MHC class I antigens, immune surveillance, and tumor immune escape
Abstract
Oncogenic transformation in human and experimental animals is not necessarily followed by the appearance of a tumor mass. The immune system of the host can recognize tumor antigens by the presentation of small antigenic peptides to the receptor of cytotoxic T-lymphocytes (CTLs) and reject the nascent tumor. However, cancer cells can sometimes escape these specific T-cell immune responses in the course of somatic (genetic and phenotypic) clonal evolution. Among the tumor immune escape mechanisms described to date, the alterations in the expression of major histocompatibility complex (MHC) molecules play a crucial step in tumor development due to the role of MHC antigens in antigen presentation to T-lymphocytes and the regulation of natural killer cell (NK) cell function. In this work, we have (1) updated information on the mechanisms that allow CTLs to recognize tumor antigens after antigen processing by transformed cells, (2) described the altered MHC class I phenotypes that are commonly found in human tumors, (3) summarized the molecular mechanisms responsible for MHC class I alteration in human tumors, (4) provided evidence that these altered human leukocyte antigens (HLA) class I phenotypes are detectable as result of a T-cell immunoselection of HLA class I-deficient variants by an immunecompetent host, and (5) presented data indicating the MHC class I phenotype and the immunogenicity of experimental metastatic tumors change drastically when tumors develop in immunodeficient mice. © 2003 Wiley-Liss, Inc.
Abbreviations:
MHC, major histocompatibility complex; CTL, cytotoxic T-lymphocyte; NK, natural killer cell; TAP, transporter associated antigen processing; LMP, low molecular mass polypeptide; TCR, T-cell receptor; KIR, killer cell inhibitory receptor; HLA, human leukocyte antigens; LOH, loss of heterozygosity; IFN, interferon; APM, antigen processing machinery; SCID, severe combined immunodeficiency; HC, heavy chain.
Evidence has accumulated in recent years indicating that tumors appear and develop despite an active and sometimes efficient immune response. The appearance of a particular tumor is not due to the existence of a deteriorated immune system, at least in early stages of tumor development, but rather to the acquisition by the cancer cells of new genetic and phenotypic characteristics that allow them to escape anti-tumor immune responses (Mareel et al., 1990; Seymour et al., 1999; Villunger and Strasser, 1999). Somatic evolution of genetically unstable tumor cells leads to the development of sophisticated immune escape variants in primary tumor lesions that are selected out by T-lymphocyte responses. As a result, different altered tumor phenotypes are produced (Marincola et al., 2000; Garrido and Algarra, 2001; Seliger et al., 2002). These altered tumor phenotypes drastically affect the recognition of tumor antigens, and therefore, immune escape variants appear in the primary tumors and later in metastatic lesions. The combination of somatic evolution versus immune selection in cancer development is a modern view of the clonal expansion of tumor cells, which may also provide an explanation for why the escapee tumor phenotype is a crucial step in the natural evolution of human and experimental cancers.
This review will concentrate on describing the antigen processing machinery (APM) that allows tumor antigens to be recognized by T-lymphocytes, and on the generation of tumor immune escape variants that are frequently found in a variety of human tumors. In particular, we will analyze the major histocompatibility complex (MHC) class I-altered profiles, which allow tumor cells to blind T-lymphocyte immune responses because of the role that these MHC molecules play in antigen presentation. Any alteration affecting the expression of MHC class I molecules on tumors will have a profound effect on the recognition of tumor antigen peptides by T-lymphocytes. We will also update the current knowledge about the structure and function of the antigen processing machinery of tumor cells, which produces a functional MHC class I-tumor peptide complex. Additional evidence will be provided indicating that T-lymphocytes are responsible for selecting the MHC class I-negative or -deficient tumors by destroying the antigenic variants and in some way “favoring” the appearance of the MHC class I tumor escape variants.
ROLE OF MHC CLASS I MOLECULES IN ANTIGEN PRESENTATION TO T-CELLS AND INTERACTION WITH NATURAL KILLER (NK) CELLS
The molecular mechanisms involved in the recognition of tumor cells by cytotoxic T-lymphocytes (CTLs) and NK cells have been partially elucidated in recent years (Boon et al., 1994; Trowsdale, 2001). The class I molecules encoded by the MHC are cell surface glycoproteins that play a fundamental role in the regulation of immune responses. MHC class I molecules are necessary for the presentation of peptide antigens to CTLs (Townsend et al., 1986) and for the immune regulatory activity exerted by NK cells (Ljunggren and Kärre, 1990).
MHC class I molecules comprise the classical (class Ia) human leukocyte antigens (HLA)-A, -B, and -C antigens in humans and H-2K, D, and L in mice, and the nonclassical (class Ib) E, F, and G, in humans and Qa and Tla antigens in mice (Bjorkman et al., 1987). They form a trimolecular complex consisting of a 45-kDa heavy chain (HC), peptide antigen, and the nonpolymorphic 12-kDa β2-microglobulin (β2-m) light chain. The HLA-A, -B, and -C and the H-2K, D, and L HCs are highly polymorphic (Bjorkman and Parham, 1990). In humans, the class I HCs are encoded by genes located within the MHC region on chromosome 6, whereas β2-m is encoded by a gene mapped on chromosome 15. In mice, these antigens are encoded in chromosome 17 and chromosome 2, respectively. The classical HLA/H-2 class I molecules are expressed on the surface of most mammalian cells with only a few exceptions (Le Bouteiller, 1994). It is estimated that there are up to 250,000 of each HLA class I molecule on the surface of a somatic cell (Parham and Ohta, 1996).
Most glandular or squamous epithelia, as well as the surrounding connective tissue, express MHC class I antigens. However, the intensity of expression varies between different locations. For instance, skeletal smooth muscle and gastric mucosa are weakly positive (Ferrón et al., 1989; Fernandez et al., 1991), whereas the central and peripheral nervous system are considered negative (Daar et al., 1984). The nonclassical MHC class I molecules have a much more limited degree of polymorphism and a more restricted tissue distribution (Braud et al., 1999).
MHC class I molecules bind antigens in the form of peptides that are presented to CTLs on the surface of tumor or virus-infected cells. These antigenic peptides are generated from degraded endogenous proteins (tumor-associated antigens (TAAs) in tumor cells) by the APM. This process, known as antigen processing (Fig. 1), is carried out by a large, multicatalytic protease complex called the proteasome (Maffei et al., 1997; Van Endert, 1999). Their different subunits, i.e., low-molecular-mass polypeptides (LMPs), are involved in the efficiency of proteosome functions to produce antigenic peptides capable of binding MHC class I molecules. These peptides are transported into the lumen of the endoplasmatic reticulum (ER) by the transporter associated with antigen processing-1 and -2 (TAP1 and TAP2) (Abele and Tampé, 1999). The folding and assembly of a complete MHC class I molecule depends on the association of the HC first with β2-m and then with the peptide in the ER. This process involves a number of accessory proteins with a chaperone-like function, such as calnexin, calreticulin, Erp57 (endoplasmic reticulum glycoprotein 57), and tapasin (Fig. 1) (Grandea and Van Kaer, 2001). The exit of the trimolecular complex from the ER through the Golgi secretory pathway and its display on the cell surface are referred to as antigen presentation. The correct functioning of these antigen processing and presentation machinery components gives rise to cells with normal surface expression of the MHC class I molecules (Koopman et al., 1997; Parmer and Cresswell, 1998). Any defect in these processes will lead to nonexpression of MHC class I molecules on the cell surface.
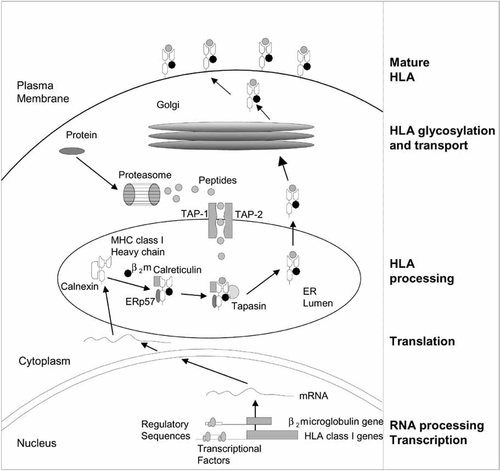
The antigen processing machinery (APM). Endogenous proteins are degraded by the multicatalytic protease complex (the proteosome) to peptides of 9 amino acids. These peptides are transported to the endoplasmatic reticulum (ER) by the transporter associated antigen processing (TAP) transporter and associated with some major histocompatibility complex (MHC) class I molecules to be exported to the cell membrane for surveillance by T-lymphocytes.
MHC class I-bearing cells are essential structures that interact with the corresponding recognition molecule, the T-cell receptor (TCR), on CD8+ CTL cells (Fig. 2). This interaction triggers a cascade of T-signaling events that ultimately lead to cell proliferation, cytokine production, and target cell lysis (Johnsen et al., 1999; Seliger et al., 2000). MHC class I antigens also regulate the lytic activity of NK immune cells, which is related to their ability to kill target cells lacking MHC class I expression (Fig. 2). Although NK cells do not express TCR receptors, the detection of targets by NK cells is mediated by two major families of receptor molecules belonging to the killer immunoglobulin-like receptor (KIR) and to the C-type lectin superfamily. These receptors have different specificities and can be divided into two groups of inhibiting or activating receptors (Moretta et al., 1996; Lopez-Botet et al., 2000).
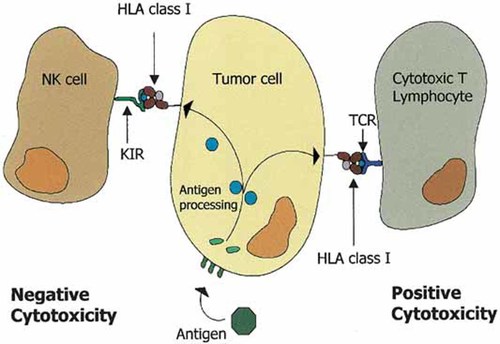
MHC class I-peptide interactions with T-lymphocytes and natural killer (NK) cells. T-lymphocytes recognize tumor antigens as small peptides presented by a particular MHC class I molecule. This recognition by the specific T-cell receptor activates T-lymphocytes to specifically kill the tumor target (right side). In contrast, the same MHC class I molecule will inhibit the NK cell by interacting with NK inhibitory receptors (left side). Only the MHC class-I negative variants will be suitable targets for NK cells.
It appears evident that any alteration in the expression of any of the MHC class I subunits can affect normal MHC cell surface expression and alter both T and NK cell-mediated immunity. In human as well as experimental tumors, such alterations may affect the tumorigenic phenotype as well as metastatic capacity (Villunger and Strasser, 1999; Garrido and Algarra, 2001).
ESCAPE OF TUMOR CELLS FROM THE IMMUNE RESPONSE: LOSS OF MHC CLASS I ANTIGENS
The loss of MHC class I molecules is a frequent mechanism in experimental and spontaneous tumors to escape recognition and destruction by CTLs (Garrido et al., 1993, 1997; Hicklin et al., 1999) (Fig. 3). According to the “missing self” hypothesis (Ljunggren and Kärre, 1990), these losses should make tumor cells more susceptible to NK immune effector mechanisms, since NK effector cells monitor MHC class I cell surface expression through their specific receptor (KIRs), and eliminate those cells with downregulated HLA/H-2 class I molecules (Moretta et al., 1996).
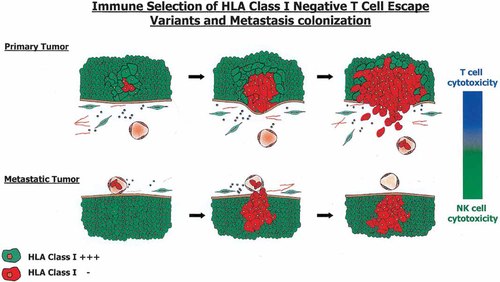
Natural history of MHC antigens during tumor development. Primary tumors originate from MHC class I-positive epithelia (green). Human leukocyte antigens (HLA) class I-negative or-deficient variants that appear during tumor development (red) escape T-cell recognition, invade, and start to metastasize. The metastatic colonies are composed of homogeneous MHC class I-negative tumor cell variants.
However, despite an active and a priori normal immune response in a healthy immune system, tumor cells grow, invade, and metastasize in the host (Klein et al., 1960; Boon et al., 1994). Data obtained in different laboratories in the past 20 years indicate that this is due in many instances to a highly sophisticated and not yet well understood selection of MHC class I-deficient tumor escape variants (Festenstein and Garrido, 1987; Garrido et al., 1997). This escape strategy used widely by tumor cells allows them to behave as stealth targets to immune effectors (Ljunggren and Kärre, 1990). This is not surprising, since MHC genes control the synthesis of molecules that are at the center of the immune function mediated by T-lymphocytes and NK cells.
The loss of an MHC antigen associated with the H-2Kk class I molecule was first described in 1976 in a mouse lymphoma, and the detection of HLA losses in human tumors followed in 1977 (Garrido et al., 1976; Pellegrino et al., 1977). In subsequent years, an increasing proportion of tumors was found to have such alterations (Garrido et al., 1993; Hicklin et al., 1999).
Total or selective losses of HLA class I antigens have been reported in different human tumor samples (Fig. 4) (Garrido et al., 1993). The frequency of such losses has been evaluated by studying series of tumor samples with immunohistological techniques or by flow cytometry in disrupted tumor cell suspensions using monoclonal antibodies (mAbs) directed against HLA class I monomorphic, HLA-A or -B locus-specific or HLA allelic epitopes (Garrido et al., 1997; Koopman et al., 2000). The rates of HLA class I losses in some tumors are close to 100%, being reported as 96% in cervical carcinomas (Koopman et al., 2000), 96% in breast carcinomas (Cabrera et al., 1996), 87% in colorectal carcinomas (Cabrera et al., 1998), and 70% in laryngeal carcinomas (Cabrera et al., 2000).
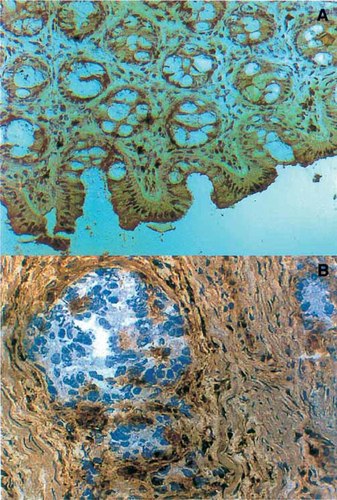
Expression of HLA antigens in normal colon mucosa and a colorectal carcinoma. Normal colon mucosa reacts with a monoclonal antibody (mAb) directed against a monomorphic determinant of HLA class I molecules with an immunoperoxidase technique (A). The malignant cells of a colorectal carcinoma are HLA class I-negative (Phenotype I), whereas the surrounding stroma is positive (B).
It is, therefore, important to define the MHC class I deficiencies of tumor cells precisely, since heterogeneous populations of tumor cells exist in tumor tissues. Our laboratory has been developing new strategies and extensively analyzing the MHC altered phenotypes found in a variety of human tumors (Cabrera et al., 2003). The description of these MHC altered phenotypes (Garrido and Algarra, 2001) has proved to be useful in establishing additional strategies to analyze the mechanisms responsible for such alterations.
-
Phenotype I: HLA class I total loss. This phenotype is characterized by the absence of any HLA class I antigen expression in tumor cells and is present to a different extent in different tumors.
-
Phenotype II: HLA haplotype loss. Tumors can partially or entirely lose one of the two HLA haplotypes present in the tumor cell.
-
Phenotype III: HLA A, B, or C locus product downregulation. This altered phenotype is found when both products of HLA A, B, or C loci are coordinately downregulated. Loss of class I locus expression has been documented in several tumors.
-
Phenotype IV: HLA allelic loss. This alteration is defined as the loss of a single HLA class I allele. The use of anti-HLA class I mAbs that define individual HLA class I alleles is required to establish this diagnosis in tissues.
-
Phenotype V: Compound phenotypes. To produce this phenotype a combination of two different alterations is required, for instance, an HLA haplotype loss and an HLA B–C locus downregulation (a combination of Phenotypes II and III.). The final result is a tumor cell expressing only one HLA class I allele.
-
Phenotype VI: Unresponsiveness to interferons. Some tumor cells express basal levels of HLA class I antigens but have lost the capacity to upregulate these molecules in response to different cytokines, including α and γ interferons.
-
Phenotype VII: Downregulation of classical HLA A–B–C molecules and appearance of HLA-E molecules.
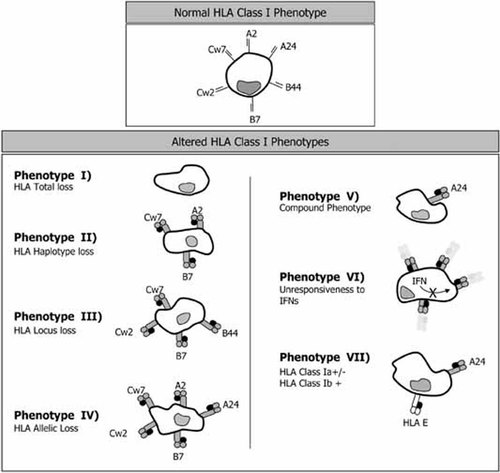
Normal and altered HLA class I phenotypes found in human tumors. Normal cells express 6 HLA class I alleles (2 HLA-A, 2 HLA-B and 2, HLA-C). HLA molecules can be totally or partially absent from tumor cells (Phenotype I–Phenotype V). In addition, tumor cells may not respond to INFs (Phenotype VI), or may also express aberrant HLA-E molecules in cells with low expression of HLA A, B, or C classical class I antigens (Phenotype VII).
MOLECULAR MECHANISMS LEADING TO MHC CLASS I LOSSES
Different mechanisms can lead to the total or partial loss of HLA expression. These MHC losses can be produced at any step required for HLA synthesis, assembly, transport, or expression on the cell surface (Garrido et al., 1997; Ruiz Cabello and Garrido, 1998). Indeed, reports have implicated as major mechanisms in these defects (1) the presence of HC and β2-m gene mutations (D'Urso et al., 1991; Browning et al., 1996; Benitez et al., 1998; Pérez et al., 1999), (2) alterations in regulatory factors induced by the cis–trans mechanism (Blanchet et al., 1991), (3) alterations in glycosylation and transport (Cromme et al., 1994; Johnsen et al., 1999), and (4) HLA gene deletions associated with loss of heterozygosity (LOH, Torres et al., 1996).
A variety of tumor cell lines derived from melanoma, colon, head–neck, and breast tumors have been used to define the molecular mechanisms responsible for MHC class I alterations (Garrido et al., 1993).These mechanisms are summarized in Table I. Additional analyses of the different HLA class I altered phenotypes commonly found in tumor tissues and described in the previous section are also shown (Garrido et al., 1997; Ruiz-Cabello and Garrido, 1998).
| MHC phenotype | Mechanisms | References |
|---|---|---|
| Phenotype I | Defects in β2-m synthesis and mutations |
Benitez et al. (1998) |
| Loss or downregulation of APM components (LMP or TAP) |
Seliger et al. (2000) |
|
| Impaired transcriptional factor |
Sanda et al. (1995) |
|
| MHC class I genes hypermethylation |
Serrano et al. (2001) |
|
| Phenotype II | LOH, gene loss at 6p21 |
Torres et al. (1996) |
| Phenotype III | Regulatory defect (transcriptional activator or suppressors) |
Méndez et al. (2001) |
| Phenotype IV | HLA class I gene mutations | |
| Somatic recombination within genes |
Browning et al. (1996) |
|
| Nonsense mutations |
Koopman et al. (2000) |
|
| Missense mutations |
Wang et al. (1999) |
|
| Deletions and insertions |
Koopman et al. (2000) |
|
| Phenotype V | LOH at 6p21 + downregulation of A-, B-locus | |
| LOH at 6p21 + mutation |
Koopman et al. (2000) |
|
| Phenotype VI | Downregulation of transcriptional factor binding to IRSE |
Abril et al. (1996) |
| Altered TAP-1 and LMP2 expression by a defective IFN-γ signaling pathway |
Dovhey et al. (2000) |
|
| Phenotype VII | Aberrant expression of HLA-E + low HLA la expression |
Marin et al. (2003) |
- MHC, major histocompatibility complex; β2-m, β2 microglobulin; APM, antigen processing machinery; TAP, transporter associated antigen processing; LMP, low molecular mass polypeptide; LOH, loss of heterozygosity; HLA, human leukocyte antigens; IRSE, interferon response sequence element; IFN, interferon.
Phenotype I, HLA class I total loss, can be associated with a lack of synthesis or synthesis of a truncated β2-m. There are indications that β2-m mutations are involved in some cases of HLA class I total loss observed in colorectal carcinomas (Browning et al., 1996), melanomas (Benitez et al., 1998; Wang et al., 1999), lung adenocarcinomas (Chen et al., 1996), and Burkitt's lymphoma (Rosa et al., 1983). A summary of such mutations was recently published (Garrido and Algarra, 2001). Another mechanism that causes defects in the assembly and stability of HLA class I molecules involves interference with the normal function of APM components, including the transporter associated with peptides (TAP). This interference leads to failure to transport peptides from the cytoplasm to the lumen of the ER, and hence failure of the class I processing pathway (Rosenberg and Bennink, 1993; Vitale et al., 2002). TAP defects can lead to the complete loss of class I molecules (Phenotype I), and are frequently associated with the absence of reactivity to antibodies directed against TAP proteins in HLA-negative tumor cells. Other alterations include the coordinated downregulation of several APM components (Ritz et al., 2001) and structural defects in the MHC genes that induce total loss of HLA class I molecules. These later defects will not be corrected by cytokine treatment, hence such treatment will not restore HLA expression.
Phenotype II, HLA haplotype loss, has been found in tumors of different histological origin (Torres et al., 1996; Mendez et al., 2001). The detection of microsatellite markers in chromosome 6 using PCR amplification of these short tandem repeats has provided an easy and effective way to diagnose this altered HLA phenotype. In a pancreatic adenocarcinoma, HLA haplotype loss was demonstrated in the fresh tumor and the tumor-derived cell line, indicating that such loss was not due to an in vitro event (Torres et al., 1996). The majority of these studies also revealed LOH at other loci of chromosome 6 by deletion of a full chromosome 6 or a large genomic region. Current figures for LOH for the HLA region in chromosome 6 are 46% in cervix carcinomas, 36% in head and neck, 17% in colorectal, and 14% in breast carcinomas (Feenstra et al., 1999; Jimenez et al., 1999; Koopman et al., 2000; Maleno et al., 2002). This is the most common mechanism of alteration in HLA class I expression (Ramal et al., 2000).
The mechanism of HLA A, B, or C locus downregulation, Phenotype III, is associated with alterations in MHC class I gene transcription, since the mRNA levels in these tumor cell lines can be upregulated with cytokines, and low expression of transcription factors that bind to locus-specific DNA motifs can induce HLA-B locus downregulation (Soong and Hui, 1992). In melanomas, selective HLA-B locus downregulation correlates with increased c-myc transcription which interferes with HLA-B transcription at the promoter level (Peltenburg and Schrier, 1994).
Phenotype IV, HLA allelic loss, might be the result of point mutations, partial deletions of HLA class I genes, chromosomal breakage, or somatic recombination (Brady et al., 2000; Serrano et al., 2000). These mechanisms can not be overridden by cytokine treatment.
Phenotype V, the compound phenotype observed in some tumors (Ikeda et al., 1997; Real et al., 1998), requires a combination of at least two different alterations. For instance, an HLA haplotype loss and HLA-B and -C locus downregulation (combination of Phenotypes II and III) give rise to a tumor cell expressing a single HLA class I molecule.
Phenotype VI, unresponsiveness to interferons (IFNs), is the result of the downregulation of transcriptional factor binding to interferon response sequence element (IRSE) (Abril et al., 1996) and altered TAP-1 and LMP-2 expression by a defective IFN-γ signaling pathway (Dovhey et al., 2000). Phenotype VII, low HLA class Ia and aberrant expression of HLA class Ib, appears when the tumor drastically reduces the expression of HLA A–B–C molecules and at the same time expressed HLA-E, a nonclassical HLA class I molecule that produces a strong NK inhibition capacity after interacting with the CD94/NKG2a inhibitory receptor (Marin et al., 2003).
EVIDENCE FOR T-CELL IMMUNOSELECTION OF MHC CLASS I-DEFICIENT TUMOR VARIANTS
Immune surveillance against cancer was postulated many years ago (Ehrlich, 1909; Thomas, 1959; Burnet, 1970), but clinical and experimental evidence for a direct role of the immune system in protecting against spontaneous malignancies has been slow to appear. Nevertheless, the idea seemed to fit some clinical observations. For example, cancer is rare in children and young adults, but appears more frequently with advancing age, when the efficiency of the immune system is declining. Also, some solid tumors were found to contain large numbers of lymphocytes, suggesting that the cellular immune system was recognizing the malignant neoplasm as foreign and fighting it.
It has mostly been assumed that both innate (NK cell) and acquired (CTL cell) immune mechanisms may contribute to the host's anti-tumor immunity by destroying transformed cells or by creating a local environment that suppresses tumor growth. These mechanisms have received significant support with the discovery of the pathway of antigen presentation to T-lymphocytes (Townsend et al., 1986), the identification of tumor antigens recognized by T-lymphocytes (Boon et al., 1994), and the partial elucidation of the molecular mechanisms that allow NK cells to perform their function (Moretta et al., 1996).
MHC class I total or partial loss is a widespread mechanism used by tumor cells to evade the immune system (Garrido et al., 1993; Marincola et al., 2000). It has been proposed that the major force contributing to the appearance of these MHC class I-negative tumor clones is T-cell immunoselection (Kaklamanis and Hill, 1992; Lehmann et al., 1995; Jäger et al., 1997). This hypothesis implies that T-cells can recognize tumor antigens presented by HLA class I-positive tumor cells, thereby performing effective immune surveillance. But when HLA class I-defective tumor variants appear, T-cells cannot “see” these targets, and these tumor clones acquire a growth advantage that allows them to take over the other clonal tumor populations. Nevertheless, the origin of these MHC-negative tumor variants remains unknown, and there is thus far no reported experimental or clinical data to substantiate this hypothesis (Tomlinson and Bodmer, 1999; Villunger and Strasser, 1999).
Our laboratory recently obtained direct evidence that a T-cell immune mechanism is responsible for the selection of tumor cells with a specific MHC class I phenotype (Garcia-Lora et al., 2001). The data indicate that a particular tumor can produce MHC class I-negative or -positive metastatic colonies depending on the immune status of the host (Fig. 6). The studies were performed with an H-2 class I negative fibrosarcoma tumor clone (Garrido et al., 1986; Perez et al., 1990; Algarra et al., 1991) that generated H-2 class I-negative spontaneous lung metastases in immunocompetent BALB/c mice. In contrast, the same tumor clone produced MHC class I-positive metastatic nodes in athymic nu/nu mice (Garcia-Lora et al., 2001) (Fig. 6). This phenomenon was observed in metastatic nodules generated after a period of in vivo growth, but not in the primary tumors growing locally in the footpad. An analysis of the molecular mechanisms implicated in the origin of these MHC class I-deficient metastatic nodes in immunocompetent mice suggested the coordinated suppression of multiple components of the MHC class I APM (Garcia-Lora et al., 2003). Treatment with IFN-γ transcriptionally induces the expression of these downregulated APM components, thereby also enhancing MHC class I surface expression. Such deficiencies are not present in metastases obtained from immunodeficient nu/nu BALB/c mice. In this connection, it has been shown that inoculation of immunocompetent C57BL/6 mice with mixtures of TAP1-positive and TAP1-negative cells produced tumors composed exclusively of TAP1-negative cells, indicating selection and evasion of immune surveillance in cells with the TAP deficiency (Johnsen et al., 1999).
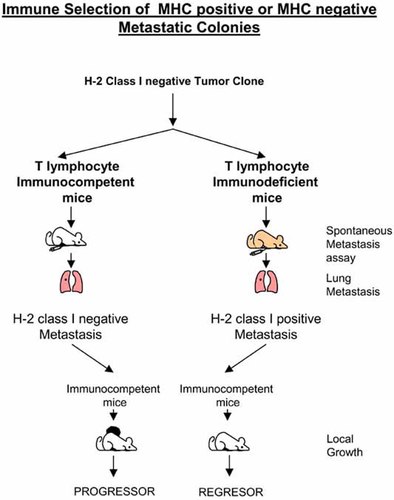
The MHC class I phenotype of a lung metastasis originated from a tumor clone is dependent on the immune status of the tumor-bearing mouse. H-2 class I-negative cells in an immune-competent host. H-2 class I-positive cells in nude/nude T-cell-deficient animals. Metastases originated in immunodeficient animals are rejected by immunocompetent syngeneic mice.
These findings support the hypothesis that the MHC phenotype of metastatic nodes is influenced by the T-cell repertoire of the host, since in the absence of this T-cell pressure, the metastatic nodes “recovered” not only H-2 class I expression but also the APM functioning necessary to produce stable MHC class I molecules on the cell surface. These results showed that in this tumor model the major factor that contributed to the appearance of MHC class I-deficient tumor variants is T-cell immunoselection.
It was also recently shown that lymphocytes and IFN-γ play a central role in providing an immunocompetent host with a mechanism of tumor surveillance against carcinogen-induced sarcomas and spontaneous tumors, a finding that suggest a role for the immune system in editing tumors with low immunogenicity (tumor escape variants, Shankaran et al., 2001). These studies used a strain of mice that completely lacked functional lymphocytes. Lymphocyte depletion was accomplished by inactivating a lymphocyte-specific gene called RAG2. When the mice were injected with the chemical carcinogen MCA, more than 50% developed tumors, compared to only 19% of the wild-type animals. Similar results were obtained in mice that lacked either receptor for IFN-γ or one of the proteins (Stat1) required for the receptor to function (Kaplan et al., 1998). It was also reported that these tumor-suppressor systems prevented the formation of spontaneous tumors. Our findings of greater production of metastases in nude mice (5–7 per mouse) than in wild-type animals (1 per mouse) indicate that an effective immune system also acts during metastatic evolution of the tumor (Garcia-Lora et al., 2001).
It might be predicted that tumors arising in immunodeficient patients such as kidney transplant recipients or HIV-infected individuals, who have received long-term treatment with immunosuppressive drugs, will not require the production of HLA-deficient clones to escape T-cell responses, since T-cell function will presumably be markedly decreased (Garrido and Algarra, 2001).
The tumor metastases generated in our model appeared to be immunoselected depending on the immune status of the host. Those metastases that were not immunoselected should be more immunogenic than those that were immunoselected. In fact, the metastastic colonies generated in immunodeficient nu/nu mice (H-2 positive) were more immunogenic than the metastases generated in immunocompetent mice (H-2 negative, Garcia-Lora et al., 2003). In this connection, it has been reported that chemically induced sarcomas produced in nude and severe combined immunodeficiency (SCID) mice were more immunogenic than similar sarcomas induced in congenic immunocompetent mice (Svane et al., 1996; Engel et al., 1997), and that tumors originated in RAG2 −/− knockout mice were more immunogenic that those originated in wild-type animals (Shankaran et al., 2001).
At this point, it is important to consider that MHC class I downregulation is not the only escape mechanism available to tumors to avoid T-cell responses. Others mechanisms, such as downregulation of the tumor antigens (Jäger et al., 1997), alteration of the apoptosis program (Hahne et al., 1996), expression of inhibitory cytokines (Chouaib et al., 1997), or immunological ignorance (Ochsenbein et al., 1999) have also been described.
Taken together, the studies reviewed above provide new evidence that support the original concept of cancer immunosurveillance. In addition, the identification of a particular immune escape mechanism in human or mouse tumors also implies the existence of active immune surveillance.
FUTURE PERSPECTIVES
We are just starting to understand that immunesurveillance against cancer cells may be more than an attractive theory. Evidence obtained in different laboratories, including ours indicates that the tumor escape from cell immune responses is a frequent finding in clinical tumor samples and can be detected when the HLA class I antigens are analyzed with immunohistological techniques in fresh tumor sections or tumor cell lines. Another important consequence of these findings is that the immunogenicity of tumors can be inferred to depend on the immune status of the host, i.e., tumors induced in immunodeficient mice are rejected by immunocompetent individuals.
The identification of a particular tumor immune escape mechanism will no doubt have a profound influence on T-cell-based immunotherapy protocols currently being used for cancer patients, and will help to select particular individuals suitable for such therapies. But the cancer cell can evolve to fight back the immune system, generating new escape variants. We are, therefore, facing the very difficult task of trying to destroy all possible metastatic variants growing in particular individual. Restoration of the normal tumor MHC class I phenotype may be a new way to restore an efficient immune response in cancer patients, but at present remains hypothetical, and no such procedures have been tested thus far.
Acknowledgements
We thank all the members of the research group of the Departamento de Análisis Clínicos, Hospital Universitario Virgen de las Nieves, Granada for their contribution to this work, and K. Shashok for checking the use of English in the manuscript.




