtained. Data from mutation studies were analyzed by one-way ANOVA, followed by Tukey's multiple comparison tests using the computer software PRISM (GraphPad Software, Inc., San Diego, CA).
RESULTS
Mapping of the mouse claudin-11 promoter in TM4 cells
TM4 cells, a Sertoli cell line expressing claudin-11 gene, was used in this study (Fig. 1A). To locate the active promoter region of the claudin-11 gene, a 900 bp 5′-flanking sequence of the gene was used in the study. Progressive 5′-deletion mutants were constructed and analyzed in TM4 cells (Fig. 1B). Results of the transient transfection study revealed that the maximal promoter activity (5.9-fold) was obtained when 5′-sequence was deleted to nt −367. Further removal of sequence to nt −247 completely abolished the promoter activity. These results indicated that the core promoter of claudin-11 gene is located within the region between nt −367 and nt −247 in TM4 cells (Fig. 1B).
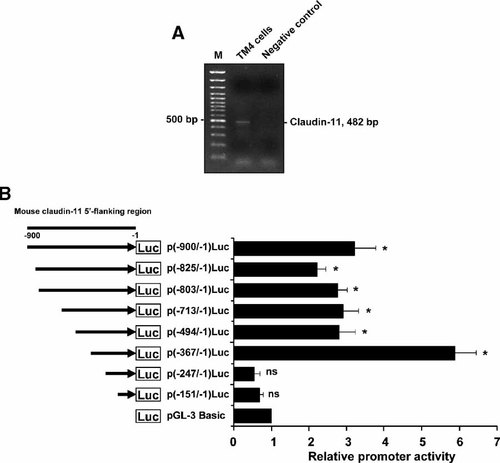
Expression of mouse claudin-11 mRNA and progressive 5′-deletion analysis of claudin-11 promoter in TM4 cells. A: Claudin-11 mRNA level was determined by RT-PCR in TM4 cells using a pair of primers specific to claudin-11 gene. The authenticity of the PCR product was confirmed by sequencing analysis. PCR without template was served as negative control. B: Progressive 5′-deletion analysis of mouse claudin-11 5′-flanking region was performed between nt −900 and −1. Corresponding regions were amplified by PCR, followed by subsequent cloning of the amplified fragments into promoterless pGL-3 Basic vector. Various claudin-11 promoter-luciferase constructs were co-transfected with the pCMV-β-gal vector. The relative promoter activity was normalized by β-galactosidase activity and represented as the fold induction when compared to the promoterless pGL-3 Basic vector. Values represent the mean ± SD of three independent experiments each performed in duplicate. *P < 0.01 versus pGL-3 Basic; ns, not significant versus pGL-3 Basic.
Mutational analysis of putative NF-Y, GATA, E2F, and MZF1 motifs within the core promoter region
Two putative GATA binding sites, namely GATA-b (5′-CGGATTGGCGC-3′, located from nt −270 to −259, with overlapping sequences with NF-Y and E2F motifs) and GATA-c (5′-TGCGATTGGC-3′, located from nt −300 to −291, with an overlapping sequence of NF-Y motif), and one MZF1 motif (5′-CTGCCCCTCCTCA-3′, located from nt −351 to −338) were identified within the core promoter region (Fig. 2A). To examine the functional significance of these motifs in regulating the claudin-11 gene transcription in TM4 cells, site-directed mutants were constructed and transfected into TM4 cells. Single mutation of GATA-c and GATA-b motifs caused a 75 and 26% reduction of promoter activity, respectively, while mutation of MZF1-d motif had no significant effect. These results showed that mutation of either one of the GATA motifs could partially abolish the promoter activity.
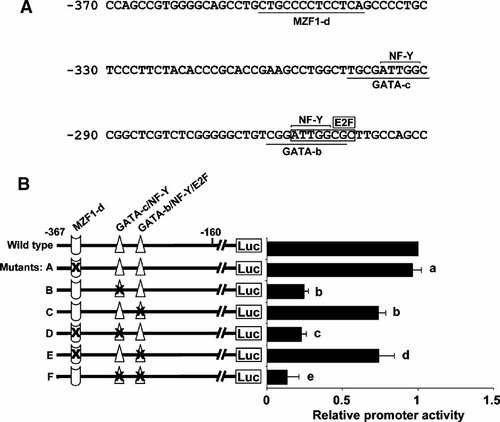
Mutational analysis of the putative MZF1, GATA, NF-Y, and E2F motifs on transcriptional activity of mouse claudin-11 5′-flanking region. A: Two putative GATA binding sites, namely GATA-b and GATA-c and one MZF1 binding site, namely MZF1-d are shown along the −370/-251 region and underlined. The overlapping regions of NF-Y and E2F on the GATA-b and GATA-c motifs are overlined and boxed as indicated. Numbers on the left side refer to the position of the claudin-11 gene relative to the translation start site. B: A diagrammatic representation of the mutated promoter constructs (mutants A–F) is shown. Mutated motifs are marked with black crosses. Wild type [p(−367/−1)Luc] or mutated promoter-luciferase constructs were co-transfected with the pCMV-β-gal vector into TM4 cells. The relative promoter activity was normalized by β-galactosidase activity and represented as the fold induction when compared to wild type [p(−367/−1)Luc] construct. Values represent the mean ± SD of three independent experiments each performed in duplicate. a, not significant versus wild type; b, P < 0.001 versus wild type; c, not significant versus mutant B; d, not significant versus mutant C; e, P < 0.01 versus mutants B and C.
To examine whether there is any functional cooperation among these cis-acting elements, constructs containing double mutations were analyzed. As depicted in Figure 2B, no further change in promoter activity was observed when either GATA-c or GATA-b was concurrently mutated with MZF1-d (mutants D and E), suggesting that MZF-1 motif may not involve in claudin-11 gene transcription in TM4 cells. However, a significant reduction was observed when the two GATA motifs (GATA-c and GATA-b, mutant F) were concurrently mutated (mutant F vs. mutant B or C), indicating that these two regulatory motifs functionally co-operate with one another to stimulate the basal claudin-11 gene transcription.
Analysis of DNA–protein interactions of the GATA motifs by EMSAs
Results from mutational analysis suggest that the two GATA motifs (GATA-c and GATA-b) within the core region are required for the basal promoter activity of claudin-11 gene. We then sought to examine and identify the transcription factors that bound to these two motifs. EMSAs showed that DNA–protein complexes were formed in a dose-dependent manner when synthetic oligonucleotides containing either GATA-c or GATA-b motifs interacted with nuclear extracts prepared from TM4 cells (Fig. 3A,C). As depicted in Figure 3A,C, two DNA–protein complexes were observed when using double-stranded oligonucleotide containing GATA-c or GATA-b motif, respectively. Formation of the complexes was inhibited dose-dependently by the addition of cold competitors (100- to 500-fold excess) (Fig. 3A (lanes 4–6), C (lanes 3–5)). Antibody inhibition assays for GATA-c probe revealed that transcription factors including GATA-1 and NF-YA were present in complex B since incubation of nuclear extract with antibodies against GATA-1 and NF-YA could abolish the DNA–protein interactions (Fig. 3B, lanes 2,3, arrowhead). To our surprise, incubation of nuclear extract with anti-Smad3 and anti-CREB antibodies could supershift the complex B and diminish the intensity of complex A (Fig. 3B, lanes 5,7), suggesting that these two transcription factors might somehow involve in claudin-11 gene transcription. Addition of rabbit serum had no effect which indicated the specificity of antigen–antibody interaction (Fig. 3B, lane 8). Taken together, these results suggested that interaction of GATA family, NF-YA, Smad3, and CREB transcription factors over the GATA-c motif were responsible for the formation of DNA–protein complexes.
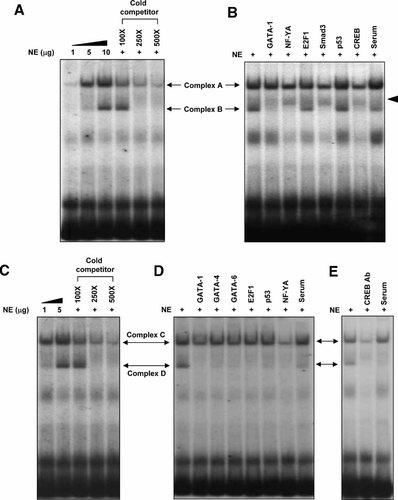
EMSAs of the GATA-c and GATA-b motifs using nuclear extracts (NE) from mouse TM4 cells. Synthetic oligonucleotides containing the GATA-c and GATA-b motifs were annealed to form double-stranded DNA, end-labeled with 32P and incubated with nuclear extracts from TM4 cells. DNA–protein complexes (complexes A–D) were formed with an increasing amount (1–10 µg) of TM4 nuclear extracts in the presence of radiolabeled probes containing the putative GATA-c (A, lanes 1–3) or GATA-b (C, lanes 1,2) motif. Nuclear extracts (10 and 5 µg) were incubated with the radiolabeled GATA-c (A, lanes 4–6) and GATA-b (C, lanes 3–5) probes in the presence of an increasing amount of cold competitors (100- to 500-fold excess), respectively. B,D,E: Nuclear extracts were pre-incubated with specified antibodies or rabbit serum before addition of the radiolabeled GATA-c (B) or GATA-b (D and E) probes.
Similar studies for GATA-b probe showed that GATA-1, GATA-6, and NF-YA were present in complex C when addition of antibodies against GATA-1, -6, and NF-YA, but not GATA-4 and E2F1, could partially abolish the complex formation, while the unrelated antibody such as anti-p53 antibody and serum had no effect on complex C formation (Fig. 3D). However, the protein component of complex D has not yet confirmed as addition of serum could effectively inhibit the formation of complex D, which makes the results difficult to interpret.
Interactions of the GATA motifs with CREB protein
Since the anti-CREB antibody could supershift the DNA–protein complex of the GATA-c probe (Fig. 3B, lane 7), we decided to examine whether similar interaction would be found within the GATA-b motif. It is of interest to note that anti-CREB antibody not only diminished the intensity of complexes A and B (Fig. 3B), but also that of the complex C (Fig. 3E). These results suggest that CREB might act as a major transcription factor to drive the claudin-11 promoter activity via these two GATA motifs.
GATA family, NF-Y, and CREB activate claudin-11 promoter activity in TM4 cells
To confirm the involvement of GATA family, NF-YA, and CREB in controlling the claudin-11 promoter activity, expression vectors coding for different transcription factors were co-transfected with the p(−367/−1) Luc construct. As shown in Figure 4A, there were 7-, 3.5-, and 3-fold increases in promoter activities in TM4 cells when GATA-1, GATA-4, and GATA-6 expression constructs were co-transfected with p(−367/−1)Luc, respectively. There was a 4.5-fold increase in promoter activity when wild-type NF-YA13 expression vector was used, whereas only 2-fold increase was observed when dominant negative NF-YA13m29 mutant having mutations in its DNA-binding domain was co-transfected into TM4 cells (Mantovani et al., 1994) (Fig. 4A). These results suggest that NF-YA is not a crucial transcription factor that interacts directly with the GATA motifs. The regulatory effect of NF-YA on the claudin-11 promoter is possibly through its interaction with CREB or GATA that bind to the GATA motifs. Apparently, CREB is a crucial transcription factor involved in claudin-11 transcription as over-expression of CREB could significantly increase the promoter activity by 15-fold (Fig. 4B). The promoter activity was similar to the control (pCMV vector alone) when a dominant-negative mutant (KCREB) having mutations in its DNA-binding domain was co-transfected (Fig. 4B), suggesting that the DNA-binding domain is essential for CREB-mediated claudin-11 gene transcription.
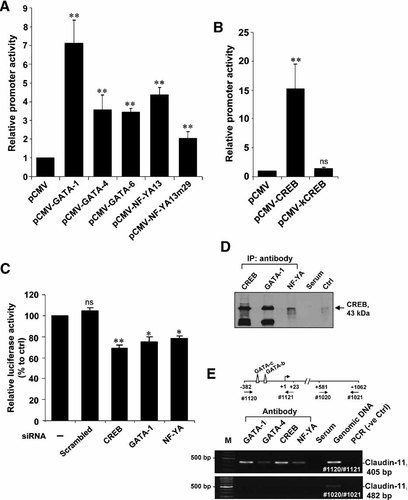
Effects of various transcription factors on claudin-11 promoter activity and in vivo binding of transcription factors on claudin-11 promoter region. A: The p(−367/−1)Luc construct was co-transfected with the expression vectors (pCMV-GATA-1, pCMV-GATA-4, pCMV-GATA-6, pCMV-NF-YA13, and pCMV-NF-YA13m29) into TM4 cells. B: The p(−367/−1)Luc construct was co-transfected with either empty expression vector pCMV or expression vectors encoding either CREB or KCREB. C: The p(−367/−1)Luc construct and siRNA oligos were co-transfected into TM4 cells. The relative promoter activity was normalized by β-galactosidase activity and represented as the fold induction when compared to pCMV alone (lower part). Values represent the mean ± SD of three independent experiments each performed in duplicate. D: TM4 nuclear extract were immunoprecipitated with anti-CREB, anti-GATA-1, anti-NF-YA antibody, or normal rabbit serum. The immunocomplexes were subjected to SDS–PAGE analysis followed by immunoblotting by anti-CREB antibody. Nuclear extract without immunoprecipitation were served as a positive control to indicate the presence of target protein. E: ChIP analysis for the association of various transcription factors with claudin-11 promoter. Nuclear proteins and genomic DNA were cross-linked by formaldehyde and isolated genomic DNA was immunoprecipitated with antibodies against GATA-1, -4, CREB, and NF-YA (lanes 2–5). After immunoprecipitation, the claudin-11 promoter (-382 to +23) and the open reading frame (+581 to +1062) was amplified by PCR using primer pairs: #1120/#1121 and #1020/#1021, respectively. *P < 0.01 versus corresponding control; **P < 0.001 versus corresponding control; ns, not significant versus corresponding control.
RNA interference assay was performed to examine the role of endogenous transcription factors including CREB, GATA-1, and NF-YA in regulation of claudin-11 gene transcription. siRNA oligos against specific transcription factors were transiently transfected into TM4 cells. Co-transfections of siRNA for CREB, GATA-1, and NF-YA with p(−367/−1)Luc construct resulted in reductions in promoter activities (reduced by 31.1, 24.8, and 21.7%, respectively) (Fig. 4C). However, no significant inhibition was observed for non-specific siRNA (Fig. 4C).
Since GATA, NF-YA, and CREB could also interact with the GATA motifs as demonstrated in EMSA, it is worthy to investigate whether GATA or NF-YA would interact with CREB to regulate the promoter activity. Co-immunoprecipitation was performed by incubating TM4 nuclear extract with antibodies against GATA-1 and NF-YA, respectively, a specific CREB protein band was found in the immunoblot probed with anti-CREB antibody (Fig. 4D). No band was found when antibody was replaced by serum. The result of co-immunoprecipitation suggested that CREB could physically interact with GATA-1 or NF-YA to regulate gene transcription.
In vivo binding of GATA, NF-YA, and CREB protein to the claudin-11 promoter
In order to determine whether endogenous GATA, NF-YA, and CREB proteins are recruited by the claudin-11 promoter in vivo, ChIP assay was performed using TM4 cells (Fig. 4E). Positive PCR signal was detected using primer pair #1120/#1121 when immunoprecipitation was performed using antibodies against GATA, CREB, or NF-YA. No signal was detected from control antibody (rabbit serum) and the PCR negative control (no template). The whole set of immunopreciptated DNA was also used for the amplification of open reading frame for claudin-11 gene using primer pair #1020/#1021 and no specific DNA could be detected except mouse genomic DNA was used in the PCR. These results suggest that GATA, NF-YA, and CREB proteins are recruited at the site of promoter region to regulate claudin-11 gene transcription.
Smad proteins exert negative regulatory effect at the GATA-c motif
EMSA analysis has shown that anti-Smad3 antibody could diminish the formation of complexes A and B (Fig. 4B, lane 5), suggesting that Smad protein might interact with the cis-acting motifs of claudin-11 promoter to regulate its transcription. Since the core promoter region of claudin-11 does not contain any putative Smad-binding element (SBE), we therefore search for the cis-acting motifs involved and the regulatory mechanism mediated by Smad proteins. Co-transfection of deletion constructs including p(−247/−1)Luc and p(−367/−1)Luc with Smad3 and Smad4 expression vectors confirmed that Smad proteins exerted their actions on the sequence between nt −247 and nt −367 (Fig. 5A). To determine whether Smad proteins interact with the GATA and MZF1-d motifs between nt −247 and nt −367, we co-transfected the mutated construct (−367/−1) carrying mutation of particular motif together with the Smad3 or Smad4 expression vectors into TM4 cells (Fig. 5B,C). Co-transfection of either Smad3 or Smad4 with wild-type construct repressed the promoter activities by 40 and 50%, respectively compared to pcDNA3.1 vector alone. Constructs carrying either GATA-b or MZF1-d mutation co-transfected with Smad3 or Smad4 expression plasmid have similar reduction in promoter activities as the wild-type, suggesting that GATA-b or MZF1-d are not the cis-acting motifs that interact with Smad proteins since mutation could not alleviate the Smad-mediated repression. However, there was no reduction in promoter activity when Smad3 or Smad4 expression vector was co-transfected with the GATA-c mutant construct. These results suggest that mutation of GATA-c blocked the Smad-mediated claudin-11 repression and that Smad-mediated claudin-11 repression is via the GATA-c motif.
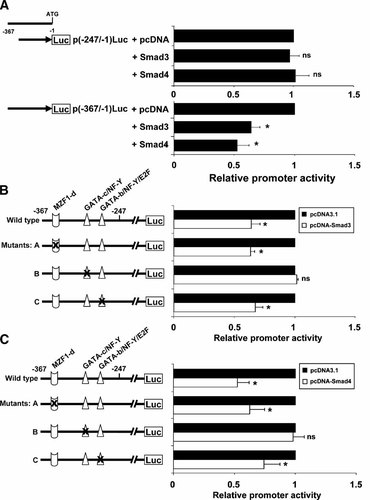
Identification of Smad-interacting motif on claudin-11 promoter in TM4 cells. A: The p(−247/−1)Luc and p(−367/−1)Luc constructs was co-transfected with pcDNA or expression vectors encoding Smad3 and Smad4 proteins. The p(−367/−1)Luc constructs and mutated constructs were co-transfected with either Smad3 (B) or Smad4 (C) expression vectors. The promoter activity was normalized by β-galactosidase activity. The relative promoter activity is represented as the fold induction when compared to corresponding control (pcDNA3.1). Values represent the mean ± SD of three independent experiments each performed in duplicate. *P < 0.001 versus pcDNA vector alone; ns, not significant versus pcDNA vector alone.
Smad proteins inhibit CREB and GATA transactivation
Since Smad proteins repress claudin-11 gene transcription via the GATA-c motif on which a positive regulatory effect in gene transcription was elicited by CREB and GATA interaction, the involvement of Smad proteins in the repression of CREB and GATA transactivation for claudin-11 gene was examined. In this regard, cells were co-transfected with expression vector encoding CREB or GATA and an increasing amount of either Smad3 or Smad4 expression plasmid (0, 0.25, 0.5, and 1 µg), along with the p(−367/−1)Luc construct (Fig. 6A,B). It was shown that co-expression of Smad3 or Smad4 inhibited CREB and GATA transactivation in a dose-dependent manner (Fig. 6A,B). This Smad inhibition was albeit to a greater extent in CREB transactivation than GATA transactivation and Smad4 inhibited transactivation more effective than Smad3. Taken together, these results suggested that Smad proteins are involved in the repression of claudin-11 promoter through inhibition of CREB and GATA transactivation in TM4 cells.
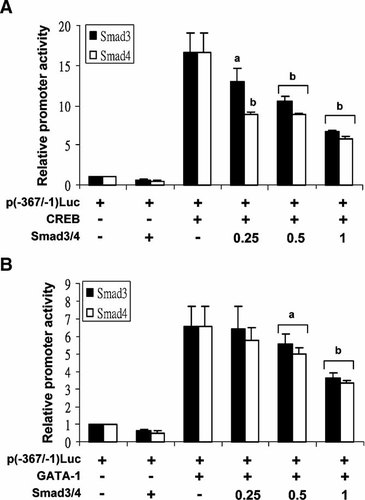
Inhibition of CREB and GATA transactivation by Smad proteins. TM4 cells were co-transfected with 1 µg of CREB (A) or GATA-1 (B) expression vector and increasing amounts of either Smad3 or Smad4 expression plasmids, along with p(−367/−1)Luc. The promoter activity was normalized by β-galactosidase activity. The relative promoter activity is represented as the fold induction when compared to the control [p(−367/−1)Luc]. Values represent the mean ± SD of three independent experiments each performed in duplicate. a, P < 0.01 versus p(−367/−1)Luc+CREB or p(−367/−1)Luc+GATA-1; b, P < 0.001 versus p(−367/−1)Luc+CREB or p(−367/−1)Luc+GATA-1.
Smad proteins repress claudin-11 promoter activity via recruitment of HDAC
To search for a clue to the mechanism by which Smad proteins repress CREB and GATA transactivation for claudin-11 gene, we assessed the involvement of histone deacetylases (HDAC) by the HDAC inhibitor, Trichostatin A (TSA). In TM4 cells, CREB, and GATA-1 transactivation for claudin-11 promoter repressed by co-transfection of Smad3, Smad4, or both constructs was fully recovered by TSA treatment at a concentration of 200 ng/ml (Fig. 7A,B). In the absence of Smad proteins, transcription activity by p(−367/−1)Luc along with co-expression of either CREB or GATA was not affected by TSA treatment. These results imply that HDACs are involved in the Smad-mediated claudin-11 gene repression.
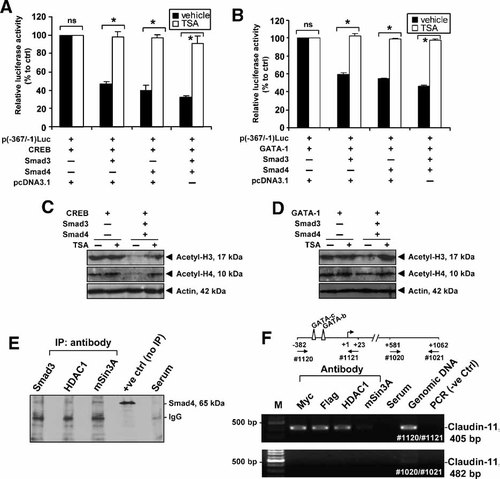
Effect of TSA and the acetylation status of histones on claudin-11 promoter activity in TM4 cells and the interaction of Smad proteins with HDAC1 and mSin3A within claudin-11 promoter region. A,B: The p(−367/−1)Luc along with pCMV-CREB or pCMV-GATA-1 were co-transfected with pcDNA3.1, pcDNA3.1-Smad3, or pcDNA3.1-Smad4 expression vectors. Cells were treated either with vehicle (ethanol, black bars) or TSA (200 ng/ml, white bars) for 24 h before harvest. The promoter activity was normalized by β-galactosidase activity. The relative promoter activity is represented as the fold induction when compared to corresponding control group (vehicle). Values represent the mean ± SD of three independent experiments each performed in duplicate. C,D: The p(−367/−1)Luc along with pCMV-CREB or pCMV-GATA-1 were co-transfected with pcDNA3.1, pcDNA3.1-Smad3, and pcDNA3.1-Smad4 expression vectors. Cells were treated with either vehicle (ethanol) or TSA (200 ng/ml) for 24 h before protein extraction. Extracted proteins were probed with anti-acetyl-histone H3 (acetyl-H3), anti-acetyl-histone H4 (acetyl-H4), or anti-actin (actin) antibodies. E: TM4 nuclear extract were immunoprecipitated with anti-Smad3, anti-HDAC1 and anti-mSin3A antibody, or normal rabbit serum. The immunocomplexes were subjected to SDS–PAGE analysis followed by immunoblotting using anti-Smad4 antibody. Nuclear extract without immunoprecipitation were served as a positive control to indicate the presence of target protein. F: ChIP assay for the detection of Smad proteins and HDAC associations over claudin-11 promoter region. Myc-tagged Smad3 and Flag-tagged Smad4 were expressed in TM4 cells. Nuclear proteins and genomic DNA were cross-linked by formaldehyde and isolated genomic DNA was immunoprecipitated with antibodies against Myc, Flag, HDAC1 or mSin3A, and serum. After immunoprecipitation, the claudin-11 promoter (−382 to +23) and the open reading frame (+581 to +1062) were amplified by PCR using primer pairs #1120/#1121 and #1020/#1021, respectively. *P < 0.001; ns, not significant.
Smad expression is correlated with claudin-11 promoter deacetylation
Because TSA treatment could reverse the negative effect of Smads on claudin-11 promoter activity, one would expect to observe change in the histone acetylation status in TM4 nuclear extract. To address this point, we analyzed the acetylation status of histones H3 and H4 in TM4 cells co-transfected with Smad3, Smad4, and p(−367/−1)Luc in the absence or presence of TSA. It was shown that treatment with TSA in TM4 cells overexpressing Smad3 and Smad4 led to a rebound in the level of both acetylated histones H3 and H4 in both CREB- and GATA-1- transactivation (Fig. 7C,D). These results indicate that TSA indeed alters/blocks the Smad-mediated histone deacetylation in CREB- and GATA-1 transactivation of claudin-11 gene.
Smad protein associates with HDAC1 and a co-repressor, mSin3A
To get further insights into the model of Smad-mediated repression, we analyzed the association of HDAC1, a histone deacetylase, and mSin3A, a co-repressor, with Smad proteins. Such interaction was confirmed by co-immunoprecipitation analysis of TM4 nuclear extract with specific antibodies against Smad3, HDAC1, and mSin3A followed by immunoblotting with anti-Smad4 antibody (Fig. 7E, lanes 1–3). The specificity of these interactions was confirmed with the serum control immunoprecipitation (Fig. 7E, lane 5). These results support the existence of physical interaction between Smad proteins, HDAC1 and the co-repressor, mSin3A, suggesting the formation of a negative regulatory complex to repress claudin-11 expression.
Binding of Smad proteins and HDAC1 on claudin-11 promoter region
Constructs encoding Myc-tagged Smad3 and Flag-tagged Smad4 were co-transfected into TM4 cells, nuclear extracts were prepared for ChIP assay to examine the in vivo binding of Smad3 and Smad4 on claudin-11 promoter (Fig. 7F). Positive PCR signal was detected using primer pair #1120/#1121 when immunoprecipitation was performed using antibodies against Myc, Flag, HDAC1, or mSin3A, whilst no signal was detected in serum and negative PCR control. No signal was detected except genomic DNA (positive control) when primer pair #1020/#1021 specific for open reading frame of claudin-11 gene was used.
DISCUSSION
In the present study, we have characterized the regulatory elements involved in claudin-11 gene transcription. The core promoter of claudin-11 is relatively short (∼100 bp in length). Within this region, an overlapping GATA-c/NF-Y motif plays a crucial role in controlling the claudin-11 gene transcription as dual transcriptional control of claudin-11 gene was observed by differential binding of various transcription factors within this motif. Binding of several positive regulatory factors including GATA, NF-YA, and CREB to the promoter enhances transcription; whilst the presence of Smad proteins could strongly inhibit transcription even in the presence of either GATA or CREB transcription factors. This suggests that a change in the level of either a particular transcription factor or combination of transcription factors in the seminiferous epithelium may provide a mechanism to control precisely claudin-11 expression, leading to the reassembly and disassembly of the BTB.
GATAs are essential transcription factors in gonadal development, testosterone production, and regulation of gene expression in testicular somatic cells such as Sertoli cells. Of the six members in this family, GATA-1, -4, and -6 are found in the testis (Tremblay and Viger, 2001; LaVoie, 2003; Viger et al., 2004). Previous studies have shown that GATA-1 is expressed in mouse Sertoli cells from stages VII to IX of spermatogenic cycle (Yomogida et al., 1994), whereas GATA-4 is present in mouse testis and localized to Sertoli cells and Leydig cells throughout all developmental stages (Viger et al., 1998; Ketola et al., 1999, 2002). CREB is present in stages I–VIII of the spermatogenic cycle and the amount decreases to an undetectable level during stages IX–XIV (Waeber et al., 1991). Taken collectively, Sertoli cells at stages I–VIII of the spermatogenic cycle exhibit the highest level of positive regulators for claudin-11 expression. Because of its ability to aggregate with GATA-1 and NF-YA, the decrease in CREB during stages IX–XIV could result in an overall decrease in the level of positive regulators at the promoter. Smad3, one of the negative regulators of claudin-11 gene transcription, is known to be involved in is shown to express in the cytoplasm of Sertoli cells throughout the spermatogenic cycle (Xu et al., 2003). In addition, there is a shift in the localization of Smad3 from cytoplasm to nucleus at stages VII–VIII, suggesting that Smad3 may involve in the stage-specific expressions (Xu et al., 2003). In fact, pre-leptotene/leptotene spermatocytes must traverse through the BTB between adjacent Sertoli cells during stages VIII–IX (for reviews, see Russell, 1977; Cheng and Mruk, 2002). The timely increase in Smad3 protein at stage VIII followed by a decrease in CREB protein at stage IX alters the ratio of positive/negative regulators which favors claudin-11 gene repression and disassembly of BTB at stages VIII–IX. Soon afterwards, Smad3 protein migrates back to cytoplasm resulting an increase in overall ratio of positive/negative regulators in the nucleus to promote claudin-11 transcription and reconstruction of the BTB. The forced expression of Smad proteins in this study mimics the increased expression of Smad proteins at stage VIII. Results shown in this study indeed support our hypothesis that the change of positive/negative regulators ratio could be a possible mechanism to modulate claudin-11 gene transcription.
Chromatin remodeling and histone modifications have emerged as a major mechanism of controlling gene expression. Chromatin of transcriptionally active regions is closely associated with hyperacetylated histones H3 and H4, whereas transcriptionally inactive chromatin is enriched by deacetylated histones (for review, see Strahl and Allis, 2000). The recruitment of histone acetyltransferases (HAT) and histone deacetylases (HDAC) at the specific DNA regulatory sequence could alter the acetylation status of histones, thus controlling gene expression (for review, see Strahl and Allis, 2000). In this study, we have investigated the involvement of this mechanism in Smad-mediated claudin-11 gene repression. It is possible that binding of Smad proteins at the GATA-c motif helps recruiting HDAC1 and a co-repressor, mSin3A. Moreover, inhibition of HDAC activity by TSA could reverse Smad-mediated claudin-11 gene repression along with an increase in acetylated H3 and H4 protein levels. Taken collectively, the co-operation of HDAC1 and mSin3A with Smad proteins is necessary for an effective repression of the claudin-11 promoter via the modulation of the acetylation status of histones along the GATA-c motif.
Another intriguing finding in this study is the identification of a novel CREB binding site. We have demonstrated that CREB is capable of interacting with NF-YA and GATA-1 (Fig. 4C,D) and is a component of the DNA–protein complex (Fig. 3B,E). Smad proteins, the negative regulators, could significantly affect CREB transactivation (Fig. 6A) at the GATA-c motif (Fig. 5B,C). Taken these results collectively, CREB is a major component of the claudin-11 gene transcription. The results of overexpression studies using wild-type and mutant CREB plasmids indicate that CREB promotes claudin-11 transcription via its direct DNA interaction since overexpression of KCREB expression plasmid carrying mutation at the DNA binding motif failed to induce claudin-11 gene transcription. To our knowledge, this is the first demonstration that CREB transcription factor can bind the overlapping GATA/NY-F motif, GATTGG, which is composed of the forward GATA consensus sequence, GATT, and the reverse NF-Y consensus sequence, ATTGG.
The significance of basic-domain-leucine-zipper (b-zip) transcription factor family members (including CREB and CREM) in spermatogenesis has been demonstrated by knockout mice studies (Hummler et al., 1994; Blendy et al., 1996; Nantel et al., 1996; Rudolph et al., 1998). Disruption of CREB and CREM in mice could severely interrupt spermatogenesis, resulting in male infertility, yet not affecting other systems. Subsequent investigations have been focused on the identification of the CREM target genes since CREMτ was found to be a testis-specific CREM isoform expressed predominantly in germ cells (for review, see MacLean and Wilkinson, 2005). Given the fact that the localization of CREB is restricted to Sertoli cells (Waeber et al., 1991), it is believed that CREB is an important member of the b-zip family that regulates gene expression in Sertoli cells. For instance, KCREB serving as an antagonist CREB could completely inhibit the expression of c-fos in Sertoli cells (Hall et al., 1988; Scobey et al., 2001). Our previous study has indicated that nectin-2 gene transcription in Sertoli cells is controlled by the functional co-operation of Sp1, AP-1, and CREB families (Lui et al., 2006b). The present study shows CREB also acting as an important trans-factor in regulating claudin-11 gene expression in Sertoli cells. It is of interest to further investigate the role of CREB in regulating junction dynamics as it seems to be the master transcription factor in modulating the expression of transmembrane proteins of both TJ and adherens junctions in the testis.
Acknowledgements
We thank Robert Czolij for GATA-1 expression construct, Michael Parmacek for GATA-4 and GATA-6 expression constructs, Sheng-Cai Lin for CREB expression constructs, Karl WK Tsim for KCREB dominant negative mutant, Roberto Mantovani for NF-YA13 expression construct and NF-YA13m29 dominant negative mutant, and Peter ten Dijke for Myc-tagged Smad3 and Flag-tagged Smad4 expression constructs. This work was supported in part by grants from the Hong Kong Research Grant Council (HKU7536/05M) and The University of Hong Kong (CRCG).




