Baseline red blood cell distribution width and perforin, dynamic levels of interleukin 6 and lactate are predictors of mortality in patients with sepsis
Xin Li and Zhongnan Yin contributed equally to this work.
Abstract
Background
Sepsis is a critical illness often encountered in the intensive care unit. However, prognostic biomarkers for sepsis have limited sensitivity. This study aimed to identify more sensitive predictors of mortality through repeated monitoring of laboratory parameters.
Methods
Patients with sepsis (Sepsis 3.0 criteria met) were recruited and divided into the survivor and nonsurvivor groups after 28 days. Data on blood biochemistry, lymphocyte subsets, and cytokines were obtained on the first and seventh hospitalization days. Univariate and multivariate Cox regression analyses were performed to explore the correlation between these variables and patient mortality.
Results
Forty patients with sepsis were included. The mortality rate was 37.5%. Red blood cell distribution width-standard deviation (RDWSD) (hazard ratio [HR] = 1.107 [95% CI: 1.005–1.219], p = 0.040) and perforin level (HR = 1.001 [95% CI: 1–1.003], p = 0.035) on the first day, as well as lactate (HR = 112.064 [95% CI: 2.192–5729.629], p = 0.019) and interleukin 6 (IL-6) (HR = 1.005 [95% CI: 1.001–1.008], p = 0.014) levels on the seventh day, were independent risk factors of mortality. If the patients were divided into two groups based on RDWSD (normal: n = 31; increased: n = 9), the Kaplan–Meier curves showed that the group with increased RDWSD had a lower survival (p = 0.025).
Conclusion
Baseline RDWSD and perforin, along with dynamic IL-6 and lactate levels, were independent predictors of mortality in patients with sepsis.
1 INTRODUCTION
Sepsis is an infection-induced systemic inflammatory response involving multiple cytokines and mediators. It is a commonly encountered life-threatening condition in intensive care units (ICUs) worldwide.1 Although substantial research and improvements in the clinical workflow have accelerated the diagnosis and treatment of sepsis,2 the disease remains a heavy burden on society, with a disproportional impact on older individuals.3 A previous study found that more than half of sepsis cases occurred in adults aged over 65 years.4 Given the increased burden of comorbidities and immune weakening associated with population aging,3 sepsis mortality could increase at a considerable rate over the next two decades.5 At present, the Acute Physiology and Chronic Health Evaluation (APACHE)6 and Sequential Organ Failure Assessment (SOFA)7 are widely used to assess the prognosis of critically ill patients. However, these assessments are complicated and time-consuming, and their sensitivity and specificity are limited. Therefore, more sensitive biomarkers are needed to predict the outcome of sepsis.
This study aimed to identify potentially more sensitive biomarkers for sepsis prognosis through repeated monitoring of relevant laboratory data and multivariate regression analysis.
2 MATERIALS AND METHODS
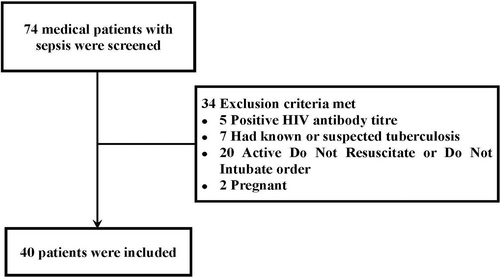
This prospective cohort study was conducted in the medical intensive care unit (MICU) of a university-affiliated teaching hospital in China from December 2019 to February 2022. The study protocol was approved by the ethics committee of Peking University Third Hospital (approval no. M2019396). All patients or their legally authorized representatives provided written informed consent to participate in the study. During the study period, 40 patients with sepsis who were eligible for the study were included. Patients were subsequently divided into the survivor and nonsurvivor groups based on their 28-day survival.
The inclusion criteria were as follows: (1) patients who met the Sepsis 3.0 diagnostic criteria8 and (2) age of ≥18 years.
The exclusion criteria were as follows: (1) pregnancy, (2) human immunodeficiency virus infection or tuberculosis, and (3) a “do-not-resuscitate” or discontinuation of tracheal intubation order.
Demographic and clinical data, including age, sex, weight, height, body mass index (BMI), and comorbidities were collected. Arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2), APACHE II, and SOFA scores was also collected on the first and seventh days of admission, as well as mechanical ventilation time and length of stay at discharge or death.
Laboratory data, including routine blood tests, red blood cell (RBC) distribution width-standard deviation (RDWSD), RBC distribution width-coefficient of variation (RDWCV), lactate, creatinine, urea nitrogen, bilirubin, lymphocyte subsets, and cytokines were measured within 24 h of the first day of admission and on the seventh day of hospitalization.
2.1 Flow cytometry
Peripheral venous blood was collected directly into the anticoagulant tubes, which were centrifuged at 1800 × g for 10 min. The cells were treated with ammonium–chloride–potassium lysis buffer and stained with fluorescence-labeled antibodies for 15 min in 1 × phosphate-buffered saline. All experiments were performed using the Beckman CytoFLEX S flow cytometer (Beckman Coulter). Kaluza Analysis 2.1 software was used for data analysis. The following antibodies were used in these experiments: CD45-APC (clone HI30), CD3-FITC (clone HIT3a), CD4-PE (clone RPA-T4), CD8-PerCP/Cy5.5 (clone SK1), and CD8-PE/DazzleTM (clone SK1). All antibodies were purchased from BioLegend.
2.2 Statistical analysis
The SPSS software version 21.0 (SPSS Inc.) was adopted for statistical analysis. Continuous variables were expressed as median (interquartile range [IQR]), and categorical variables were expressed as numbers (%). Subsequently, the clinical data of 28-day survivors and nonsurvivors were compared. Continuous variables were compared using the nonparametric Mann–Whitney U test, whereas categorical variables were compared using the chi-squared test. Cox proportional hazard regression analysis was undertaken to assess the factors associated with 28-day mortality. The variables significantly associated with 28-day nonsurvival in the univariate analysis were used in the Cox proportional hazard regression analysis. The Kaplan–Meier survival curve was used to show the probability of sepsis survival at day 28 according to the RDWSD classification. Survival curves were compared using a logarithmic rank test.
3 RESULTS
3.1 Demographic data
Figure 1 shows the patient selection process. Forty patients with sepsis were included in this study, of which 24 were males (60%). The median age of the patients was 80 years (IQR: 67–84). The 28-day mortality rate was 37.5%. According to the 28-day survival, the patients were divided into a survivor group (n = 25) and a nonsurvivor group (n = 15). Detailed information was presented in Table 1.
| Variable | Survivors (n = 25) | Nonsurvivors (n = 15) | p-value |
|---|---|---|---|
| Age (range), years | 78 (52–84) | 83 (76–85) | 0.105 |
| Male sex, n (%) | 12 (48) | 12 (80) | 0.046* |
| BMI (kg/m2) | 24.91 (20–29) | 22.34 (19–28) | 0.224 |
| Source of infection | |||
| Lung | 20 (80) | 11 (73) | 0.922 |
| Other infections | 5 (20) | 4 (27) | 0.922 |
| Comorbidities | |||
| COPD | 1 (4) | 1 (7) | 1.000 |
| DM | 9 (36) | 3 (20) | 0.476 |
| Hypertension | 13 (52) | 10 (67) | 0.364 |
| Cerebrovascular disease | 12 (48) | 6 (40) | 0.622 |
| ARDS | 13 (52) | 10 (67) | 0.364 |
| Duration of MV (days) | 6 (2–10) | 7 (2–14) | 0.594 |
| Length of hospital stay (days) | 17 (12–25) | 15 (6–20) | 0.088 |
- Note: Values are expressed as n (%) or median (25%–75%) unless otherwise stated.
- Abbreviations: ARDS, acute respiratory distress syndrome; BMI, body mass index; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; ICU, intensive care unit; MV, mechanical ventilation.
- * p-value < 0.05 was considered statistically significant.
3.2 Comparison of laboratory data between the survivor and nonsurvivor groups
Routine blood tests, liver and kidney function, coagulation, and other related parameters of the enrolled patients were collected on the first and seventh day. In addition, the APACHE II and SOFA scores of patients were assessed on the first and seventh day according to their condition.
The results showed that on the first and seventh day of admission, RDWCV and RDWSD levels in the survival group were lower than those in the nonsurvival group, and the differences were statistically significant. In addition, APACHE II scores, creatinine, and blood urea nitrogen levels were significantly different between the two groups (see Table 2).
| Variable | D1 | D7 | ||||
|---|---|---|---|---|---|---|
| Survivors (n = 25) | Nonsurvivors (n = 15) | p-value | Survivors (n = 25) | Nonsurvivors (n = 10) | p-value | |
| WBC, 109/L | 9.7 (7.57–12.00) | 14.36 (7.91–21.51) | 0.067 | 9.03 (6.77–13.56) | 13 (7.96–25.06) | 0.154 |
| N, 109/L | 8.01 (6.29–10.50) | 11.97 (7.26–20.74) | 0.076 | 6.74 (4.45–11.63) | 11.8 (6.88–23.83) | 0.053 |
| RBC, 1012/L | 3.67 (3.11–4.1) | 3.64 (2.96–4.29) | 0.780 | 3.63 (3.16–4.16) | 2.86 (2.45–3.52) | 0.017* |
| HGB, g/L | 106 (92–120.5) | 112 (90–131) | 0.511 | 108 (98–118) | 82.5 (73.75–111.75) | 0.031* |
| RDWCV | 13.4 (12.7–14.8) | 15 (13.6–16.3) | 0.037* | 13.3 (12.75–15.05) | 15.75 (13.35–17.03) | 0.048* |
| RDWSD | 45.1 (41.05–49.9) | 51.8 (46.5–57.7) | 0.022* | 45.2 (41.5–49.8) | 52.35 (49–57.73) | 0.006* |
| LYM, 109/L | 0.9 (0.55–1.47) | 0.92 (0.55–1.44) | 0.801 | 1.26 (0.83–2.03) | 0.69 (0.44–1.26) | 0.034* |
| PLT, 109/L | 198 (132.5–252) | 155 (77–188) | 0.139 | 203 (156–297) | 158 (108.5–200.75) | 0.074 |
| Lac | 1.3 (0.95–2.05) | 2.34 (1.2–3.4) | 0.099 | 1.4 (0.9–1.9) | 2.65 (1.85–3.63) | 0.001* |
| ALB, g/L | 28.7 (26.55–31.45) | 29.9 (26.8–33.2) | 0.371 | 31 (29.5–35.3) | 29.4 (27.13–30.95) | 0.018* |
| Cr, μmol/L | 60 (47–105) | 131 (92–293) | 0.008* | 67 (52–101) | 172 (111.25–232.75) | 0.000* |
| BUN, mmol/L | 7.03 (4.21–13.33) | 17.04 (12.8–20.89) | 0.001* | 8.06 (6.1–12.46) | 20.24 (13.38–30.14) | 0.001* |
| TBIL, μmol/L | 11.9 (7.05–20.1) | 21.6 (13.1–38.9) | 0.012* | 15.3 (10.95–22.15) | 14.65 (11.75–36.73) | 0.511 |
| INR | 1.28 (1.18–1.43) | 1.29 (1.12–1.42) | 0.944 | 1.17 (1.09–1.25) | 1.40 (1.16–1.49) | 0.041* |
| PCT, ng/ml | 0.65 (0.23–2.86) | 1.52 (0.17–11.76) | 0.586 | 0.16 (0.10–0.47) | 1.18 (0.16–5.65) | 0.054 |
| PaO2/FiO2 (mmHg) | 160 (107–190.25) | 170 (97–256) | 0.665 | 271 (205–364) | 211 (165–330) | 0.214 |
| APACHE II score | 18 (14–23) | 22 (18–31) | 0.017* | 7 (5–13) | 20 (13.75–28.25) | <0.001* |
| SOFA score | 7 (6–9.5) | 9 (6–14) | 0.311 | 3 (2–5) | 7 (3.75–10) | 0.005* |
- Abbreviations: ALB, albumin; APACHE II, Acute Physiology and Chronic Health Evaluation II; BUN, blood urea nitrogen; Cr, creatinine; D1, day 1; D7, day 7; HGB, hemoglobin; INR, international normalized ratio; Lac, lactic acid; LYM, lymphocyte; N, neutrophil; PCT, procalcitonin; PLT, platelets; RBC, red blood cell; RDWCV, red blood cell distribution width-coefficient of variation; RDWSD, red blood cell distribution width-standard deviation; SOFA, Sequential Organ Failure Assessment; TBIL, total bilirubin; WBC, white blood cell.
- * p-value < 0.05 was considered statistically significant.
3.3 Comparison of lymphocyte subsets between the survivor and nonsurvivor groups
Blood lymphocyte subsets, including T-cell subset, natural killer T (NKT) cells, natural killer (NK) cells, and B cells, were detected by flow cytometry on the first and seventh day. The results showed that the proportion of CD3+ T lymphocytes in the survival group was significantly higher than that in the nonsurvival group, both on the first day and on the seventh day. There were no significant differences between the two groups in other lymphocyte subsets, except NKT cells on the first day (Table 3).
| Variable | D1 | D7 | ||||
|---|---|---|---|---|---|---|
| Survivors (n = 25) | Nonsurvivors (n = 15) | p-value | Survivors (n = 25) | Nonsurvivors (n = 10) | p-value | |
| CD3+ T | 66.68 (60.73–72.89) | 46.75 (41.92–62.88) | 0.008* | 72.23 (61.91–77.52) | 47.40 (35.90–58.93) | 0.003* |
| CD4+ T | 57.48 (45.25–66.51) | 66.77 (56.28–71.23) | 0.089 | 57.74 (50.80–62.87) | 48.23 (31.83–72.79) | 0.283 |
| CD8+ T | 38.58 (27.22–48.92) | 27.64 (23.87–34.99) | 0.079 | 37.63 (23.85–42.07) | 37.2 (20.43–48.17) | 0.834 |
| NKT | 6.77 (3.48–9.65) | 3.28 (2.14–6.28) | 0.028* | 7.38 (3.84–10.27) | 5.53 (2.92–7.26) | 0.317 |
| NK | 18.52 (11.79–32.85) | 24.99 (15.55–36.18) | 0.412 | 15.87 (8.99–22.31) | 20.47 (13.16–35.76) | 0.166 |
| B | 9.16 (5.09–18.69) | 13.72 (8.04–32.46) | 0.160 | 8.14 (5.00–16.44) | 13.73 (5.07–35.23) | 0.334 |
- Abbreviations: D1, day 1; D7, day 7; NK, natural killer cells; NKT, natural killer T cells.
- * p-value < 0.05 was considered statistically significant.
3.4 Comparison of cytokines between the survivor and nonsurvivor groups
As shown in Table 4, the differences in soluble Fas (sFas) and interferon-gamma on the first day were statistically significant between the two groups. Perforin on the first day showed a trend to increase in the nonsurvivor group (p = 0.059).
| Variable | D1 | D7 | ||||
|---|---|---|---|---|---|---|
| Survivors (n = 25) | Nonsurvivors (n = 15) | p-value | Survivors (n = 25) | Nonsurvivors (n = 10) | p-value | |
| IL-2 | 2.74 (0–78.09) | 7.32 (4.4–86.73) | 0.191 | 22.54 (0.12–171.14) | 50.33 (2.81–145.32) | 0.899 |
| IL-4 | 7.02 (1.72–31.31) | 6.36 (0–12.32) | 0.547 | 8.83 (2.89–31.81) | 4.50 (3.50–26.12) | 0.834 |
| IL-10 | 9.62 (4.97–37.93) | 22.48 (9.83–49.06) | 0.102 | 8.50 (4.38–26.58) | 14.82 (9.65–52.44) | 0.240 |
| IL-6 | 107.95 (28.63–331.66) | 239.03 (89.64–682.59) | 0.167 | 40.16 (15.24–104.74) | 93.44 (39.56–242.93) | 0.120 |
| IL-17A | 14.53 (2.55–57.71) | 15.57 (7.54–37.66) | 0.748 | 10.61 (2.95–69.23) | 13.41 (5.92–28.48) | 0.950 |
| TNF-α | 9.62 (2.03–46.52) | 4.76 (1.58–10.09) | 0.235 | 10.13 (3.03–38.13) | 9.67 (5.95–16.7) | 0.705 |
| sFas | 551.58 (137.74–2763.62) | 3489.86 (1214.64–6034.79) | 0.001* | 948.07 (209.33–2657.07) | 2447.72 (1380.89–4316.58) | 0.078 |
| sFasL | 0 (0–13.87) | 0 (0–6.95) | 0.395 | 0.1 (0–19.53) | 1.49 (0–15.43) | 0.964 |
| INF-γ | 159.76 (10.23–444.01) | 12.14 (6.7–101.78) | 0.043* | 98.81 (7.76–422.52) | 19.47 (14.11–40.79) | 0.239 |
| Granzyme A | 65.86 (5.43–533.07) | 40.35 (13.71–171.53) | 0.944 | 107.51 (18.04–498.64) | 38.69 (12.22–1878.71) | 0.570 |
| Granzyme B | 20.65 (0–1352.04) | 89.25 (21.88–1152.65) | 0.549 | 128.62 (0–1593.49) | 56.32 (7.33–853.59) | 0.734 |
| Perforin | 2179.07 (1615.45–2592.45) | 3666.33 (1397.78–5434.08) | 0.059 | 1831.6 (1495.91–3213.74) | 2357.49 (1782.01–5199.04) | 0.240 |
| Granulysin | 3641.5 (2852.49–5912.70) | 3908.82 (3358.64–5151.61) | 0.791 | 5309.28 (2382.53–7296.13) | 3795.83 (3351.4–6160.59) | 0.769 |
- Abbreviations: D1, day 1; D7, day 7; IL, interleukin; INF-γ, interferon gamma; sFas, soluble Fas; sFasL, soluble Fas ligand; TNF-α, tumor necrosis factor alpha.
- * p-value < 0.05 was considered statistically significant.
3.5 Univariate and multivariate risk analyses of the 28-day mortality in patients with sepsis
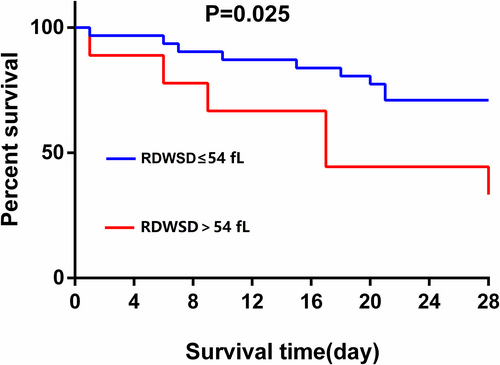
The patients with sepsis were firstly divided into the survivor and nonsurvivor groups according to their 28-day survival. Results indicated that RDWSD (hazard ratio [HR] = 1.107 [95% CI: 1.005–1.219], p = 0.040) and perforin (PFN) (HR = 1.001 [95% CI: 1–1.003], p = 0.035) on the first day, as well as lactate (Lac) (HR = 112.064 [95% CI: 2.192–5729.629], p = 0.019) and interleukin 6 (IL-6) (HR = 1.005 [95% CI: 1.001–1.008], p = 0.014) on the seventh day, were independent risk factors for the 28-day mortality, as shown in Table 5. In addition, based on a normal range of RDWSD (37–54 fL) at our hospital laboratory, patients were further divided into a group with RDWSD >54 fL and a group with RDWSD ≤54 fL. Subsequently, the Kaplan–Meier survival curve (Figure 2) was plotted to show the 28-day survival probability of the patients in different RDWSD categories, followed by a pairwise comparison using the log-rank test. The results showed that a relatively high RDWSD was associated with a poorer prognosis of sepsis (p = 0.025).
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| D1 lab results | ||||
| WBC, 109/L | 1.053 (1.015–1.092) | 0.006* | ||
| Neu, 109/L | 1.053 (1.015–1.092) | 0.006* | ||
| RDWCV | 1.156 (1.005–1.330) | 0.042* | ||
| RDWSD | 1.062 (1.014–1.113) | 0.012* | 1.107 (1.005–1.219) | 0.040* |
| BUN, mmol/L | 1.068 (1.022–1.115) | 0.003* | ||
| APACHE II score | 1.103 (1.025–1.187) | 0.009* | ||
| CD3+ T | 0.959 (0.929–0.989) | 0.009* | ||
| sFas | 1 (1–1) | 0.001* | ||
| Perforin | 1 (1–1.001) | 0.014* | 1.001 (1–1.003) | 0.035* |
| D7 lab results | ||||
| WBC, 109/L | 1.076 (1.012–1.143) | 0.018* | ||
| Neu, 109/L | 1.087 (1.016–1.163) | 0.016* | ||
| RBC, 1012/L | 0.217 (0.064–0.728) | 0.013* | ||
| HGB, g/L | 0.954 (0.917–0.993) | 0.020* | ||
| RDWCV | 1.247 (1.035–1.504) | 0.021* | ||
| RDWSD | 1.071 (1.013–1.132) | 0.016* | ||
| Lac | 6.712 (2.224–20.110) | 0.001* | 112.064 (2.192–5729.629) | 0.019* |
| Cr, umol/L | 1.008 (1.003–1.013) | 0.003* | ||
| IL-6 | 1.002 (1–1.003) | 0.025* | 1.005 (1.001–1.008) | 0.014* |
- Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; BUN, blood urea nitrogen; CI, confidence interval; Cr, creatinine; D1, day 1; D7, day 7; HR, hazard ratio; IL-6, interleukin 6; Lac, lactic acid; Neu, neutrophil; RBC, red blood cell; RDWCV, red blood cell distribution width-coefficient of variation; RDWSD, red blood cell distribution width-standard deviation; sFas, soluble Fas; WBC, white blood cell.
- * p-value < 0.05 was considered statistically significant.
4 DISCUSSION
In this prospective study of a cohort of 40 patients with sepsis admitted to an MICU, blood RDWSD and PFN level on the first day, as well as lactate and IL-6 levels on the seventh day, were found to be independently associated with 28-day mortality. Kaplan–Meier curve study further showed that patients with a higher RDWSD had a lower survival. To our knowledge, the current study was the first to show that the blood PFN level on the first day of admission was an independent risk factor for mortality at 28 days in sepsis patients.
The mean RDW, which consists of RDWSD and RDWCV, is a measure of the heterogeneity of RBC size.9 It is commonly assessed in complete blood counts. A normal reference range for RDW is 12%–15% in adults. At present, multiple studies have suggested that RDW may indicate the health status of an individual. Moreover, it can also reflect the degree of inflammation due to factors such as the half-life of RBC circulation and cell membrane deformation, which may be affected by inflammation.10 Previous studies reported that escalated RDW was associated with an increased risk of mortality in sepsis patients.11, 12 In a retrospective study of 566 patients with severe sepsis and septic shock, the 28-day mortality of patients with a RDW < 14% was merely 13.1%, while the mortality of those with a RDW > 15.8% increased to 44.9%.13 Therefore, an increase in RDW may reflect the extent of underlying inflammation and serves as a prognostic marker of mortality.
Previously, RDW was often considered as a diagnostic marker to distinguish thalassemia from iron deficiency anemia.9 However, recent studies have revealed the clinical significance of RDW in several non-hematological diseases, such as acute or chronic heart failure,14 acute kidney injury,15 tumors,16, 17 and sepsis.12, 18 In a retrospective study on sepsis, Jo et al.13 found that the level of RDW was significantly higher in patients of the nonsurvival group as compared to those in the survival group, and was correlated with the 28-day mortality. However, this study only focused on the baseline measurements, but did not monitor the dynamic change of RDW. Alternatively, Kim et al.19 discovered that an increase in RDW from the baseline within 72 h of admission was a strong independent risk factor for the mortality of patients with sepsis even after the adjustment of confounders. Our present findings were largely consistent with this result. We found that the levels of RDWCV and RDWSD on both the first day and the seventh day were significantly different between the survivor and nonsurvivor groups, and more notably, RDWSD on the first day of admission was identified as an independent risk factor for the 28-day mortality of patients with sepsis.
Although the mechanisms underlying the association between elevated RDW and the mortality of patients with sepsis are not fully understood, two hypotheses involving inflammation and organ dysfunction have been raised by previous literature. First, elevated RDW is probably associated with a systemic inflammatory response, which features high levels of pro-inflammatory factors (such as IL-6 and tumor necrosis factor alpha [TNF-α]) and an increased level of oxidative stress in sepsis.20 This inflammatory state can compromise bone marrow function and RBC homeostasis, thereby causing side effects,19 including impaired iron metabolism due to oxidative stress, shortened RBC life span, and dysregulated bone marrow response to erythropoietin that increases the level of RDW.21, 22
In the current study, we observed the changes of blood lymphocyte subsets and inflammatory mediators/cytokines from the first day to the seventh day of admission and found that PFN was an independent risk factor for the 28-day mortality of patients with sepsis. The blood level of PFN of the survival group was lower than that of the nonsurvivor group. PFN is expressed by innate and adaptive T cells, including NK cells, NKT cells, cytotoxic CD8+ T cells (CTLs), Treg cells, and γδ T cells.20 During inflammation, the cytotoxic cellular granule secretion pathway relies on PFN to deliver granzyme (Gzm) proteases to the cytosol of target cells, where they induce apoptosis and other biological effects.23 PFN has been defined as a pore-forming effector molecule involved in cytolytic killing.24 However, it may exhibit immunomodulatory effects in addition to the aforementioned activities.25 CTLs are key players in adaptive immune responses against certain viruses and intracellular parasites.26, 27 They become cytokine-secreting and cytocidal through a series of complex events, which involve the clonal activation of CTL precursors, as well as the rapid division of lymphoblastoid cells expressing Fas ligand (FasL) and PFN- and Gzm-containing lytic granules. Cell death induced by the apoptotic process occurs in a programmed and active manner in different physiological (tissue remodeling and morphogenesis) and pathophysiological conditions. A previous study28 showed that programmed necrosis of RBCs proceeded in a molecular manner similar to that of nucleated cell necroptosis. This process is characterized by multiple features, including dependence on receptor-interacting protein 1 phosphorylation, mixed lineage kinase domain-like protein, and extrinsic ligands of the TNF superfamily (e.g., FasL), antagonism of caspase 8 and necrosome assembly. Fas is a major death receptor of the extrinsic pathway of apoptosis, activating apoptosis when bound to its ligand. A death signal is then generated, which activates caspase 8, followed by caspase 3, thereby inducing extrinsic apoptosis and, consequently, cell damage.29 Our study found that the difference in the level of soluble (s) Fas between the survivor and the nonsurvivor groups on the first day of admission was significant. The lower RDW of the survival group of our patients was likely related to the abnormal secretion of the lymphoid subsets and dysregulated cytokines and mediators of the inflammatory immune response during sepsis. In addition, RDW is associated with renal insufficiency, which is closely related to malnutrition and inflammation.30 As a result, it is speculated that an increase in RDW may be the reflection of multiple deleterious pathological processes, including oxidative stress, inflammatory responses, renal dysfunction, and malnutrition, which can occur simultaneously during sepsis.
In the current study, we also used Cox multivariate regression to show that RDWSD and PFN were independent risk factors for 28-day mortality of sepsis, while the APACHE II score was not an independent risk factor for 28-day mortality, although its differences between the survival and nonsurvival groups on the first day and on the seventh day of admission were statistically significant. This result suggests that RDWSD and PFN may serve as an accurate marker in predicting the 28-day mortality of sepsis. In the study by Juhyun et al.31 the diagnostic and prognostic value of IL-6 for sepsis and septic shock was superior to that of pentraxin 3 (PTX3) and procalcitonin (PCT) when the IL-6 detection was perfected within 6 h after the definite diagnosis of sepsis or septic shock. Liu et al.32 included 3713 patients with sepsis and found that lactic acid was an independent prognostic predictor of mortality in patients with sepsis. Considering the large sample sizes of these studies, it was understandable that the prognostic value of lactic acid and IL-6 could be evident on the first day of admission. However, due to the small sample size of our study, no difference was found in lactic acid and IL-6 on the first day, while during repeated monitoring, lactic acid and IL-6 on the seventh day were found to be independent risk factors for the 28-day mortality of sepsis. These results suggest that dynamic observation of lactic acid and IL-6 is warranted for prediction of mortality in individual patients or in small cohorts.
The study also found that the proportion of men in the survivor and nonsurvivor groups was different (48% vs. 80%, p = 0.046). In their study, Padkin et al.33 found that men were predominant (58.8%) in the cohort of patients with severe sepsis. In the French EPISEPSIS study,34 the number of male patients with sepsis was twice that of female patients, indicating that the former was more susceptible to sepsis.35 Interestingly, some studies showed that the level of RDW was correlated with sex,36 whereas others found that it was only correlated with age.37 In our study, after adjusting for age, sex, APACHE II, and SOFA scores, RDW still demonstrated a good discriminability for in-hospital mortality, superior to that of APACHE II and SOFA scores.
Our study had several limitations. Firstly, the patients included in this study were MICU patients with an median age of 80 years, which may account for the higher 28-day mortality as compared to other reported data. In addition, the sample size was small, which may have led to a sample-related bias.
5 CONCLUSIONS
Baseline RDWSD was an independent predictor of 28-day mortality in patients with medical sepsis. In addition, the baseline level of blood PFN and the dynamic changes of IL-6 and lactate were also associated with the outcome of sepsis. The clinical value of these potential biomarkers for sepsis prognosis needs validation in prospective studies with large sample-sized cohorts and in different populations of sepsis.
FUNDING INFORMATION
This study was funded as a clinical cohort construction program of Peking University Third Hospital (No.BYSYDL2021019).
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
Open Research
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.




