Transcriptional regulation of VCAM-1 expression by tumor necrosis factor-α in human tracheal smooth muscle cells: Involvement of MAPKs, NF-κB, p300, and histone acetylation
Abstract
Tumor necrosis factor-α (TNF-α) has been shown to induce the expression of adhesion molecules in airway resident cells and contribute to inflammatory responses. Here, the roles of mitogen-activated protein kinases (MAPKs) and NF-κB in TNF-α-induced expression of vascular cell adhesion molecule (VCAM)-1 were investigated in human tracheal smooth muscle cells (HTSMCs). TNF-α-enhanced expression of VCAM-1 protein and mRNA as well as phosphorylation of p42/p44 MAPK, p38, and JNK were significantly attenuated by inhibitors of MEK1/2 (U0126), p38 (SB202190), and JNK (SP600125). Transfection with dominant negative mutants of MEK1/2, ERK1, ERK2, p38, and JNK attenuated TNF-α-induced VCAM-1 expression. Furthermore, TNF-α-induced VCAM-1 expression was significantly blocked by a selective NF-κB inhibitor helenalin. TNF-α-stimulated translocation of NF-κB into the nucleus and degradation of IκB-α was blocked by helenalin, but not by U0126, SB202190, or SP600125. VCAM-1 promoter activity was enhanced by TNF-α in HTSMCs transfected with VCAM-1-Luc, which was inhibited by helenalin, U0126, SB202190, and SP600125. Most surprisingly, VCAM-1 expression was also significantly blocked by a selective inhibitor of p300, curcumin. NF-κB transcription factor and p300 were associated with the VCAM-1 promoter, which was dynamically linked to histone H3 acetylation stimulated by TNF-α, as determined by chromatin immunoprecipitation assay. Moreover, the resultant enhancement of VCAM-1 expression increased the adhesion of polymorphonuclear cells (PMNs) to monolayer of HTSMCs, which was blocked by helenalin, U0126, SB202190, or SP600125. These results suggest that in HTSMCs, activation of MAPK pathways, NF-κB, and p300 is essential for TNF-α-induced VCAM-1 expression. J. Cell. Physiol. 207: 174–186, 2006. © 2005 Wiley-Liss, Inc.
Elevated levels of pro-inflammatory cytokines including tumor necrosis factor-α (TNF-α) in the bronchoalveolar lavage fluid have been detected in allergic asthmatic patients (Chung, 2000). TNF-α has been shown to be a potent stimulator in inflammatory responses (Broide et al., 1992; Amrani et al., 2000a) through up-regulation of genes encoding cytokines, chemokines, proteases, cyclooxygenase, cPLA2 and adhesion molecules (Vassalli, 1992; Tracey and Cerami, 1993; Amrani et al., 2000b; Lin et al., 2004). The induction of cell adhesion molecules such as intercellular adhesion molecule (ICAM)-1 mediates the tight adhesive of polymorphonuclear cells (PMNs) and thus facilitates PMNs migration across the vascular endothelial barrier (Dustin et al., 1988; Smith et al., 1989; Javaid et al., 2003). Vascular cell adhesion molecule (VCAM)-1 is also an inducible cell surface glycoprotein on several cell types and implicates in a number of inflammatory responses (Elices et al., 1990; Chan and Aruffo, 1993; Cook-Mills, 2002). To reach the airway lumen and submucosa, circulating PMNs must first be recruited across the vascular endothelium (Butcher, 1991; Georas et al., 1992; Moreland et al., 2002) and then migrate through the interstitial matrix before interacting with airway epithelium. The migratory processes of PMN may occur and contribute to the airway damage seen in the inflammatory responses of asthma (Montefort et al., 1992; Tosi et al., 1992; Bloemen et al., 1993).
Although the roles of cytokines and adhesion molecules in PMNs adhesion to endothelial cells are well described, little is known about the mechanism(s) underlying the interaction between PMNs and tracheal smooth muscle cells (TSMCs). Several reports have described the induction of VCAM-1 by cytokines such as IL-1β, TNF-α, and IFN-γ in various cell types (Kaiserlian et al., 1991; Mulligan et al., 1993; Ju et al., 2002). Moreover, the promoters of both ICAM-1 and VCAM-1 genes contain nuclear factor-κB (NF-κB) binding sites, regulated by TNF-α through mitogen-activated protein kinases (MAPKs) activation in several cell types (Barnes and Karin, 1997; Bian et al., 2001; Chen et al., 2001).
Cytokines such as TNF-α have been reported to activate all of these MAPKs (McEwan, 2002; Pfeffer, 2003), including p42/p44 MAPK (Boulton et al., 1990), p38 MAPK (Han et al., 1994), and the c-Jun-N-terminal kinase (JNK) (Kyriakis et al., 1994), activated by phosphorylation of a tyrosine and a threonine residue catalyzed by a dual specificity MAPK kinase. The relationship between the activation of these pathways and expression of adhesion molecules has been controversial. For example, TNF-α-induced VCAM-1 and ICAM-1 expression in mouse Sertoli cells does not require the activation of p38 kinase, whereas activation of JNK is essential for these responses (Szlosarek and Balkwill, 2003). In endothelial cells, JNK and PKC activation are required for TNF-α-mediated ICAM-1 expression. In contrast, cardiac cells require p38 MAPK activation for VCAM-1 and ICAM-1 expression (Kacimi et al., 1998). Moreover, p38 MAPK and JNK oppositely regulate TNF-α-induced VCAM-1 expression in chondrosarcoma cells (Ju et al., 2002). In A549 cells, TNF-α induces ICAM-1 expression through activation of NF-κB, but not p42/p44 MAPK, p38, and JNK (Chen et al., 2001). It is therefore, important to examine whether the activation of these MAPK pathways by TNF-α linked to VCAM-1 expression in human HTSMCs. In this regard, several genes regulated by MAPKs are mediated through activation of NF-κB for transcription (Bian et al., 2001). Recent studies have demonstrated that pro-inflammatory gene transcription is tightly regulated by the transcriptional co-activators, p300, and cAMP-response element-binding protein binding protein (CBP). Current models suggest that the binding of these co-activators to transcription factor activates histone acetyltransferases (HATs) near specific nucleosomes in target gene promoter regions (Vo and Goodman, 2001). Several studies have reported that p300 and CBP, phosphoproteins (Goodman and Smolik, 2000; Chan and La Thangue, 2001), are regulated by protein kinases, such as MAPK (Ait-Si-Ali et al., 1999; Poizat et al., 2005) or Akt (Huang and Chen, 2005).
To address these questions, experiments were performed to investigate the roles of MAPK pathway, NF-κB, and p300 in TNF-α-induced VCAM-1 expression in HTSMCs. We found that co-activation of p42/p44 MAPK, p38, and JNK are required for maximal induction of VCAM-1 expression by HTSMCs. These findings suggest that up-regulation of VCAM-1 enhanced the adhesion of PMNs to TNF-α-challenged HTSMCs. These effects were mediated, at least in part, through these MAPK signaling pathways, NF-κB, and p300.
MATERIALS AND METHODS
Materials
DMEM/F-12 medium, FBS, and Trizol were purchased from Invitrogens (Carlsbad, CA). Hybond C membrane, enhanced chemiluminescence (ECL) Western blotting detection system and Hyperfilms were from Amersham Biosciences (Buckinghamshire, England). Polyclonal antibodies VCAM-1, IκB-α, and NF-κB p65 were from Santa Cruz (Santa Cruz, CA). Anti-GAPDH antibody was from Biogenesis (Boumemouth, UK). PhosphoPlus p42/p44 MAPK, p38 MAPK, and SAPK/JNK antibody kits were from New England Biolabs (Beverly, MA). U0126, SB202190, SP600125, curcumin, and helenalin were from Biomol (Plymouth Meeting, PA). Bicinchoninic acid (BCA) protein assay kit was from Pierce (Rockford, IL). Enzymes and other chemicals were from Sigma (St. Louis, MO).
Cell culture
HTSMCs were isolated from human trachea and cultured as previously described (Lee et al., 2004). When the cultures reached confluence, cells were treated with 0.05% (w/v) trypsin/0.53 mM EDTA for 5 min at 37°C. The cell suspension was diluted with DMEM/F-12 containing 10% FBS to a concentration of 2 × 105 cells/ml. The cell suspension was plated onto (1 ml/well) 12-well culture plates and (10 ml/dish) 10-cm culture dishes for the measurement of protein expression and mRNA accumulation, respectively. Experiments were performed with cells from passages 3 to 8.
Preparation of cell extracts and Western blot analysis
Growth-arrested HTSMCs were incubated with TNF-α at 37°C for the indicated time. The cells were washed with ice-cold PBS, scraped, collected, and centrifuged at 45,000g for 1 h at 4°C to yield the whole cell extract, as previously described (Lee et al., 2004). Samples were denatured, subjected to SDS–PAGE using a 10% running gel, and transferred to nitrocellulose membrane. Membranes were incubated overnight at 4°C with the anti-VCAM-1 or anti-GAPDH antibody used at a dilution of 1:2,000 in 5% (w/v) BSA in TTBS [(50 mM Tris-HCl, 150 mM NaCl, 0.05% (w/v) Tween 20, pH 7.4)]. Membranes were incubated with a 1:1,500 dilution of anti-goat or anti-mouse horseradish peroxidase antibody for 1 h. The immunoreactive bands detected by ECL reagents were developed by Hyperfilm-ECL.
Plasmids and transfection
The plasmids encoding dominant negative mutants MEK1/2 (MEK K97R), ERK1, ERK2 (ERK K52R), JNK, and p38 were kindly provided by Dr. K.L. Guan (Department of Biological Chemistry, University of Michigan), Dr. M.H. Cobb (Department of Pharmacology, University of Texas Southwestern Medical Center), Dr. J. Pouyssegur (Center de Biochimine, Center National de la Research Scientifique, Universite de Nice, Nice, France), Dr. C.C. Chen (Department of Pharmacology, National Taiwan University, Taipei, Taiwan), and Dr. J. Han (The Scripps Research Institute, La Jolla, CA), respectively. All plasmids were prepared by using QIAGEN plasmid DNA preparation kits.
HTSMCs cells were plated at 3 × 105 cells/ml in 12-well culture plates for 24 h. Cells were transfected with 1 µg of dominant negative mutants (MEK1/2, ERK1, ERK2, JNK, or p38 for each well) using DNA PLUS-Lipofectamine and incubated at 37°C for 3 h. One milliliter of DMEM/F-12 medium containing 10% FBS was added and incubated for additional 19 h. The cells were washed twice with PBS and maintained in DMEM/F-12 containing 1% FBS for 24 h before treatment with TNF-α. The transfection efficiency (approximate 60%) was determined by transfection with EGFP.
Total RNA extraction and RT-PCR analysis
Total RNA was isolated from HTSMCs treated with TNF-α in 10-cm culture dishes with Trizol. RNA concentration was spectrophometrically determined at 260 nm. First strand cDNA synthesis was performed with 2 µg of total RNA using random hexamers as primers in a final volume of 20 µl (5 µg/µl random hexamers, 1 mM dNTPs, 2 U/µl RNasin, and 10 U/µl moloney murine leukemia virus reverse transcriptase). The reaction was carried out at 37°C for 60 min. cDNAs encoding β-actin and VCAM-1 were amplified from 3 to 5 µl of the cDNA reaction mixture using specific gene primers. Oligonucleotide primers for β-actin and VCAM-1 were as follow: β-actin: 5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′(sense), 5′-CTAGAAGCATTTGCGGTGGACGATG-3′ (anti-sense); VCAM-1: 5′-GGAACCTTGCAGCTTACAGTGACAGAGCTCCC-3′(sense), 5′-CAAGTCTACATATCACCCAAG-3′ (anti-sense).
The amplification profile includes 1 cycle of initial denaturation at 94°C for 5 min, 30 cycles of denaturation at 94°C for 1 min, primer annealing at 62°C for 1 min, and extension at 72°C for 1 min then 1 cycle of final extension at 72°C for 5 min. The expression of β-actin was used as an internal control.
NF-κB translocation
HTSMCs were seeded in a 10-cm dish. After they reached 90% confluence, the cells were starved for 24 h in serum-free DMEM/F-12 medium. After stimulation with 150 µg/ml TNF-α, the cells were washed, scraped, and centrifuged to prepare cytosolic and nuclear fractions, as previously described (Wang et al., 2005). The translocation of NF-κB and degradation of IκB-α were identified by Western blot analysis using IκB-α and NF-κB (p65) antibody, respectively. The nuclear fraction was also used to identify ATF-2 phosphorylation against anti-phospho-ATF-2 antibody.
Immunofluorescence staining
HTSMCs were plated on six-well culture plates with coverslips. Cells were treated with 150 µg/ml TNF-α and washed twice with ice-cold PBS. Immunofluorescence staining was performed as previously described (Wang et al., 2005).
Measurement of VCAM-1 luciferase activity
For construction of the VCAM-1-luc plasmid, human VCAM-1 promoter, a region spanning −1,716 to −119 bp (kindly provided by Dr. W.C. Aird, Department of Molecular Medicine, Beth Israel Deaconess Medical Center, Boston, MA) was cloned into pGL3-basic vector. VCAM-1-luc plasmid was transfected into HTSMCs as described above. To assess promoter activity, cells were collected and disrupted by sonication in lysis buffer (25 mM Tris, pH 7.8, 2 mM EDTA, 1% Triton X-100, and 10% glycerol). After centrifugation, aliquots of the supernatants were tested for luciferase activity using the luciferase assay system. Firefly luciferase activities were standardized for β-galactosidase activity.
Chromatin immunoprecipitation assay
To detect the in vivo association of nuclear proteins with human VCAM-1 promoter, Chromatin immunoprecipitation (ChIP) analysis was conducted as previously described (Nie et al., 2003) with some modifications. DNA immunoprecipitated by anti-p300 (sc-585; Santa Cruz), anti-acetylated histone H3 (06-599; Upstate) and NF-κB p65 were from Santa Cruz antibody was purified. The DNA pellet was resuspended in H2O and subjected to PCR amplification with the forward primer 5′-AAATCAATTCACATGGCATA-3′ and the reverse primer 5′-AAGGGTCTTGTTGCAGAGG-3′, which were specifically designed from the VCAM-1 promoter region (−403 to −30). PCR products were analyzed on ethidium bromide-stained agarose gels.
Neutrophil adhesion assay
Peripheral blood PMNs were isolated from whole venous blood by dextran sedimentation followed by density separation over Ficoll–Hypaque and hypotonic lysis. PMNs were used within 4 h of purification. HTSMCs grew on glass-coverslips pretreated with 150 µg/ml of TNF-α for 24 h. Leukocyte-HTSMCs adhesion was measured by a parallel plate chamber according to the methods as previously described (Wang et al., 2005). The number of neutrophils adhering to HTSMCs was analyzed with an inverted light microscope connected to an image analysis system.
Analysis of data
Data were expressed as the mean ± SEM and analyzed with a one-way ANOVA to make comparisons with Bonferroni's test at a P < 0.05 level of significance.
RESULTS
TNF-α induces de novo VCAM-1 protein and gene expression
To determine the effect of TNF-α on VCAM-1 protein and mRNA expression, HTSMCs were treated with various concentrations of TNF-α for the indicated time. The amount of de novo synthesis of VCAM-1 protein was determined using Western blot analysis. As shown in Figure 1A, TNF-α induced VCAM-1 protein expression in a time- and concentration-dependent manner. A significant stimulation was observed within 4 h. A maximal response was achieved within 16 h and slightly declined by 24 h. The amount of VCAM-1 protein expression was increased with increasing concentrations of TNF-α (Fig. 1B). The blot was stripped and re-probed with an anti-GAPDH antibody to demonstrate equivalent amount of GAPDH expression (Fig. 1A). To determine if the effect of TNF-α on VCAM-1 expression involved at the transcriptional level, VCAM-1 mRNA was determined by RT-PCR. As shown in Figure 1C, TNF-α induced VCAM-1 mRNA accumulation in a time-dependent manner. A maximal response was obtained within 4–5 h upon TNF-α exposure.
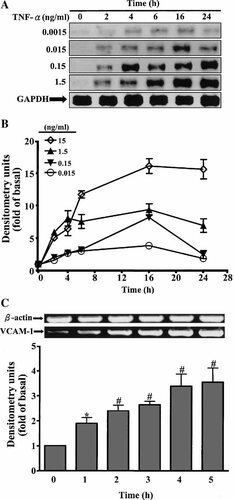
TNF-α induces VCAM-1 expression in HTSMCs. A: For VCAM-1 protein expression, HTSMCs were treated with TNF-α for the indicated times. The cell lysates were subjected to 10% SDS–PAGE and transferred to nitrocellulose membrane and then blotted using an antiserum reactive with an anti-VCAM-1 or total GAPDH (as a control) polyclonal antibody. B: Data are summarized from the time course study and expressed as mean ± SEM of four independent experiments. C: Time dependence of TNF-α-induced VCAM-1 mRNA expression, the cells were incubated with TNF-α (150 pg/ml) for the indicated times. The isolated RNA samples were analyzed by RT-PCR, using the primer specific for VCAM-1 and β-actin, as described in the text. The intensity of PCR product bands shown was quantitated by scanning densitometry and standardized to equivalent β-actin mRNA levels. Data are expressed as mean ± SEM of three independent experiments. *P < 0.05; #P < 0.01, as compared with the basal level.
TNF-α induces VCAM-1 expression via p42/p44 MAPK phosphorylation
We next investigated whether TNF-α-induced VCAM-1 expression was mediated via p42/p44 MAPK in HTSMCs. Figure 2A showed that pretreatment with MEK1/2 inhibitor U0126 (Favata et al., 1998) for 1 h prior to TNF-α exposure for 24 h caused an attenuation of VCAM-1 expression in a concentration-dependent manner. At a concentration of 10 µM, U0126 almost completely inhibited TNF-α-induced VCAM-1 expression.
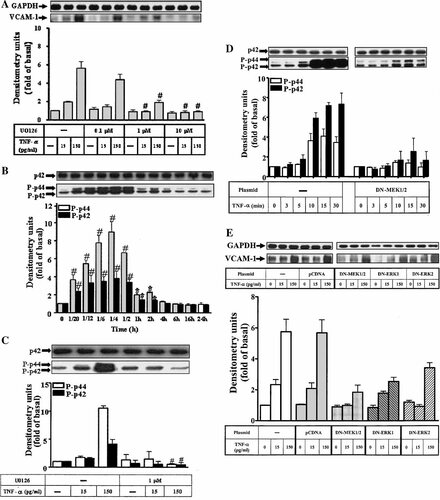
Effect of MEK1/2 inhibitor on TNF-α-stimulated VCAM-1 expression and p44/p42 MAPK phosphorylation in HTSMCs. A: Cells were preincubated with U0126 for 1 h, and then incubated with TNF-α for 24 h. B: HTSMCs were incubated with 150 pg/ml TNF-α for various times and C: pretreated with U0126 (1 µM) for 1 h and then stimulated with TNF-α for 10 min. D, E: Involvement of MEK1/2 and p42/p44 MAPK in TNF-α-induced expression of VCAM-1 in HTSMCs, cells were transfected with plasmids encoding dominant negative mutants MEK1/2 (MEK K97R), ERK2 (ERK K52R), or ERK1 for 2 h, shifted to DMEM/F-12 containing 10% FBS for 24 h, serum free for 24 h and then stimulated with TNF-α for 10 min or 24 h. After incubation, the cell lysates were subjected to 10% SDS–PAGE and transferred to nitrocellulose membrane to determine the phosphorylation of p42/p44 MAPK (D) and the level of VCAM-1 protein expression (E) as described in Figure 1. The membrane was stripped and re-probed with an anti-GAPDH or p42 antibody as an indicator of protein loading in each lane. Data are expressed as mean ± SEM of three independent experiments. *P < 0.05; #P < 0.01, as compared with the cells incubated with TNF-α alone.
To determine whether p42/p44 MAPK phosphorylation was necessary for the TNF-α-induced VCAM-1 expression, activation of these kinases was assayed by blotting with an antibody specific for the phosphorylated, active form of p42/p44 MAPK. As shown in Figure 2B, TNF-α (150 pg/ml) stimulated a transient phosphorylation of p42/p44 MAPK in HTSMCs. A maximal response was obtained within 15 min and declined to the basal level within 4 h. It is well-known that activation of p42/p44 MAPK is mediated through an upstream component of MAPK kinases, MEK1/2. Therefore, activation of p42/p44 MAPK was further investigated using a MEK1/2 inhibitor U0126. Pretreatment with U0126 significantly attenuated TNF-α-stimulated p42/p44 MAPK phosphorylation (Fig. 2C).
To ascertain that the activation of MEK1/2 and p42/p44 MAPK was required for TNF-α-induced expression of VCAM-1, HTSMCs were transfected with dominant negative mutants MEK1/2 (MEK K97R), ERK1, or ERK2 (ERK2 K52R), and then treated with TNF-α for 0–30 min or 24 h. As shown in Figure 2D–F, transfection with dominant negative mutants of MEK1/2, ERK1, or ERK2 significantly reduced p42/p44 MAPK phosphorylation and VCAM-1 expression induced by TNF-α. Taken together, these results suggested a link between activation of MEK1/2-p42/p44 MAPK pathway and VCAM-1 expression induced by TNF-α.
TNF-α induces VCAM-1 expression via p38 MAPK phosphorylation
To determine whether p38 MAPK was involved in TNF-α-induced VCAM-1 expression in HTSMCs, a p38 MAPK inhibitor, SB202190 (Singh et al., 1999) was used. As shown in Figure 3A, pretreatment with this inhibitor significantly attenuated VCAM-1 expression (approximate 53%; P < 0.05, as compared with that of cells stimulated with TNF-α alone). To ascertain that TNF-α promoted p38 MAPK phosphorylation, activation of this kinase was assayed by blotting with an antibody specific for the phosphorylated, active form of p38 MAPK. As shown in Figure 3B, TNF-α (150 pg/ml) caused a time-dependent phosphorylation of p38 MAPK with a maximal response within 10 min and declined to basal level by 6 h in HTSMCs. TNF-α-stimulated p38 MAPK phosphorylation was significantly inhibited (approximate 50%; P < 0.05, as compared with that of cells stimulated by TNF-α alone) by 30 µM SB202190 (Fig. 3C). To examine whether the activation of p38 MAPK was required for TNF-α-induced VCAM-1 expression, HTSMCs were transfected with a dominant negative mutant p38. As shown in Figure 3D,E, transfection with dominant negative mutant of p38 significantly reduced p38 MAPK phosphorylation and VCAM-1 expression induced by TNF-α. Moreover, the nuclear fraction prepared by centrifugation was also used to examine if ATF-2 phosphorylation was a downstream component of p38 activation. As shown in Figure 3F, (150 pg/ml) TNF-α-stimulated ATF-2 phosphorylation was significantly inhibited by 30 µM SB202190. These results suggested that activation of p38 MAPK was, at least in part, involved in TNF-α-induced VCAM-1 expression in HTSMCs.
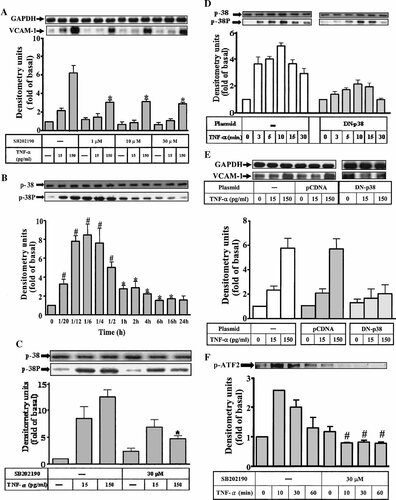
Effect of SB202190 on TNF-α-stimulated VCAM-1 expression and p38 MAPK phosphorylation. A: HTSMCs were preincubated with SB202190 for 1 h, and then incubated with TNF-α for 24 h. B: Time dependence of TNF-α-stimulated p38 MAPK phosphorylation, cells were incubated with 150 pg/ml TNF-α for various times and C: pretreated with SB202190 (30 µM) for 1 h and then stimulated with TNF-α for 10 min. D, E: Cells were transfected with plasmid encoding dominant negative mutant p38 for 2 h, shifted to DMEM/F-12 containing 10% FBS for 24 h, serum free for 24 h and then stimulated with TNF-α for 10 min or 24 h. After incubation, the cell lysates were subjected to 10% SDS–PAGE and transferred to nitrocellulose membrane to determine the phosphorylation of p38 MAPK (D) and the level of VCAM-1 protein expression (E) as described in Figure 1. F: Time dependence of TNF-α-stimulated ATF2 phosphorylation, cells were incubated with 150 pg/ml TNF-α for various times and inhibition of TNF-α-stimulated ATF2 phosphorylation by pretreatment with SB202190 (30 µM) for 1 h. The nuclear fraction prepared by centrifugation was subjected to 10% SDS–PAGE and analyzed with anti-phospho-ATF2 antibody as described. Membranes were stripped and re-probed with GAPDH or p38 as a control. Data are expressed as mean ± SEM of three independent experiments. *P < 0.05; #P < 0.01, as compared with the cells incubated with TNF-α alone.
TNF-α induces VCAM-1 expression via JNK phosphorylation
To determine whether JNK also involved in TNF-α-induced VCAM-1 expression in HTSMCs, the pharmacological inhibitor of JNK (Bennett et al., 2001) was used. As shown in Figure 4A, pretreatment of HTSMCs with SP600125 significantly blocked VCAM-1 expression (approximate 66%; P < 0.05, as compared with that of cells stimulated with TNF-α alone) in a concentration-dependent manner. The activation of JNK kinase was assayed by blotting with an antibody specific for the phosphorylated, active forms of JNK. As shown in Figure 4B, TNF-α (150 pg/ml) stimulated a time-dependent phosphorylation of JNK with a maximal response within 15 min and then declined to the basal level within 1 h in HTSMCs. Correspondingly, pretreatment of HTSMCs with SP600125 also resulted in a significant attenuation of JNK phosphorylation (P < 0.05, as compared with that of cells stimulated with TNF-α alone) (Fig. 4C). To further ascertain that the activation of JNK was required for the TNF-α-induced expression of VCAM-1, HTSMCs were transfected with a dominant negative mutant JNK and then treated with TNF-α (150 pg/ml) for 0–30 min or 24 h. As shown in Figure 4D,E, transfection with dominant negative mutant of JNK significantly reduced TNF-α induced JNK phosphorylation and VCAM-1 expression. These results suggest that activation of JNK may be involved in TNF-α-induced VCAM-1 expression.

Effect of JNK inhibitor on TNF-α-stimulated VCAM-1 expression and JNK phosphorylation in HTSMCs. A: Cells were preincubated with SP600125 for 1 h, and then incubated with TNF-α for 24 h. B: HTSMCs were incubated with 150 pg/ml TNF-α for various times and (C) pretreated with SP600125 (30 µM) for 1 h, and then stimulated with TNF-α for 10 min. D, E: Involvement of JNK1/2 in TNF-α-induced expression of VCAM-1 in HTSMCs, cells were transfected with plasmids encoding dominant negative mutants JNK for 2 h, shifted to DMEM/F-12 containing 10% FBS for 24 h, serum free for 24 h and then stimulated with TNF-α for 15 min or 24 h. After incubation, the cell lysates were subjected to 10% SDS–PAGE and transferred to nitrocellulose membrane to determine the phosphorylation of JNK1/2 (D) and the level of VCAM-1 protein expression (E) as described in Figure 1. The membrane was stripped and re-probed with an anti-GAPDH or p42 antibody as an indicator of protein loading in each lane. Data are expressed as mean ± SEM of three independent experiments. *P < 0.05; #P < 0.01, as compared with the cells incubated with TNF-α alone.
NF-κB inhibitors suppress TNF-α-induced VCAM-1 expression
Inflammatory responses following stimulation with cytokines such as TNF-α are highly dependent on activation of the NF-κB transcription factor. Moreover, NF-κB is one of the major mediators of the intracellular functions of TNF-α. In addition, TNF-α has been shown to involve in VCAM-1 gene expression through NF-κB cascade (Cybulsky et al., 1991; Barnes and Karin, 1997). Therefore, the involvement of NF-κB activation in VCAM-1 expression following stimulation with TNF-α was investigated using pharmacological inhibitors, helenalin, a specific sesquiterpene lactone compound, is known to inhibit NF-κB (Lyss et al., 1998). As shown in Figure 5A, pretreatment of HTSMCs with helenalin for 1 h prior to TNF-α exposure caused an attenuation of VCAM-1 protein expression (P < 0.05, n = 3, as compared with that of cells exposed to TNF-α alone) in a concentration-dependent manner. At the highest concentration used, helenalin almost completely inhibited VCAM-1 protein expression induced by TNF-α in HTSMCs.
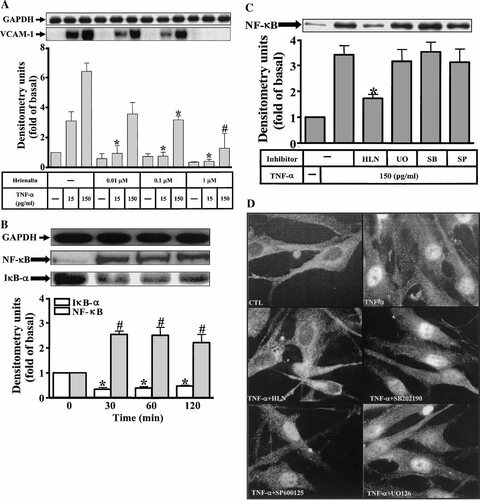
Effect of NF-κB inhibitor on TNF-α-stimulated VCAM-1 expression in HTSMCs. A: Cells were preincubated with helenalin for 1 h and then incubated with TNF-α for 24 h. The cell lysates were subjected to 10% SDS–PAGE and transferred to nitrocellulose membrane to determine the level of VCAM-1 protein expression as described. B: Time dependence of TNF-α-induced NF-κB translocation and IκB-α degradation, cells were treated with 150 pg/ml TNF-α for various times or (C) in the presence of U0126 (1 µM), SB202190 (30 µM), SP600125 (10 µM), and helenalin (1 µM) for 1 h and then treated with TNF-α for 30 min, cells were harvested and centrifuged to prepare cytosolic and nuclear fractions. The resultant fractions were subjected to 10% SDS–PAGE and analyzed with anti-NF-κB (p65) or anti-IκB-α antibody as described. D: Nuclear translocation of NF-κB determined by immunofluorescence staining, HTSMCs were pretreated with or without U0126 (1 µM), SB202190 (30 µM), SP600125 (10 µM), or helenalin (1 µM) for 1 h and then were stimulated with 150 pg/ml TNF-α for 30 min. Cells were fixed and labeled with anti-NF-κB (p65) antibody and an FITC-conjugated secondary antibody. Individual cells were imaged as described in Methods. Figure represents one of three similar experiments.
It has been shown that cells activated by cytokines leads to the degradation of IκB-α, accompanied by NF-κB translocation into the nucleus. To determine if TNF-α induces IκB-α degradation and NF-κB translocation, the cells were stimulated by 150 pg/ml TNF-α for the indicated times. The cytosolic and nuclear fractions were then used for determination of IκB-α degradation and NF-κB translocation, respectively. As shown in Figure 5B, TNF-α stimulated a rapid translocation of NF-κB (p65) into nucleus and degradation of IκB-α, the effect was sustained for over 120 min.
Since activation of p42/p44 MAPK, p38, JNK, and NF-κB translocation are necessary for the VCAM-1 protein expression in HTSMCs induced by TNF-α, it would be important to differentiate whether phosphorylation of these MAPKs was associated with NF-κB activation. To examine this possibility, activation of NF-κB was assessed following TNF-α stimulation in the presence of inhibitors for MEK1/2, p38, JNK, and helenalin, respectively. TNF-α-stimulated activation of NF-κB was significantly inhibited by preincubation with helenalin (Fig. 5C), but not by U0126, SB202190, and SP600125. The transloaction of NF-κB in TNF-α-stimulated HTSMCs was further confirmed by immunofluorescence staining. As shown in Figure 5D, TNF-α-induced NF-κB translocation was blocked by helenalin, but not by the pharmacological inhibitors of these MAPKs. Taken together, these results demonstrated that TNF-α-stimulated NF-κB translocation was essential for VCAM-1 up-regulation and independent on activation of p42/p44 MAPK, p38 MAPK, and JNK in HTSMCs.
MAPKs and NF-κB are required for TNF-α-induced VCAM-1 mRNA expression
The possible regulation of VCAM-1 mRNA transcription by these kinases was also investigated. As shown in Figure 6A, pretreatment of HTSMCs with inhibitors of MEK1/2 (U0126), p38 (SB202190), JNK (SP600125), and NF-κB (helenalin) significantly attenuated TNF-α-induced VCAM-1 mRNA accumulation assessed by RT-PCR. These results further indicated that in HTSMCs, regulation of VCAM-1 expression through activation of p42/p44 MAPK, p38, JNK, and NF-κB occurred mainly at the transcriptional level. To rule out the observed effect was due to a change in mRNA stability, cells were treated with TNF-α (150 pg/ml) for 4 h to induce VCAM-1 transcription, and then the transcription was stopped by addition of U0126 (1 µM), SB202190 (30 µM), and SP600125 (10 µM). Figure 6B showed that the stability of VCAM-1 mRNA was not significantly changed by these drugs. To further insure that TNF-α-induced VCAM-1 mRNA expression required ongoing transcription and translation, as shown in Figure 7, pretreatment of HTSMCs with a transcriptional inhibitor (actinomycin D) or a translational inhibitor (cycloheximide) almost completely blocked TNF-α-induced VCAM-1 expression.
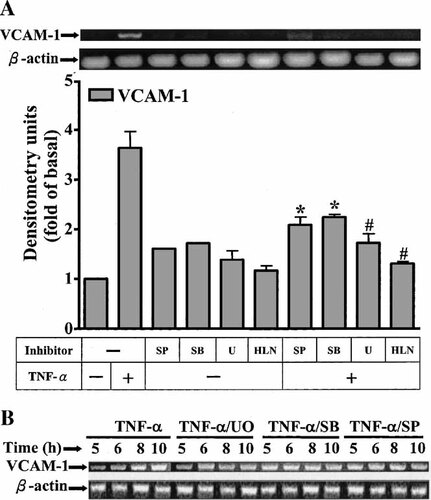
Involvement of MAPKs and NF-κB in VCAM-1 mRNA expression stimulated by TNF-α. A: HTSMCs were treated with U0126 (1 µM), SB202190 (30 µM), SP600125 (10 µM), or helenalin (1 µM) for 1 h and then incubated with 150 pg/ml TNF-α for 2 h. B: To determine the effect of MAPK inhibitors on the stability of VCAM-1 mRNA induced by TNF-α, cells were treated with TNF-α for 4 h and then added U0126 (1 µM), SB202190 (30 µM), SP600125 (10 µM) for 1, 2, 4, and 6 h. VCAM-1 mRNA was determined as described in Figure 1. Data are expressed as mean ± SEM of three independent experiments. *P < 0.05; #P < 0.01, as compared with the cells incubated with TNF-α alone.
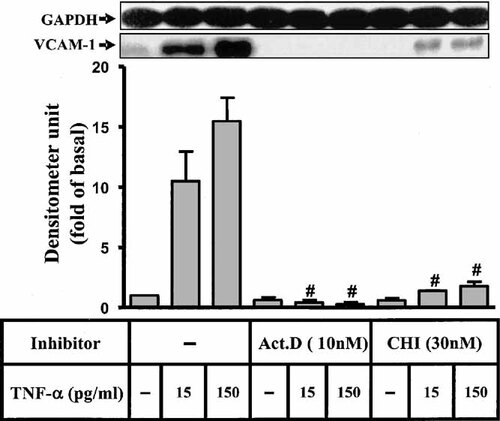
TNF-α-induced VCAM-1 mRNA expression requires ongoing transcription and translation. HTSMCs were preincubated with 10 nM of (A) actinomycin D (Act. D) and 30 nM of (B) cycloheximide (CHI) for 1 h and then incubated with TNF-α for 24 h. The cell lysates were subjected to 10% SDS–PAGE and transferred to nitrocellulose membrane to determine the level of VCAM-1 protein expression as described in Figure 1. Data are expressed as mean ± SEM of three independent experiments. #P < 0.01, as compared with the cells incubated with TNF-α alone.
TNF-α-induced VCAM-1-luc promoter activity requires MAPKs and NF-κB
The regulation VCAM-1 gene transcription by MAPKs and NF-κB pathways was further confirmed by gene luciferase activity assay. VCAM-1 luciferase reporter gene was transfected into HTSMCs, which were then stimulated with TNF-α (150 pg/ml). Figure 8A showed that TNF-α stimulated VCAM-1-luciferase activity within 4 h and sustained for over 6 h. Moreover, TNF-α-stimulated activation of VCAM-1 luciferase activity was significantly inhibited by pretreatment with helenalin, U0126, SB202190, and SP600125 (Fig. 8B). These data suggest that TNF-α-stimulated VCAM-1 luciferase gene activity involved at a transcription level mediated through activation of MAPKs and NF-κB pathways.
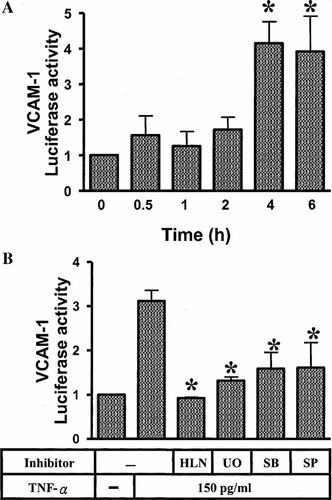
Involvement of MAPKs and NF-κB pathways in TNF-α-induced VCAM-1-luc promoter activity. HTSMCs were transiently transfected with VCAM-1-luc reporter gene and then treated with 150 pg/ml TNF-α for various times (A), and pretreated with U0126 (1 µM), SB202190 (30 µM), SP600125 (10 µM), or helenalin (1 µM) for 1 h and then stimulated with 150 pg/ml TNF-α for 4 h (B). Luciferase activity was determined in the cell lysates as described. Results are presented as means ± SEM from three separate experiments. *P < 0.05; #P < 0.01, as compared with the cells incubated with TNF-α alone.
In vivo association of p300 and NF-κB with the VCAM-1 promoter in TNF-α-stimulated HTSMCs
Curcumin, a selective p300 inhibitor, specifically represses the p300/CBP HAT activity-dependent transcriptional activation from chromatin and then inhibits the acetylation of HIV-Tat protein in vitro by p300 as well as proliferation of the virus (Balasubramanyam et al., 2004). Most surprisingly, TNF-α-induced VCAM-1 expression was significantly inhibited by preincubation with curcumin (Fig. 9A), suggesting that p300 may involve in VCAM-1 promoter regulation. To further investigate the roles of transcription factors and the VCAM-1 promoter regulatory elements in TNF-α-induced VCAM-1 transcription, in vivo association of these transcription factors with the VCAM-1 promoter was evaluated by the ChIP assay. To determine whether the transcription factors could specifically associate with the VCAM-1 promoter, PCR amplifications were conducted on an equal amount of immunoprecipitated DNA, followed by 45 cycles of PCR with the specific primer pairs encompassing position −403 to −30 region of the human VCAM-1 promoter, which contained NF-κB binding sites (Fig. 9). After TNF-α treatment, an enrichment of p65-, p300-, and histone H3-associated VCAM-1 promoter DNA appeared at 30 min and sustained up to 2 h, respectively, as compared to the nonimmune IgG immunoprecipitated control (Fig. 9B). These data suggested that NF-κB (p65), p300, and histone H3 involved in TNF-α-induced VCAM-1 transcription in HTSMCs.
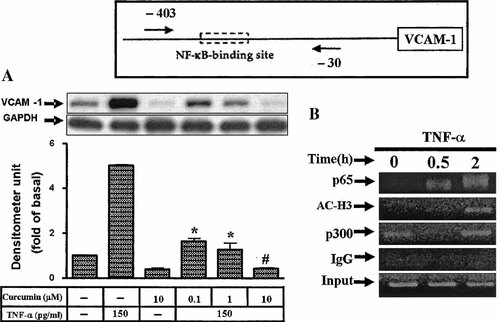
Effect of TNF-α on the in vivo DNA association of transcription factors at the VCAM-1 promoter. A: Cells were preincubated with cucurmin for 1 h and then incubated with TNF-α for 24 h. The cell lysates were subjected to 10% SDS–PAGE and transferred to nitrocellulose membrane to determine the level of VCAM-1 protein expression as described in Figure 1. Data are expressed as mean ± SEM of three independent experiments. *P < 0.05; #P < 0.01, as compared with the cells incubated with TNF-α alone. B: Confluent and serum-starved HTSMCs in 100-mm dishes were incubated with TNF-α(30 ng/ml) for the times indicated. The in vivo protein-DNA complexes were cross-linked by formaldehyde treatment and chromatin pellets were extracted and sonicated. The associated VCAM-1 promoter DNA was amplified by PCR as described in Materials and Methods. The sequence of the human VCAM-1 promoter region (−403/−30) amplified by PCR primer pairs indicated by the arrows. An enrichment of the VCAM-1 promoter DNA is shown after PCR amplification of immunoprecipitates of p65-, p300-, and histone H3-associated DNA from cells treated with TNF-α for 2 h. The input represents PCR products from chromatin pellets prior to immunoprecipitation. The results are representatives of two independent experiments with similar results.
Adhesion of neutrophils on HTSMCs treated with TNF-α
TNF-α has been recognized as a potent proinflammatory mediator that increases the expression of adhesion molecules (Ballestas and Benveniste, 1995; Barton et al., 1995) and the adhesiveness between leukocytes and several cell types (Butcher, 1991). To test the functional activity of VCAM-1 expressed on HTSMCs, we assessed the ability of purified PMNs adhering to TNF-α-stimulated HTSMCs. As shown in Figure 10, the amount of PMN adhesion to HTSMCs monolayer was significantly increased (approximate 15-folds) by the incubation with 150 pg/ml TNF-α for 24 h (P < 0.05, n = 3, as compared with the basal level). The enhanced adhesion was attenuated by pretreatment with 1 µM U0126, 10 µM SP600125, 30 µM SB202190 or 1 µM helenalin prior to TNF-α exposure. To further determine the surface molecules responsible for PMNs adhesion to HTSMCs monolayers, PMNs adhesion was assessed in the presence of antibodies to VCAM-1 and ICAM-1. PMNs adhesion to TNF-α-stimulated HTSMCs monolayers was significantly inhibited by preincubation wth these adhesion molecule antibodies (data not shown).
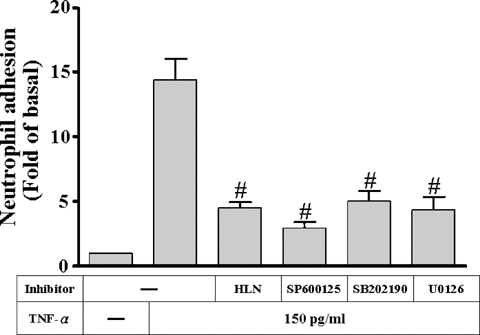
Inhibition of MAPKs and NF-κB prevents TNF-α-induced HTSMCs adhesivity. HTSMCs were pretreated with 1 µM U0126, 10 µM SP600125, 30 µM SB202190, or 1 µM helenalin for 1 h and then incubated with 150 pg/ml TNF-α for 24 h or HTSMCs were incubated with anti-VCAM-1 antibody for 1 h prior to addition of polymorphonuclear (PMN) cells. PMN adhesion assay was performed as described in “Materials and Methods.” Data are expressed as mean ± SEM of three separate experiments. #P < 0.01, as compared with the cells incubated with TNF-α alone.
DISCUSSION
Up-regulation of adhesion molecules on the surface of the airway epithelium may play a key role in recruitment and infiltration of PMN at sites of inflammation in airways (Kelley, 1990; Georas et al., 1992). TNF-α has been confirmed to induce the expression of VCAM-1 in lung epithelial cells, but little is known about the intracellular signaling pathways leading to its expression, except NF-κB (Chen et al., 2001). TNF-α has also been shown to activate all of three MAPK pathways in several cell types (McEwan, 2002; Pfeffer, 2003). However, in HTSMCs, whether TNF-α-induced VCAM-1 expression mediated through the activation of MAPKs and NF-κB was still unknown. In this study, TNF-α-induced VCAM-1 expression was significantly attenuated by the inhibitors of MEK1/2 (U0126), p38 (SB202190), JNK (SP600125), and NF-κB (helenalin). Furthermore, activation of NF-κB was inhibited by helenalin, but not by U0126, SB202190, and SP600125. Our results established that the mechanisms underlying activation of p42/p44 MAPK, p38, and JNK as well as the translocation of NF-κB by TNF-α may be essential for up-regulation of VCAM-1.
In this study, our results demonstrated that activation of MAPKs was necessary for TNF-α-induced VCAM-1 expression in HTSMCs. U0126, a selective inhibitor of MEK1/2 (Favata et al., 1998) attenuated TNF-α-induced VCAM-1 expression and p42/p44 MAPK phosphorylation in HTSMCs. In addition, transfection with dominant negative mutants of MEK1/2 (K97R), ERK1, or ERK2 (K52R) significantly reduced VCAM-1 expression and p42/p44 MAPK phosphorylation induced by TNF-α, suggesting that activation of MEK1/2 and p42/p44 MAPK was required for VCAM-1 expression. These results were consistent with those of TNF-α-induced expression of ICAM-1 in astrocytes (Ballestas and Benveniste, 1995). In contrast, TNF-α-induced adhesion molecule expression is not mediated through p42/p44 MAPK activation in A549 (Chen et al., 2001). This difference between these studies may be due to the various experimental conditions.
The involvement of p38 MAPK in TNF-α-induced VCAM-1 expression was also investigated using SB202190, a selective inhibitor of p38 MAPK (Singh et al., 1999) and transfecting with a dominant negative mutant p38. In HTSMCs, pretreatment with SB202190 and transfection with dominant negative mutant p38 significantly blocked p38 MAPK phosphorylation and VCAM-1 expression induced by TNF-α. These results indicated that p38 MAPK was involved in the TNF-α-induced VCAM-1 expression, consistent with the expression of VCAM-1 and ICAM-1 in cardiac cells (Kacimi et al., 1998) and in chondrosarcoma cells (Ju et al., 2002).
Next, we investigated the involvement of JNK in TNF-α-induced VCAM-1 expression using a selective JNK inhibitor SP600125 (Bennett et al., 2001). Pretreatment with SP600125 or transfection with dominant negative mutant JNK attenuated TNF-α-induced VCAM-1 expression and JNK phosphorylation, consistent with reports that activation of JNK is essential for the up-regulation of ICAM-1 and VCAM-1 in various cell types (De Cesaris et al., 1999; Szlosarek and Balkwill, 2003). Taken together, TNF-α-induced VCAM-1 expression was mediated through p42/p44 MAPK, p38 MAPK, and JNK pathways in HTSMCs
It has been known that inflammatory responses following exposure to cytokines are highly dependent on activation of NF-κB and lead to gene expression (Ghosh et al., 1998; Bian et al., 2001; Lin et al., 2004). In our study, the involvement of NF-κB in TNF-α-induced VCAM-1 expression was revealed by a selective NF-κB inhibitor, helenalin. Pretreatment with helenalin significantly attenuated TNF-α-induced VCAM-1 expression and NF-κB translocation as well as degradation of IκB-α. These results are consistent with those of reports by Chen et al. (2001) that TNF-α-induced ICAM-1 expression is mediated through NF-κB activation. Interestingly, activation of p42/p44 MAPK, p38, and JNK as well as NF-κB were required for TNF-α-induced VCAM-1 expression in HTSMCs. However, whether the activation of these three MAPKs led to NF-κB translocation and finally resulting in VCAM-1 gene expression remains unknown in HTSMCs. It has been shown that MEKK1 induces activation of IKK-α and IKK-β leading to NF-κB activation (Barnes and Karin, 1997). Thus, p42/p44 MAPK activation may be required for NF-κB activation stimulated by TNF-α. Our results demonstrated that TNF-α-stimulated NF-κB activation was not significantly inhibited by U0126, SB202190, and SP600125, indicating that MAPKs and NF-κB independently regulated VCAM-1 expression in HTSMCs. These results suggested that TNF-α-induced VCAM-1 expression was independently mediated through the activation of NF-κB and of p42/p44 MAPK, p38, and JNK pathways or these MAPKs pathways may converge at a step downstream of NF-κB transactivation. Moreover, our results showed that pretreatment with helenalin, U0126, SB202190, and SP600125 significantly attenuated VCAM-1 luciferase gene activity stimulated by TNF-α, suggesting that activation of MAPKs and NF-κB pathways regulated VCAM-1 expression at a transcription level.
In HTSMCs, regulation of COX-2 gene expression is mediated through NF-κB activation by different stimuli through different promoter elements and transcription factors, which are associated with chromatin remodeling after selective histone H4 acetylation in a stimulus-specific manner (Nie et al., 2003). Chromatin remodeling after p300/CBP associated with histone acetylation is believed to participate for active transcription of pro-inflammatory genes upon stimulation by inflammatory mediators. In this study, VCAM-1 expression was significantly inhibited by curcumin, a selective p300 inhibitor (Balasubramanyam et al., 2004). In this study, an enrichment of NF-κB p65-, p300-, and histone H3-associated VCAM-1 promoter (containing NF-κB binding sites) DNA complexes appeared in TNF-α-treated HTSMCs. These data suggested that acetylation of histone H3 by p300 may induce chromatin remodeling and then promote NF-κB binding to VCAM-1 promoter. However, whether activation of p300 mediated through MAPK or Akt remains to be investigated in the future.
To our knowledge, this study is the first that involvement of MAPKs, NF-κB, and p300 in TNF-α-induced VCAM-1 expression is observed in HTSMCs. Activation of these three MAPKs and NF-κB translocation were involved in TNF-α-induced VCAM-1 expression. Finally, association of NF-κB p65-, p300-, and histone H3 led to VCAM-1 gene transcription. Based on the observations from the literatures and our findings, a schematic pathway depicts a model for the roles of p42/p44 MAPK, p38, JNK, and NF-κB activation associated with TNF-α-induced VCAM-1 expression in HTSMCs (Scheme 1). The mechanisms by which TNF-α induced VCAM-1 expression may be an important link in the pathogenesis of airway inflammatory diseases. Therefore, understanding the mechanisms underlying TNF-α-induced VCAM-1 expression in HTSMCs is important to develop new therapeutic strategies.
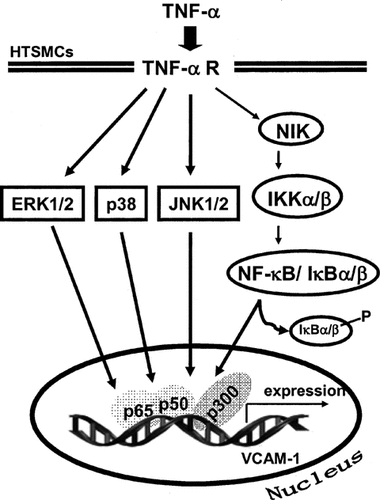
Schematic representation of the signaling pathways involved in the TNF-α-induced VCAM-1 expression in HTSMCs. Binding of the TNF-α to its receptor results in activation of MAPKs pathways, NF-κB and p300. Subsequently, VCAM-1 transcription is independently initiated by these MAPKs and NF-κB pathways, but may be regulating by p300. These signaling pathways might enforce each of the signaling pathways and contribute to sustained activation of transcription factors required for VCAM-1 expression.
Acknowledgements
The authors appreciate Dr. K.L. Guan (Department of Biological Chemistry, University of Michigan, MI, USA), Dr. M.H. Cobb (Department of Pharmacology, University of Texas Southwestern Medical Center, TX, USA), Dr. J. Han (The Scripps Research Institute, La Jolla, CA, USA), and Dr. W.C. Aird (Department of Molecular Medicine, Beth Israel Deaconess Medical Center, Boston, MA, USA) for providing dominant negative mutants MEK1/2 (MEK K97R), ERK2 (ERK2 K52R), p38 MAPK, and VCAM-1-luc constructs, respectively. The authors also thank Dr. J.K. Chen (Department of Physiology and Pharmacology, Chang Gung University) for critical reading and invaluable suggestions in this study.




