A case of hereditary hemorrhagic telangiectasia and literature review
Funding information
The National Natural Science Foundation of China (81370615, 81600097, 82070120).
Abstract
Background
To discuss the clinical features of a patient with hereditary hemorrhagic telangiectasia (HHT).
Methods
The clinical data of one patient with HHT are retrospectively analysed. In addition, we review the relevant literature.
Results
A 32-year-old male patient was admitted to the hematology outpatient department of our hospital and presented with intermittent epistaxis for 24 years. In recent years, he was diagnosed with iron deficiency anemia. The nasal endoscopic examination showed telangiectasia at the front of the right-middle turbinate and the left nasal cavity. He had ENG genetic mutation positivity.
Conclusions
Patients with HHT may suffer from many complications, including bleeding, anemia, iron deficiency, and high-output heart failure. These patients may have telangiectasias and arteriovenous malformations in various organs.
1 INTRODUCTION
Hereditary hemorrhagic telangiectasia (HHT), also known as Osler–Weber–Rendu syndrome, is an autosomal dominant genetic disease that causes abnormal vascular development.1 Epistaxis is the primary clinical manifestation of HHT patients and can occur during the preschool age. With increasing age, the frequency and severity of epistaxis gradually increase and eventually may result in anemia. The present study found that mutations in ENG, ACVRL1/ALK1, and MADH4/SMAD4 lead to abnormal plasma levels of transforming growth factor β(TGF-β)2 and vascular endothelial growth factor (VEGF)3 in HHT patients. This article aimed to improve clinicians' understanding of HHT by analysing the clinical data of a patient and reviewing the relevant literature.
2 CASE PRESENTATION
A 32-year-old male patient presented to the outpatient hematology department of our hospital with the chief complaint of “intermittent epistaxis for 24 years” in April 2021. At the beginning of the onset of nasal bleeding, bleeding can be stopped by a cotton ball pressure plug. Since June 2020, bleeding can be stopped only by electrocoagulation treatment. During that time, his epistaxis severity score (ESS, an epistaxis bleeding scale in HHT) was 9. He chose to be hospitalized due to symptoms of nasal bleeding and fatigue at a local hospital. Routine blood examination showed small cell hypochromic anemia, and no abnormalities were found in coagulation function or bone marrow–related examinations. His previous hemoglobin level was as low as 56 g/L and gradually increased after oral iron supplementation. He underwent septoplasty, but the epistaxis severity score did not decline. In December 2020, he received nasal endoscopy for nasal bleeding in another hospital. The examination suggested dilatation of blood vessels at the front of the right-middle turbinate and multiple telangiectasias in the left nasal cavity. At that time, he accepted nasal gauze and other hemostatic treatments. In April 2021, he was treated in the outpatient department of our hospital, and genetic testing was conducted. The results indicated ENG gene variation (Figure 1).
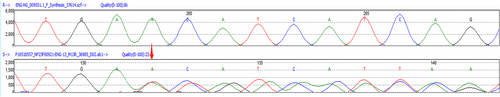
The investigation of the patient's family indicated that there was no history of consanguinity in the family. The patient's grandfather had epistaxis since middle age. His parents and brothers had no epistaxis, but his parents and brothers were not fully screened and imaged for HHT.
3 DISCUSSION
More than 80% of HHT patients have known genetic mutations, while approximately 20% of patients meet clinical diagnostic criteria with no confirmed genetic mutations.4 The current literature classifies HHT into different types.5 Among HHT patients, the known gene mutations include ENG mutation (HHT1), ACVRL1 (ALK1) mutation (HHT2), and MADH4 (SMAD4) mutation (JP-HHT), and very few patients have other pathogenic gene mutations.6 Additionally, HHT3 refers to a genetic mutation found on chromosome 5, and HHT4 refers to a genetic mutation on chromosome 7; however, no specific pathogenic gene has been detected for these two types.7 Bone morphogenetic protein (BMP9), encoded by GDF2, is a specific ALK1 ligand. HHT caused by GDF2 gene mutation is called the HHT5 type.8
ENG, ACVRL1, and MADH4 encode endothelial cells expressing proteins that participate in cell signaling through BMP/TGF-β superfamily ligand signaling pathways. The proteins regulate SMAD and non-SMAD signaling pathways9 and play an important role in angiogenesis and remodeling. Genetic variation destroys the integrity of endothelial cells, affects smooth muscle differentiation, leads to an abnormal cytoskeleton, destroys the integrity of blood vessels, and eventually leads to an increased VEGF concentration.3
The diagnostic criteria of HHT (Curacao) include the following: (1) recurrent and spontaneous epistaxis; (2) telangiectasias, which occur frequently at characteristic parts (lips, mouth, fingers, and nose); (3) visceral telangiectasia, such as gastrointestinal telangiectasia, pulmonary AVM, liver AVM, brain AVM, or spinal AVM; and (4) family history, where a first-degree relative has HHT. HHT can be diagnosed if three or more of these criteria are met. If only two conditions are met, HHT may be highly suspected. HHT can be ruled out if fewer than two conditions are met,10 while symptoms of HHT are age-related and often atypical in childhood. Suspected children often lack skin mucosal telangiectasis and epistaxis, making it difficult to diagnose. For a person/child who has a first-degree relative with confirmed HHT, his risk of HHT is 50%, regardless of whether he has any symptoms. Another way HHT can be diagnosed is via positive genetic testing. In this case, the patient had a recurrent spontaneous nosebleed and was positive for an ENG gene mutation, so the diagnosis was considered HHT. Although this patient has no telangiectasia of the skin mucosa, such as the fingertip, lip, or oral mucosa, at present, it is still necessary to actively evaluate whether there is an arteriovenous malformation of internal organs. Morbidity and mortality are significantly increased in patients with arteriovenous malformations of the brain, pulmonary. The HHT patients are at risk of refractory high-output cardiac failure, complicated portal hypertension or pulmonary hypertension, and so on. For this patient, cranial magnetic resonance imaging (MRI)/magnetic resonance angiography (MRA), enhanced lung CT, abdominal color Doppler ultrasound or liver CT/MRI, colonoscopy, and comprehensive otolaryngologic evaluation can detect potential arteriovenous malformations. The hematologic assessment included complete blood count (CBC), reticulocyte count, folate, vitamin B12, reticulocyte counts, serum iron, total iron-binding ability, and ferritin.
The treatment of HHT patients should not only target the existing clinical symptoms, such as nasal bleeding, but also actively prevent complications.11 The treatment involves a multidisciplinary approach. For this patient, the main clinical symptoms were recurrent nosebleed and small cell hypochromic anemia. His anemia was treated with oral iron. In the case of nosebleeds, the clinician may choose moisturizing topical therapies, nasal packing, or topical use of tranexamic acid infiltration gauze or oral tranexamic acid. For recurrent nosebleed, electrocoagulation, septodermoplasty, and even nasal closure are options when the above treatment is not effective. It is important to note that HHT is not a clotting factor deficiency disorder, so clinicians should evaluate patients for hypercoagulability or prior thrombotic events when using antifibrinolytic drugs to treat HHT-related bleeding.
According to a literature review, anti-VEGF therapy is relatively new at present, for example, thalidomide and bevacizumab. In 2018, a retrospective study by Lyer et al.12 described the use of bevacizumab for gastrointestinal and nasal bleeding in HHT patients. Thirty-four patients received intravenous bevacizumab with a standard regimen. The results showed a significant reduction in the severity score of nosebleed and the need for red blood cell transfusion. Bevacizumab nasal spray has been studied for the treatment of epistaxis. Intranasal bevacizumab injections did not show significant benefit.11 The low serum iron levels in HHT patients were associated with increasing levels of factor VII and increased the risk of venous thrombotic events (VTE) by 2.5 times.13 Patients with VTE may be treated with anticoagulant therapy.
Treatment of HHT patients also includes immunosuppressant therapy. Tacrolimus, for example, is an activator of the ALK1-SMAD1/5/8 signaling pathway and can ameliorate defects caused by mutations in ALK1.14
4 CONCLUSIONS
In summary, HHT continues to be a rare disease in the clinic. For the treatment and prognosis of HHT, a large amount of medical evidence is still needed to provide a more theoretical basis to guide clinical work. We hope to explore the diagnosis and treatment of the disease at the gene level.
CONFLICT OF INTEREST
All authors declare that there is no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The date that supports the finding of this study are openly available in PubMed




