Heterogeneous nuclear ribonucleoprotein A3 binds to the internal ribosomal entry site of enterovirus A71 and affects virus replication in neural cells
Abstract
Enterovirus A71 (EV-A71) belongs to the genus Enterovirus of the Picornaviridae family and often causes outbreaks in Asia. EV-A71 infection usually causes hand, foot, and mouth disease and can even affect the central nervous system, causing neurological complications or death. The 5′-untranslated region (5′-UTR) of EV-A71 contains an internal ribosome entry site (IRES) that is responsible for the translation of viral proteins. IRES-transacting factors can interact with the EV-A71 5′-UTR to regulate IRES activity. Heterogeneous nuclear ribonucleoprotein (hnRNP) A3 is a member of the hnRNP A/B protein family of RNA-binding proteins and is involved in RNA transport and modification. We found that hnRNP A3 knockdown promoted the replication of EV-A71 in neural calls. Conversely, increasing the expression of hnRNP A3 within cells inhibits the growth of EV-A71. HnRNP A3 can bind to the EV-A71 5′-UTR, and knockdown of hnRNP A3 enhances the luciferase activity of the EV-A71 5′-UTR IRES. The localization of hnRNP A3 shifts from the nucleus to the cytoplasm of infected cells during viral infection. Additionally, EV-A71 infection can increase the protein expression of hnRNP A3, and the protein level is correlated with efficient viral growth. Based on these findings, we concluded that hnRNP A3 plays a negative regulatory role in EV-A71 replication within neural cells.
1 INTRODUCTION
Enterovirus A71 (EV-A71) belongs to the Enterovirus genus of Picornaviridae. It was first isolated from patients with encephalitis in 1969 in California, USA.1 Since then, outbreaks of EV-A71 infection have been reported worldwide, including in Europe, Australia, Singapore, Malaysia, Japan, Vietnam, and mainland China. EV-A71 has become a global public health threat, especially in the Asia-Pacific region.2 Taiwan experienced an epidemic of EV-A71 infection in 1998, leading to more than 100,000 people becoming infected and 78 fatalities.3 EV-A71 is a highly neurotropic virus that can invade the central nervous system (CNS) to induce acute neurologic complications, such as aseptic meningitis, encephalitis, brainstem encephalitis, motor neuron death, neurogenic pulmonary edema, hemorrhage, or even death.4, 5 According to several autopsy findings, EV-A71 infects neurons, causes neuronal degeneration, and triggers inflammatory reactions that lead to encephalitis in the location of the lesion.6, 7 Infants with cardiopulmonary failure caused by EV-A71 infection occasionally exhibit long-term neurological morbidity.4 Therefore, it is important to understand the pathogenesis of EV-A71 in neural cells.
The genome of EV-A71 is a signal-stranded, positive-sense RNA genome. The 5′-untranslated region (5′-UTR) of EV-A71 contains a cloverleaf-like structure (stem‒loop I) and an internal ribosome entry site (IRES) region (stem‒loop II–VI).8 The EV-A71 RNA genome lacks the cap structure in the 5′-UTR; thus, viral protein translation is dependent on IRES binding with the ribosome to initiate protein translation. IRES-dependent translation is regulated not only by eukaryotic translation initiation factors (eIFs) but also by some cellular proteins called IRES trans-acting factors (ITAFs).9 ITAFs can promote or suppress viral translation by regulating the activity of the IRES. Many ITAFs have been shown to bind to the IRES region with RNA-binding domains.10 The ITAFs of EV-A71 include many heterogeneous nuclear ribonucleoprotein (hnRNP) family members, such as hnRNP A1,11 hnRNP A2,12 hnRNP EI (poly(C)-binding protein 1, PCBP1),13 hnRNP D (AU-rich element-binding protein 1, AUF1)14 and hnRNP K,15 which have been reported to regulate EV-A71 translation and replication through different mechanisms. The hnRNP family is a family of RNA-binding proteins that contains more than 20 distinct proteins (hnRNPs A-U).16 In the process of messenger RNA (mRNA) maturation, which includes transcriptional regulation, capping, splicing, and polyadenylation in the nucleus, various proteins associate with mRNAs to form messenger ribonucleoprotein particles (mRNPs).17, 18 HnRNPs are the most abundant components of mRNPs.16 Additionally, numerous hnRNPs have nuclear localization sequences that cause them to move back and forth between the cytoplasm and nucleus to control mRNA translation and nucleocytoplasmic transport.17 HnRNP A3 is a member of the hnRNP A/B protein family, which includes hnRNP A0, A1, A2/B1, and A3. HnRNP A3 has structural features and sequences similar to those of other hnRNP A/B protein members. Therefore, we were interested in determining whether hnRNP A3 can regulate EV-A71 infection in neural cells.
In this study, neuroblastoma IMR-32 and differentiated IMR-32 cells were used to be infected with EV-A71, and short interfering RNA (siRNAs) targeting hnRNP A3 and hnRNP A3 plasmids were transfected into neural cells to investigate the effect of hnRNP A3 on EV-A71 replication. The interaction between hnRNP A3 and the EV-A71 5′-UTR and the effect of hnRNP A3 on IRES activity were both demonstrated in neural cells.
2 MATERIALS AND METHODS
2.1 Cell culture and virus infection
Neuroblastoma IMR-32 cells were cultured in Dulbecco's modified Eagle medium (DMEM; Thermo Fisher Scientific) supplemented with 10% HyClone fetal bovine serum (FBS; Cytiva) and 1% l-glutamine (Gibco; Thermo Fisher Scientific). For neural differentiation, IMR-32 cells were seeded in 12-well plates at a concentration of 3 × 105 cells/well for 2 days, after which the culture medium was changed to neurobasal medium (Gibco; Thermo Fisher Scientific) supplemented with 10 μM retinoic acid (Sigma-Aldrich), 1% FBS, and 1% l-glutamine. Human rhabdomyosarcoma (RD) cells were cultured in DMEM supplemented with 10% newborn calf serum, 1% penicillin/streptomycin, 1% l-glutamine, and 1% nonessential amino acids (all from Gibco; Thermo Fisher Scientific). Enterovirus A71 (4643/1998/TW) was used in this study. For virus infection, IMR-32 cells were seeded in 12-well plates at a concentration of 3 × 105 cells per well for 2 days, and differentiated IMR-32 cells were used for infection at 7 days postdifferentiation. EV-A71 (2 multiplicity of infections (MOIs) solution prepared in serum-free DMEM or neurobasal medium was added to the cells for viral adsorption. After 1 h, the viral solution was removed from the cells, and phosphate buffer saline (PBS) was used to wash the cells once. DMEM containing 2% FBS or neurobasal medium was added to the cells, and the cells were incubated at 37°C in 5% CO2.
2.2 Plaque assay
RD cells were seeded in 6-well plates at a concentration of 5 × 105 cells/well overnight. The cells were washed with PBS once, and then the serially diluted virus solution was added to the RD. After 1 h of incubation, the viral solution was removed, and the cells were washed twice with PBS. DMEM supplemented with 2% FBS and 0.3% agarose (PB1200; Bioman) was added to the cells. After 72 h of incubation, 5% formaldehyde (Sigma-Aldrich) was used to fix the cells for 24 h. Then, the agarose medium was removed, and the plaques were visualized with 0.5% crystal violet staining.
2.3 Immunofluorescence assay (IFA)
The IMR-32 cells were seeded in 12-well plates at a concentration of 3 × 105 cells/well. After 2 days, the cells were infected with EV-A71 at an MOI of 2. Differentiated IMR-32 cells were infected with 2 MOIs of EV-A71 at 7 days postdifferentiation. At the indicated time points, the culture medium was removed from the plates, and 4% cold paraformaldehyde was used to fix the cells at room temperature for 15 min. Then, 0.5% Triton X-100 was used to permeabilize the cells for 5 min. The cells were blocked with 2% FBS at room temperature for 30 min. The primary antibodies used in this study were rabbit anti-hnRNP A3 (1:100; Proteintech) and mouse anti-EV-A71 3D (1:100). The cells were incubated with primary antibodies at 4°C overnight. The secondary antibodies included DyLight 594nm-conjugated donkey anti-mouse secondary antibody or DyLight 488 nm-conjugated goat anti-rabbit secondary antibody (1:1000; Jackson ImmunoResearch Laboratories), and the cells were incubated with secondary antibodies at room temperature for 2 h.4′,6-diamidino-2-phenylindole (DAPI, 1:1000; Sigma-Aldrich) was used to stain the cell nuclei. The images were observed using a fluorescence microscope (Olympus BX51).
2.4 Western blot
The cellular lysate was harvested using CA630 protein lysis buffer (1% CA-630, 50 mM Tris, 150 mM NaCl, and 1× protease inhibitor cocktail). The protein concentration was quantified with the Bradford method (Bio-Rad Laboratories). The protein samples were separated according to molecular weight using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene fluoride membranes (GE). The membranes containing protein samples were blocked in 5% skim milk in Tris-buffered saline with Tween-20 (TBST, 20 mmol/mL Tris-HCl [pH 7.4], 150 mmol/L NaCl, and 0.1% Tween-20) at room temperature. The membranes were incubated with rabbit anti-hnRNP A3 (1:1000; Proteintech), goat anti-FBP3 (1:1000; Santa Cruz), mouse anti-EV-A71 3D (1:1000), mouse anti-Flag (1:2000; Sigma-Aldrich), mouse anti-β-actin (1:10,000; Sigma-Aldrich), mouse anti-β tubulin (1:1000; Cell Signaling), or rabbit anti-Lamin B (1:1000; Santa Cruz) antibodies overnight at 4°C. TBST was used to wash the membranes. Subsequently, the membranes were incubated with horseradish peroxidase-conjugated anti-mouse, anti-rabbit, or anti-goat secondary antibodies (1:2500; Jackson ImmunoResearch Laboratories). A chemiluminescence agent was used to visualize the proteins (PerkinElmer), and signals were detected with a ChemiTM imaging system (Bio-Rad).
2.5 siRNA and plasmid transfection
A specific siRNA targeting hnRNP A3 (5′-CAAUGUGUGCUCGACCACA-3′; 5′-UGUGGUCGAGCACACAUUG-3′; Sigma-Aldrich) and scrambled RNA (5′-GAUCAUACGUGCGAUCAGA-3′, 5′-UCUGAUCGCACGUAUGAUC-3′; Sigma-Aldrich) were prepared as a stock solution of 100 μM using RNase-free distilled water. Lipofectamine 2000 RNAiMAX (Thermo Fisher Scientific) was used for siRNA transfection. The siRNA (final concentration: 100 nM) and Lipofectamine 2000 RNAiMAX were mixed in Opti-MEM (Thermo Fisher Scientific) and incubated for 5 min at room temperature. The culture medium was removed from the cells, and Opti-MEM was added to the cells. A mixture of siRNA and Lipofectamine 2000 RNAiMAX was added to the cells, which were then incubated for 6 h at 37°C in 5% CO2. After decanting the siRNA and Lipofectamine 2000 RNAiMAX-containing Opti-MEM, fresh culture medium was added for the incubation process. After 72 h of transfection, immunoblot analysis was used to evaluate the knockdown efficiency, and virus infection experiments were performed. The hnRNP A3 plasmid was constructed as follows: the polymerase chain reaction (PCR) product of the hnRNP A3 isoform was inserted into the p3xFLAG-MYC-CMV26 vector using HindIII and Kpn I (a gift from Dr. Rei-Lin Kuo, Chang Gung University, Taiwan). The hnRNP A3 plasmids (1 μg) were transfected into cells with Lipofectamine 2000 (Thermo Fisher Scientific). The plasmids and Lipofectamine 2000 were diluted in Opti-MEM for 20 min at room temperature. The culture medium of the cells was replaced with a fresh culture medium, and then a mixture of plasmids and Lipofectamine 2000 was added to the cells. At 72 h posttransfection, the cell lysate was harvested, and a western blot analysis was performed to confirm the expression of hnNRP A3 plasmids with an anti-Flag antibody.
2.6 Real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted with TRIzol reagent (Life Technologies). Reverse transcription was performed with a RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) according to the manufacturer's instructions. qPCR assays were performed on complementary DNA (cDNA) samples with 5 μM forward and reverse primers and SYBR Green (Kapa Biosystems) in a Roche Light Cycler 480 (Roche). 18S ribosomal RNA (rRNA) was used as a reference gene. Each sample was analyzed in triplicate. The PCR program was as follows: preincubation, 95°C, 3 min, one cycle; amplification, 95°C, 10 s, 55°C, 20 s, 72°C, 10 s, 40 cycles; melting curve, 95°C, 5 s, 65°C, 1 min, one cycle; and cooling, 40°C, 10 s, one cycle. The approach was used to determine each gene's relative expression level. The sequences of primers used were as follows: EV-A71 5′-UTR forward, CCC TGA ATG CGG CTA ATC C; EV-A71 5′-UTR reverse, ATT GTC ACC ATA AGC AGC CA; hnRNP A3 forward, ATG GAA GAC AGG CAG AGT GG; hnRNP A3 reverse, GAT CCA CCT CCA CGA CCT CT; 18S rRNA forward, GTA ACC CGT TGA ACC CCA TT; and 18S rRNA reverse, CCA TCC AAT CGG TAG TAG CG.
2.7 Monocistronic expression assay
IMR-32 or differentiated IMR-32 cells were transfected with scrambled RNA or sihnRNPA3 for 3 days. Then, monocistronic mRNA containing the EV-A71 5′-UTR and firefly luciferase (FLuc) was transfected into the cells. After 6 h, the total lysate was harvested to detect FLuc activity. A dicistronic reporter plasmid (pRHF-EV-A71, a gift from Dr. Shin-Ru, Shin, Chang Gung University, Taiwan) was transfected into hnRNP A3 knockdown differentiated IMR-32 cells. After 48 h, the cell extract was harvested to detect the activity of Renilla luciferase (RLuc) and FLuc using a dual-luciferase reporter assay (Promega) according to the manufacturer's instructions.
2.8 Preparation of cell extracts
The differentiated IMR-32 cells were pelleted, washed three times with cold PBS, resuspended in 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate hydrate (CHAPS) buffer (10 mM Tris-HCl [pH 7.4], 1 mM MgCl2, 1 mM Ethyleneglycol- bis(β-aminoethyl)-N,N,Nʹ,Nʹ-tetraacetic Acid, 0.5% CHAPS, 10% glycerol, 0.1 mM Phenylmethylsulfonyl fluoride2-ME: 2-Mercaptoethanol, 5 mM 2-ME), and then incubated on ice for 30 min for swelling. The cells were centrifuged at 13,000 rpm for 10 min at 4°C, and the supernatants were collected for further analysis. The protein concentrations of the cell extracts were determined using a Bio-Rad protein assay (Bio-Rad).
2.9 Preparation of biotinylated RNA
The 5′-UTR of EV-A71, whose sequence was shown in Supporting Information S1: Table 1, was amplified by PCR from the EV-A71 full-length infectious cDNA clone using EV-A71 5′ (GCC GGT AAT ACG ACT CAC TAT AGG GAG ATT AAA ACA GCC TGT GGGT) and EV-A71 5′ (CAT GTT TGA TTG TGT TGA GGG TCA AAAT) primers that contained the T7 promoter.19 The T7 promoter–EV71 5′-UTR fragment flanked by EcoRI sites was excised from the vector pCRII-TOPO. RNA transcripts were synthesized using a MEGAscriptTM T7 Transcription Kit (Thermo Fisher Scientific) following the protocol provided by the manufacturer. Biotinylated RNA was synthesized in a 20 μL T7 MEGAscript transcription reaction by adding 1.25 μL of 20 mM biotin-16-UTP(BD). The synthesized RNAs were purified with a RNeasy Protect Mini Kit (Qiagen).
2.10 RNA‒protein pull-down assay
The reaction mixtures contained 200 µg of the differentiated IMR-32 cell extract protein and 3 µg of biotinylated EV-A71 5′-UTR RNA (or fragments of the EV-A71 5′-UTR). RNA mobility shift buffer (5 mM 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid, 40 mM KCl, 0.1 mM EDTA, 2 mM MgCl2, 2 mM dithiothreitol, 1 U of RNasin, and 0.25 mg/mL heparin) was used to bring the reaction mixture's final volume to 100 µL. The mixture was incubated for 15 min at 30°C, and then 400 µL of Streptavidin MagneSphere Paramagnetic Particles (Promega) was added for binding at room temperature for 10 min. The RNA mobility shift buffer without heparin was used to wash the beads containing RNA–protein complex five times. After the last wash, 20 µL of 6× SDS-PAGE sample buffer was added to the beads, and the mixtures were incubated at room temperature for 10 min. After boiling the eluted proteins, 10% SDS-PAGE was used to fractionate them. Western blot analysis was performed to detect the expression of hnRNP A3 and FBP3. For the competition assay, various amounts of unlabeled 5′-UTR RNA or EV-A71 VP3 RNA were added to compete with the biotinylated EV-A71 5′-UTR in the RNA‒protein pull-down assay.
2.11 Cell cycle analysis
The cells were harvested and centrifuged at 1000 rpm for 5 min to form cellular pellets. The cellular pellets were resuspended in 2.5 mL of 70% ethanol and incubated on ice for 15 min. The suspended cells were centrifuged at 1500 rpm for 5 min. The supernatant was removed, and then 500 μL of propidium iodide (PI) solution (50 μg/mL PI, 0.1 mg/mL RNase A, 0.05% Triton X-100) was added to the cell pellets, and the samples were incubated at 37°C for 40 min. Then, 3 mL of PBS was added to the samples, and the samples were then centrifuged at 1500 rpm for 5 min. The supernatant was decanted, and 1 mL of PBS was used to resuspend the cell pellets. Flow cytometry was performed to analyze the cell cycle status.
2.12 Isolation of proteins in the nucleus and cytoplasm
The nuclear extraction kit (Abcam) was used according to the manufacturer's instructions. The culture medium was removed, and the cells were washed with PBS twice. Fresh PBS was added to the cells, and the cells were scraped into 15 mL tubes. The cells were centrifuged for 5 min at 1000 rpm, after which the supernatant was discarded. Pre-extraction buffer (1×) was used to suspend the cell pellet, which was then transferred to 1.5 mL tubes. The cells were incubated on ice for 10 min and vortexed vigorously for 10 s. Then, the preparation was centrifuged for 1 min at 12,000 rpm. The supernatant was the cytoplasmic extract. The nuclear pellet was resuspended in extraction buffer containing Dithiothreitol and protease inhibitor (1×). The extract was incubated on ice for 15 min with vortexing for 5 s every 3 min. After centrifugation for 10 min at 14,000 rpm and 4°C, the supernatant containing nuclear extract was collected.
2.13 Statistical analysis
The measurement data are expressed as the mean ± standard deviation. Statistical significance was determined by Student's unpaired t test.
3 RESULTS
3.1 Knockdown of hnRNP A3 can increase EV-A71 replication
To determine whether hnRNP A3 affects EV-A71 replication, IMR-32 cells were transfected with siRNA targeting hnRNP A3 to conduct a knockdown assay. Western blot analysis was performed to confirm the knockdown efficiency of siRNA targeting hnRNP A3 (Figure 1A). According to the RT-qPCR results, EV-A71 viral RNA replication was significantly greater in hnRNP A3-knockdown IMR-32 cells than in scrambled-transfected cells (Figure 1B). The expression of the viral protein was detected through western blot analysis. EV-A71 3Dpol expression was greater in hnRNP A3-knockdown IMR-32 cells than in control cells (Figure 1C). Western blot analysis revealed that the relative level of EV-A71 3Dpol was greater in hnRNP A3-knockdown cells than in control cells (Figure 1D). The EV-A71 viral growth curves were analyzed through a plaque assay. The results indicated that viral growth was also significantly greater in hnRNP A3-knockdown IMR-32 cells than in control cells (Supporting Information S1: Figure 1A). Next, we wanted to determine whether hnRNP A3 has a similar function in differentiated IMR-32 cells. Differentiated IMR-32 cells have been shown to express mature neuronal markers, including TUJ1, MAP2, and GAD67, and these cells are susceptible to EV-A71.20 Differentiated IMR-32 cells were transfected with siRNA targeting hnRNP A3 and then infected with EV-A71 at 72 h posttransfection. The knockdown efficiency of the siRNAs was confirmed through western blot analysis (Figure 1E). RT-qPCR revealed that EV-A71 viral RNA replication was also significantly greater in hnRNP A3-knockdown differentiated IMR-32 cells than in control cells (Figure 1F). The level of the viral protein 3Dpol was greater in hnRNP A3-knockdown differentiated IMR-32 cells than in scrambled RNA-transfected cells (Figure 1G). The quantitative findings of the western blot analysis revealed that the EV-A71 3Dpol level was greater in the hnRNP A3-knockdown cells than in the control cells. According to the results, hnRNP A3 knockdown increased EV-A71 RNA and protein expression in IMR-32 and differentiated IMR-32 cells.
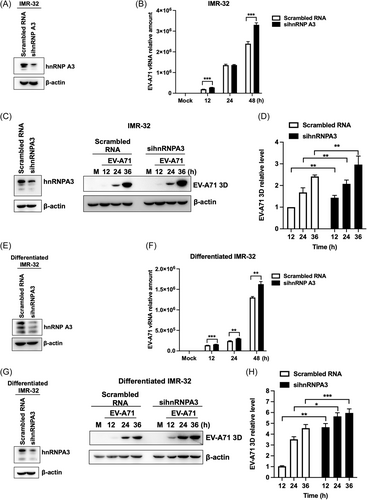
Knockdown of hnRNP A3 increases EV-A71 replication. IMR-32 cells were transfected with siRNAs against hnRNP A3 or scrambled RNA for 72 h and then infected with EV-A71 at an MOI of 2. (A) The knockdown efficiency of the siRNAs was confirmed by western blot analysis. (B) Total RNA was harvested at 12, 24, and 48 h postinfection. The expression of viral RNA was analyzed via RT-qPCR. (C) Western blot analysis was used to confirm the knockdown efficiency of sihnRNPA3 and detect the protein level of EV-A71 3Dpol. (D) The protein level of EV-A71 3Dpol was quantified using ImageJ software and normalized to the 3Dpol level in EV-A71-infected IMR-32 cells transfected with scrambled RNA at 12 h postinfection. IMR-32 cells were treated with 10 μM retinoic acid for neuronal differentiation. The differentiated IMR-32 cells were transfected with siRNA targeting hnRNP A3 or scrambled RNA and then infected with EV-A71 (MOI of 2). (E) Western blot analysis was performed to determine the knockdown efficiency of the siRNAs. (F) RT-qPCR was used to analyze the expression of the EV-A71 RNA genome. (G) The knockdown efficiency of sihnRBPA3 and the protein expression of EV-A71 3Dpol were detected through western blot analysis. (H) ImageJ software was used to quantify the protein level of EV-A71 3Dpol, and the results were normalized to the 3Dpol level of differentiated IMR-32 cells transfected with scrambled RNA at 12 h postinfection. β-Actin was used as an internal control for western blot analysis. The experiments were repeated in triplicate, and the error bars represent the mean value SD (*p < 0.05, **p < 0.01, ***p < 0.001; Student's unpaired t test). EV-A71, enterovirus A71; hnRNP, heterogeneous nuclear ribonucleoprotein; RT-qPCR, real-time quantitative polymerase chain reaction; siRNA, short interfering RNA.
3.2 Upregulation of hnRNP A3 can decrease EV-A71 replication in neuron-like cells
In previous studies, we investigated that the knockdown of hnRNP A3 could increase EV-A71 replication in IMR-32 and differentiated IMR-32 cells. Next, the effect of hnRNP A3 overexpression on EV-A71 replication in neuron-like cells was demonstrated. IMR-32 cells were transfected with plasmids containing hnRNP A3 and the tag protein 3xflag. After transfection for 72 h, western blot analysis was performed to confirm the overexpression efficiency (Figure 2A). Viral RNA replication was analyzed via RT-qPCR, and the results showed that the expression of viral RNA was significantly lower in hnRNP A3-overexpressing IMR-32 cells than in vector-transfected cells (Figure 2B). The relative level of the viral protein 3Dpol was also decreased in hnRNP A3-overexpressing cells (Figure 2C). A plaque assay was performed to measure EV-A71 growth in hnRNP A3-overexpressing IMR-32 cells. Compared with those of cells transfected with control vectors, the viral growth of cells transfected with hnRNP A3 plasmids was reduced (Supporting Information S1: Figure 1B). We also wanted to determine whether hnRNP A3 overexpression can decrease EV-A71 replication in differentiated IMR-32 cells. The transfection efficiency of the hnRNP A3 plasmids was confirmed through western blot analysis (Figure 2D). Differentiated IMR-32 cells were transfected with hnRNP A3 plasmids and then infected with EV-A71. The RT-qPCR results showed that viral RNA replication was decreased in hnRNP A3 plasmid-transfected cells compared to control cells (Figure 2E). The quantitative western blot results showed that the protein expression of EV-A71 3Dpol was also significantly lower in hnRNP A3-overexpressing cells than in vector-transfected cells (Figure 2F). As shown in this figure, we further demonstrated that the overexpression of hnRNP A3 decreased EV-A71 replication in neuron-like cells.
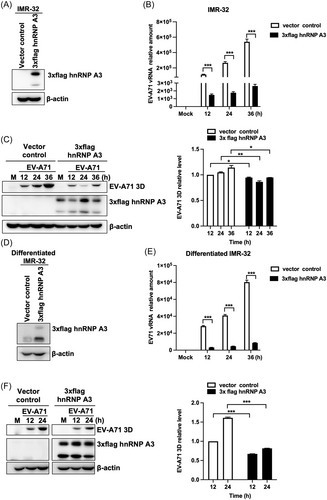
The upregulation of hnRNP A3 decreases EV-A71 replication in neuron-like cells. (A) IMR-32 cells were transfected with 3xFlag hnRNP A3 plasmids or vectors for 72 h, and western blot analysis was performed to detect the expression of hnRNP A3 with an anti-Flag antibody. At 72 h posttransfection, the IMR-32 cells were infected with 2 MOIs of EV-A71. (B) Total RNA was collected at 12, 24, and 36 h postinfection, and the expression of viral RNA was analyzed through RT-qPCR. (C) The cell lysates were harvested at the indicated time points. Western blot analysis was performed to analyze the protein levels of EV-A71 3Dpol and 3xflag hnRNP A3. The protein level of EV-A71 3Dpol was quantified using ImageJ software, and the results were normalized to the amount of 3Dpol in EV-A71-infected IMR-32 cells transfected with vectors at 12 h postinfection. The 3xFlag hnRNP A3 plasmids or vectors were transfected into differentiated IMR-32 cells for 72 h, after which the cells were infected with EV-A71 at an MOI of 2. (D) Western blot analysis was used to confirm the expression of 3xflag hnRNP A3 with an anti-Flag antibody. (E) RT-qPCR was performed to analyze the level of EV-A71 RNA at 12, 24, and 36 h postinfection. (F) EV-A71 3Dpol and 3xflag hnRNP A3 protein levels were detected using a western blot after the cell lysate was extracted at the times indicated. The expression level of 3Dpol was quantified with ImageJ software and normalized to the 3Dpol level in differentiated IMR-32 cells transfected with vectors at 12 h postinfection. The internal control for western blot analysis was β-actin. The experiments were repeated in triplicate, and the error bars represent the mean value SD (*p < 0.05, **p < 0.01, ***p < 0.001; Student's unpaired t test). EV-A71, enterovirus A71; hnRNP, heterogeneous nuclear ribonucleoprotein; RT-qPCR, real-time quantitative polymerase chain reaction.
3.3 HnRNP A3 is associated with the EV-A71 5′-UTR
We demonstrated that hnRNP A3 could affect the expression of viral RNA and protein in neuron-like cells. Therefore, we wanted to determine whether hnRNP A3 can interact with the EV-A71 5′-UTR to regulate viral replication. The EV-A71 5′-UTR contains six stem‒loop secondary structures, which are called IRESs (Figure 3A). According to the functions of these stem loops, in vitro transcription was used to synthesize the two truncated forms of the EV-A71 5′-UTR, which were labeled with biotin. Streptavidin beads were used in an RNA‒protein pull-down experiment to capture the biotin-labeled RNA and associated proteins from the cell lysate of differentiated IMR-32 cells. The results showed that the form truncated at nt 1–90, which forms a cloverleaf structure, and the form truncated at nt 91–745, which contains an IRES region, were all associated with hnRNP A3 (Figure 3B). To confirm the specific association between hnRNP A3 and the EV-A71 5′-UTR, different concentrations of nonbiotinylated EV-A71 5′-UTR RNA probes were used for the competition assay in the RNA‒protein pull-down experiment. The results showed that the association of hnRNP A3 was suppressed by increasing amounts of nonbiotinylated EV-A71 5′-UTR. FBP3, which has been demonstrated to associate with the EV-A71 IRES,21 was also outcompeted by the nonbiotinylated EV-A71 5′-UTR. (Figure 3C). To further demonstrate the binding ability between EV-A71 5′-UTR and hnRNP A3, the different amounts of nonbiotinylated EV-A71 VP3 RNA, which has a similar length with EV-A71 5′-UTR, were added for the competition assay. The results showed that nonbiotinylated EV-A71 5′-UTR RNA can outcompeted more hnRNP A3, compared to EV-A71 VP3 RNA (Supporting Information S1: Figure 2). It was demonstrated that EV-A71 5′-UTR could bind hnRNP A3 more strongly than EV-A71 VP3 RNA. According to the results, hnRNP A3 could interact with the EV-A71 5′-UTR.
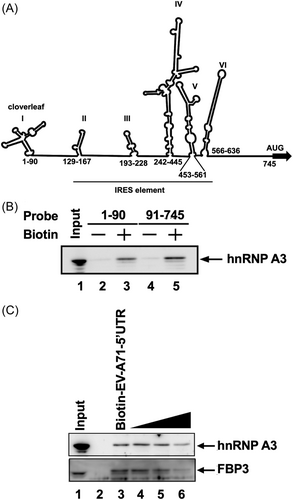
HnRNP A3 is associated with the EV-A71 5′-UTR.
(A) Schematic diagram of the EV-A71 5′-UTR RNA secondary structure. The nucleotide numbers indicate the region of each stem–loop. The stem–loop I is a cloverleaf structure. The range of the IRES element is from stem–loop II to stem–loop VI. (B) The 1–90 nt and 91–745 nt truncated forms of the EV-A71 5′-UTR were generated via in vitro transcription and biotinylation. An RNA‒protein pull-down assay was performed with the lysates of differentiated IMR-32 cells. Nonbiotinylated RNA probes were used as controls. An immunoblotting assay was used to detect the expression of hnRNP A3 in the pull-down complex. (C) To compete with the biotin-labeled EV-A71 5′-UTR for binding to hnRNP A3, the onefold, 10-fold, and 20-fold molar excess of the nonbiotinylated EV-A71 5′-UTR were added. The expression of hnRNP A3 in the pulled-down complex was analyzed by western blot analysis with an anti-hnRNP A3 antibody. FBP3 was also detected as a positive control. The total cellular lysate was loaded as input. 5′-UTR, 5′-untranslated region; EV-A71, enterovirus A71; hnRNP, heterogeneous nuclear ribonucleoprotein; IRES, internal ribosome entry site.
3.4 Downregulation of hnRNP A3 increases the translation of the EV-A71 IRES
EV-A71 viral RNA translation is dependent on the IRES. HnRNP A3 was shown to associate with the EV-A71 5′-UTR and affect viral protein translation in neuron-like cells. We further investigated whether hnRNP A3 affects the activity of the EV-A71 IRES. Monocistronic mRNA containing the EV-A71 IRES and FLuc were used to detect the activity of the EV-A71 IRES. The translation of FLuc was dependent on the EV-A71 IRES in monocistronic RNA. The expression level of FLuc was related to EV-A71 IRES activity. To determine the impact of hnRNP A3 on the EV-A71 IRES, a knockdown experiment was carried out after cell transfection with a siRNA targeting hnRNP A3. Western blot analysis was performed to confirm the knockdown efficiency of the siRNA targeting hnRNP A3 (Figure 4A). Monocistronic mRNA was transfected into IMR-32 cells after hnRNP A3 knockdown for 72 h, and the total lysate was harvested to measure the activity of FLuc at 6 h after transfection. The results showed that the activity of EV-A71 IRES-FLuc was significantly increased in EV-A71 IRES-transfected cells and was greater in hnRNP A3-knockdown IMR-32 cells than in scrambled-transfected cells. The transfection efficiency of EV-A71 IRES-FLuc RNA was confirmed by detecting the RNA level of the EV-A71 5′-UTR. The results showed that the amount of transfected EV-A71 5′-UTR RNA was not significantly different between scrambled RNA-transfected IMR-32 cells and sihnRNPA3-transfected cells (Figure 4B). Additionally, we measured the activity of EV-A71 IRES-FLuc in differentiated IMR-32 cells. Differentiated IMR-32 cells were transfected with siRNA targeting hnRNP A3 for 72 h, and western blot analysis was used to confirm the knockdown efficiency (Figure 4C). According to these findings, hnRNP A3-knockdown cells had substantially greater EV-A71 IRES FLuc activity than control cells. Under both conditions, the level of transfected EV-A71 IRES-FLuc RNA did not differ (Figure 4D). A dicistronic reporter plasmid containing RLuc and EV-A71 5′-UTR FLuc was transfected into differentiated IMR-32 cells transfected with scrambled RNA or sihnRNPA3. The translation of RLuc is cap dependent, which is as an internal control, and that of FLuc is EV-A71 IRES dependent. The total lysate was harvested for detecting RLuc and FLuc activity. The results showed that the activity of cap-dependent RLuc was not affected by hnRNP A3 knockdown. The activity of EV-A71 IRES-FLuc was also increased in the hnRNP A3 knockdown cells (Supporting Information S1: Figure 3). The downregulation of hnRNP A3 increased the activity of the EV-A71 IRES in both IMR-32 and differentiated IMR-32 cells. Therefore, hnRNP A3 acts as a negative regulator of EV-A71 IRES in neuron-like cells.
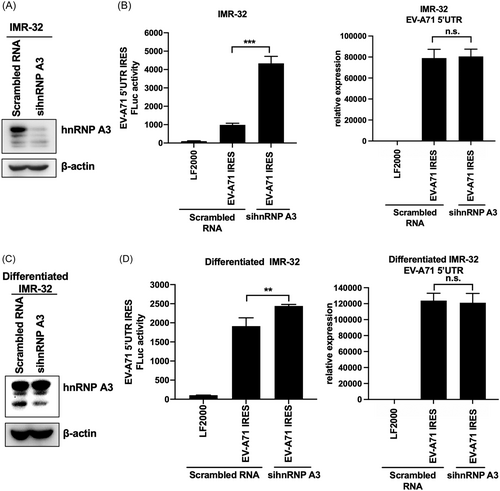
Downregulation of hnRNP A3 increases the translation of the EV-A71 IRES. IMR-32 cells were transfected with siRNA against hnRNP A3 or scrambled RNA for 72 h and then with monocistronic mRNA containing the EV-A71 5′-UTR and firefly luciferase. (A) The knockdown efficiency of the siRNAs was confirmed by western blot analysis. (B) EV-A71 5′-UTR-Fluc mRNA was transfected for 6 h, after which the total lysate was harvested to detect firefly luciferase activity. RT-qPCR was performed to confirm the transfection efficiency of EV-A71 5′-UTR-Fluc mRNA by detecting the RNA level of the EV-A71 5′-UTR. (C) Differentiated IMR-32 cells were transfected with siRNA against hnRNP A3 for 72 h, and then western blot analysis was performed to analyze the knockdown efficiency. (D) EV-A71 5′-UTR-FLuc mRNA was transfected into differentiated IMR-32 cells pretreated with sihnRNPA3. At 6 h posttransfection, the total lysate was collected for analysis of firefly luciferase activity. The transfection efficiency of EV-A71 5′-UTR-FLuc mRNA was confirmed by detecting the expression of the EV-A71 5′-UTR through RT-qPCR. β-Actin was used as an internal control for western blot analysis. The experiments were repeated in triplicate, and the error bars represent the mean value ± SD (*p < 0.05, **p < 0.01, ***p < 0.001, ns, not significant, Student′s unpaired t test). 5′-UTR, 5′-untranslated region; EV-A71, enterovirus A71; FLuc, firefly luciferase; hnRNP, heterogeneous nuclear ribonucleoprotein; IRES, internal ribosome entry site; mRNA, messenger RNA; RT-qPCR, real-time quantitative polymerase chain reaction; siRNA, short interfering RNA.
3.5 The localization of hnRNP A3 is redistributed in EV-A71-infected neuron-like cells
HnNRP A3 is a nuclear protein that is expressed in the nucleus. It is unknown how the EV-A71 5′-UTR and hnRNP A3 communicate. We investigated whether EV-A71 infection could redistribute hnRNP A3. An IFA was performed to detect the location of hnNPR A3 and EV-A71 3Dpol in EV-A71-infected IMR-32 and differentiated IMR-32 cells. The results indicated that hnRNP A3 was expressed in the nucleus in mock-infected cells. However, it was distributed in the cytoplasm in EV-A71-infected cells (Figure 5A,B). Nuclear and cytoplasmic extracts were isolated, and the results showed that the expression of hnRNP A3 in the cytoplasm increased during virus infection compared to that in the nucleus (Supporting Information S1: Figure 4). IFA was performed to detect the location of hnRNP A3 in EV-A71-infected RD cells. HnRNP A3 was also distributed in the cytoplasm from the nucleus (Supporting Information S1: Figure 5A). Overall, EV-A71 infection redistributed hnRNP A3 in neuron-like cells. This phenomenon may cause hnRNP A3 to interact with the EV-A71 5′-UTR.
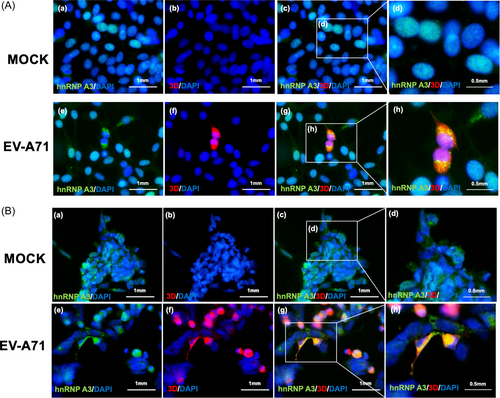
The localization of hnRNP A3 is redistributed in EV-A71-infected neuron-like cells. (A) IMR-32 cells and (B) differentiated IMR-32 cells were infected with EV-A71 at an MOI of 2, and an immunofluorescence assay was performed to detect the expression of hnRNP A3 and EV-A71 3Dpol at 8 h (IMR-32) or 12 h (differentiated IMR-32) postinfection. In the immunofluorescence assay, green indicates hnRNP A3, red indicates EV-A71 3Dpol, and DAPI was used to label the cellular nucleus. (a) mock-infection, cells were stained with anti-hnRNPA3 and DAPI; (b) mock-infection, cells were stained with anti-EV-A71 3Dpol and DAPI; (c) mock-infection, cells were stained with anti-hnRNPA3, anti-EV-A71 3Dpol, and DAPI; (d) the enlarged image of (c); (e) EV-A71-infection, cells were stained with anti-hnRNPA3 and DAPI; (f) EV-A71-infection, cells were stained with anti-EV-A71 3Dpol and DAPI; (g) EV-A71-infection, cells were stained with anti-hnRNPA3, anti-EV-A71 3Dpol, and DAPI; (h) the enlarged image of (g) Scale bar = 1 mm. DAPI, 4′,6-diamidino-2-phenylindole; EV-A71, enterovirus A71; hnRNP, heterogeneous nuclear ribonucleoprotein.
3.6 The effect of hnRNP A3 on the cell cycle distribution of neuron-like cells
The different stages of the cell cycle can affect viral replication. EV-A71 and CVA16 infection can induce S-phase arrest in infected cells to promote virus replication.22 Therefore, we investigated whether hnRNP A3 can affect EV-A71 replication by regulating the cell cycle of IMR-32 cells and differentiated IMR-32 cells. A knockdown assay was performed by transfecting IMR-32 cells with siRNA targeting hnRNP A3, and the knockdown efficiency was confirmed by western blot analysis (Figure 6A). The cell cycle status of hnRP A3-knockdown and control cells was analyzed through flow cytometry. The results showed that the percentage of cells in the G0/G1 phase was 59.59%, that of cells in the S phase was 17.09%, and that of cells in the G2/M phase was 23.32% in scrambled RNA-transfected IMR-32 cells. The percentage of hnRNP A3-knockdown cells in the G0/G1 phase was 61.96%, that in the S phase was 17.72%, and that in the G2/M phase was 20.32%. The cell cycle status did not obviously differ between the two conditions (Figure 6B). siRNA targeting hnRNP A3 was transfected into differentiated IMR-32 cells, and western blot analysis was performed to confirm the knockdown efficiency (Figure 6C). The percentages of control cells in the G0/G1 phase, S phase, and G2/M phase were 65%, 17.21%, and 17.79%, respectively, and the percentages of hnRNP A3-knockdown differentiated IMR-32 cells in the G0/G1 phase, S phase, and G2/M phase were 66.58%, 14.99%, and 18.43%, respectively (Figure 6D). There was no apparent alteration in the cell cycle status of differentiated IMR-32 cells between hnRNP A3-knockdown and control cells. The results showed that hnRNP A3 did not significantly affect the cell cycle status of neuron-like cells.
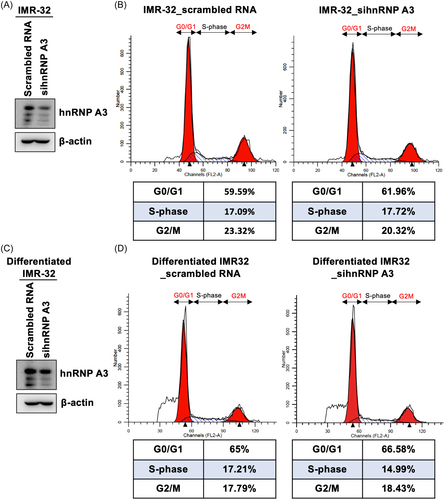
The effect of hnRNP A3 on the cell cycle distribution of neuron-like cells. (A) IMR-32 cells were transfected with siRNA targeting hnRNP A3 or scrambled RNA, and the knockdown efficiency was confirmed through western blot analysis. (B) At 72 h posttransfection, the cells were collected and stained with PI, and flow cytometry was performed to analyze the cell cycle status. (C) Differentiated IMR-32 cells were transfected with sihnRNPA3 or scrambled RNA for 72 h, and the knockdown efficiency of sihnRNPA3 was confirmed by western blot analysis. (D) Differentiated IMR-32 cells were collected and stained with PI, and the cell cycle distribution was analyzed via flow cytometry after siRNA transfection for 72 h. hnRNP, heterogeneous nuclear ribonucleoprotein; PI, propidium iodide; siRNA, short interfering RNA.
3.7 EV-A71 infection increases the expression of hnRNP A3 in neuron-like cells
HnRNP A3 can regulate EV-A71 replication by interacting with the EV-A71 5′-UTR. We wanted to determine whether EV-A71 infection affects the expression of hnRNP A3. To confirm the expression of hnRNP A3 in EV-A71-infected neural cells, IMR-32 and differentiated IMR-32 cells were infected with EV-A71, and cellular lysates were harvested at various time points. Western blot analysis was performed to measure the protein level of hnRNP A3. The results showed that the expression level of hnNPR A3 was increased with viral infection in IMR-32 and differentiated IMR-32 cells compared to that in mock-infected cells (Figure 7A,B). RD cells were infected with EV-A71, and hnRNP A3 expression was also increased after viral infection compared to that after mock infection (Supporting Information S1: Figure 5B). Therefore, EV-A71 infection could induce hnNRP A3 expression. To determine whether the increase in protein expression is related to EV-A71 replication, ultraviolet (UV)-irradiated EV-A71, which cannot actively replicate in cells, was used to infect IMR-32 cells. UV-inactivated virus did not increase the expression of the hnRNP A3 protein compared to that in active virus-infected cells (Figure 7C). Active viral replication is essential for increasing the hnRNP A3 protein level. We wanted to determine whether EV-A71 infection could also affect the RNA level of hnRNP A3. RT-qPCR was performed to analyze hnRNP A3 transcription in EV-A71-infected IMR-32 cells. The results indicated that the mRNA level of hnRNP A3 was not affected in the virus-infected cells (Figure 7D). RT-qPCR was also used to determine that the viral RNA content increased with infection time (Figure 7E). EV-A71 replication was necessary for inducing hnRPR A3 protein expression and did not affect the hnNPR A3 mRNA level.
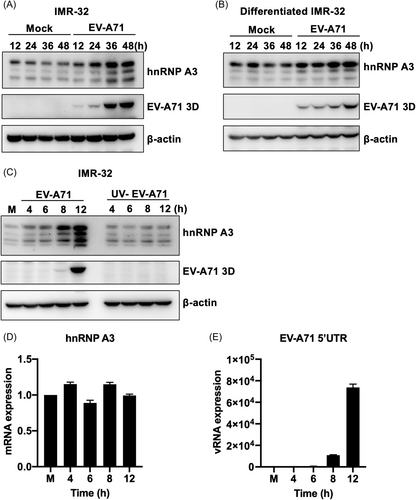
EV-A71 infection increases the expression of hnRNP A3 in neuron-like cells. (A) IMR-32 and (B) differentiated IMR-32 cells were infected with 2 MOIs of EV-A71. The total lysate was harvested at 12, 24, 36, and 48 h postinfection. Western blot analysis was performed to detect the expression of hnRNP A3 and EV-A71 3Dpol. The total lysate was collected at the indicated time points, and anti-hnRNP A3 and anti-EV-A71 3Dpol antibodies were used to detect the expression of hnRNP A3 and EV-A71 3Dpol through western blot analysis. β-Actin was used as an internal control. (C) IMR-32 cells were infected with EV-A71 or UV-inactivated EV-A71 at an MOI of 100, and the total lysate was harvested at 4, 6, 8, and 12 h postinfection. Western blot analysis was performed to detect the expression of hnRNP A3 and EV-A71 3Dpol. β-Actin was used as an internal control. IMR-32 cells were infected with EV-A71 (MOI of 100). Total RNA was collected at the indicated time points. (D) The mRNA expression of hnRNP A3 was analyzed through RT-qPCR. (E) RT-qPCR was performed to detect the expression of viral RNA. EV-A71, enterovirus A71; hnRNP, heterogeneous nuclear ribonucleoprotein; mRNA, messenger RNA; RT-qPCR, real-time quantitative polymerase chain reaction.
4 DISCUSSION
EV-A71 is a virus that typically causes uncomplicated hand–foot-and-mouth disease but can occasionally affect the CNS and induce diverse neurological complications, such as brainstem encephalitis, aseptic meningitis, and acute flaccid paralysis.4, 5 Among these complications, brainstem encephalitis is the most critical neurological manifestation because it can cause neurogenic pulmonary hemorrhage/edema, leading to death.5, 23 The exact mechanism by which EV-A71 causes neurological diseases is not fully understood. Nevertheless, it is believed that the virus can directly infect the CNS and cause inflammation or indirectly cause damage to the CNS through an immune response.5
EV-A71 infection can induce various biological effects in neural cells. The virus can proliferate in neural cells and cause neural cell lesions.24 Accumulating evidence indicates that EV-A71 can directly infect neurons in the CNS, and innate immune responses in the CNS are known to play a role in the pathogenesis of EV-A71 infection.25 However, the cellular factors implicated in EV-A71 replication in neuronal cells are unclear. A previous study demonstrated that PRSS3 could interact with the EV-A71 3A protein and thus affect viral replication.26 Additionally, Aβ1–42, a self-associating neurotoxic peptide, can inhibit EV-A71 replication in SH-SY5Y cells.27 Aberrant hnRNP function can lead to dire functional consequences, and its role in neurological diseases has been investigated in several studies.28-31 Recent studies have revealed that hnRNPs are implicated in RNA virus replication, for instance, in the replication of porcine reproductive and respiratory syndrome virus-2, severe acute respiratory syndrome coronavirus disease 2, and hepatitis E virus.32-34 Several hnRNPs, including hnRNP A1, AUF1/hnRNP D, and PCBP1/hnRNP E1,12-14 have the ability to modulate the replication of EV-A71, suggesting that multiple hnRNPs can regulate EV-A71 propagation. HnRNP A3 is expressed in human neural stem cells and controls mitotic progression.35 Furthermore, hnRNP A3 is present in inclusion bodies in the dentate gyrus and cerebellum of patients with frontotemporal lobar degeneration.36 These observations suggest that hnRNP A3 plays essential roles in neuronal lineage cells. The results of this study revealed that hnRNP A3 modulates EV-A71 replication in neuronal cells.
To confirm whether hnRNP A3 can affect EV-A71 infection, a transfection assay with siRNAs specific for the hnRNP A3 and hnRNP A3 plasmids was performed. According to the results, viral RNA and protein levels were increased in hnRNP A3-knockdown cells but decreased in hnRNP A3-overexpressing cells. HnRNP A3 was investigated as a negative regulator of EV-A71 infection in neural-like cells. EV-A71 viral RNA replication and viral protein translation are regulated by the IRES of the 5′-UTR. An RNA‒protein pull-down assay was performed for the first time to demonstrate that hnRNP A3 can bind to the highly structured EV-A71 5′-UTR. HnRNP A3 was shown to bind to the cloverleaf structure (1–90 nt) and IRES region (91–745 nt). The EV-A71 5′-UTR contains six stem–loops. Stem–loop I appears to be a cloverleaf structure that is responsible for regulating the replication of viral positive and negative strands.37 Stem–loop II can regulate IRES translation with ITAFs,11 and stem–loop III is responsible for binding with ITAFs and is required for IRES activity.38 Stem–loop VI can promote the binding of the 43S ribosomal preinitiation complex, causing scanning to continue until the initiation codon.39 The ability of the identified hnRNP ITAFs, such as hnRNP A1, PCBP1/hnRNP EI, and hnRNP K, to specifically bind to the IRES structure of the EV-A71 5′-UTR was also investigated.11, 13, 15 Nonbiotinylated EV-A71 5′-UTR RNA probes have a better affinity for ITAFs than biotinylated RNA probes, so they were used for the RNA affinity capture assay. The binding of hnRNP A3 to the biotinylated EV-A71 5′-UTR was competitively eliminated by the nonbiotinylated EV71 5′-UTR in a concentration-dependent manner. Monocistronic EV-A71 IRES FLuc mRNA was transfected into neuron-like cells, and luciferase activity was greater in hnRNP A3-knockdown cells than in scrambled RNA-transfected cells. Moreover, hnRNP A3 was shown to be an ITAF of EV-A71, and it can negatively regulate the activity of the EV-A71 IRES. However, hnRNP A1 and A2, which belong to the same protein family as hnRNP A3, cooperatively upregulate EV-A71 replication.12 In the ITAFs of EV-A71, FBP2, and AUF1/hnRNP D have been shown to negatively regulate the activity of the EV-A71 IRES.14, 40 FBP2 and AUF1 are AU-rich element-binding proteins that can control the degradation or translation of many ARE-mRNAs.41, 42 Therefore, it has been hypothesized that FBP2 destroys viral RNA and inhibits viral replication by binding to the IRES of EV-A71.40 AUF1 can inhibit viral growth by targeting the 3′-UTR of coxsackievirus B3 (CVB3) for degradation.43 Additionally, AUF-1 can compete with hnRNP A1 for binding with the IRES of EV-A71, reducing the activity of the IRES by restricting the access of positive-acting ITAFs to the IRES.14 HnRNP A3 is not an ARE-binding protein. However, future studies should explore whether hnRNP A3 may affect the activity of the IRES by competing for the binding site of the IRES with positive-acting ITAFs.
HnRNP A3 was localized to the cell nucleus after mock infection. It was relocalized to the cytoplasm during EV-A71 infection in neural cells. Some ITAFs of EV-A71, such as FBP1,44 FBP2,40 FBP3,21 hnRNP K,15 hnRNP A1,12 AUF1,14 and PTBP1,45 which are primarily localized in the nucleus, are redistributed to the cytoplasm in EV-A71-infected cells. The redistribution of hnRNP A1 is also observed in mouse hepatitis virus- and hepatitis C virus-infected cells.46, 47 Two possibilities may explain the causes of nuclear protein redistribution in virus-infected cells. One is that the freshly produced nuclear proteins cannot enter the nucleus because the nuclear proteins are trapped by unknown viral agents and damaged import pathways. The alternative idea is that pathways mediated by viruses enhance the export of nuclear proteins into the cytoplasm.44 Poliovirus infection causes alteration of the nuclear pore complex, and viral 2A protease can cleave the nucleoporins Nup153, 62, and 98, which impairs nucleocytoplasmic trafficking.48 The EV-A71 2A protease has been shown to cleave the nucleoporin Nup62 and prevent its nuclear transport.49 These possibilities may cause these nuclear proteins to be trapped in the cytoplasm and bind to the IRES.
HnRNP A3 is highly expressed in the neural progenitors of the mouse and human cortex and is involved in regulating the mitotic progression of neural progenitors.35 CVB3 polyprotein expression is significantly influenced by various cell cycle statuses. Cell cycle arrest at the G1 or G1/S phase can increase CVB3 viral protein expression. CVB3 growth is obviously reduced at the G0 and G2/M phases.50 Therefore, controlling cell cycle arrest at various phases is a strategy for controlling viral infection. However, our results showed that hnRNP A3 expression does not significantly affect the status of neuron-like cells. We infer that hnRNP A3 does not influence viral proliferation by controlling the cell cycle.
The protein level of hnRNP A3 is increased in EV-A71-infected neuron-like cells. However, the mRNA expression of hnRNP A3 was not affected by infection with EV-A71. We also investigated that hnRNP A3 protein upregulation is dependent on EV-A71 replication. According to previous research, the EV-A71 2A protease can cleave eIF4G, which lacks the eIF4E-binding domain and prevents cap-dependent translation. Cleaved eIF4G can bind and enhance the activity of the viral IRES.51 However, the expression of some proteins was increased in virus-infected cells. For example, compared to that in mock-infected cells, the protein level of PTB-associated splicing factor (PSF) was increased in CVB3-infected HeLa S3 cells. The increased protein expression is attributed to the IRES structure in the 5′-UTR of the PSF mRNA, which causes the protein to be translated via a cap-independent pathway.52 However, the hnRNP family has not been demonstrated to have an IRES structure at the 5′-UTR of the mRNA. EV-A71 RNA-dependent RNA polymerase 3D has been demonstrated to cosediment small and large subunits of ribosomes in EV-A71-infected cells and to join the functional ribosome complex. Furthermore, it has been shown that EV-A71 3Dpol positively regulates both host cap-dependent translation and EV-A71 IRES-dependent translation.53 According to our results, 3Dpol was consistently expressed in EV-A71-infected IMR-32 cells and differentiated IMR-32 cells. Therefore, this could be the cause of the increase in hnRNP A3 protein expression in neuron-like cells infected with EV-A71.
In summary, hnRNP A3 was first shown to bind to the EV-A71 5′-UTR and affect IRES activity. HnRNP A3 is a negative ITAF of the EV-A71 IRES and inhibits viral RNA replication and protein translation in neural cells. This study provides insights into the mechanisms of proteins that affect EV-A71 replication in neural cells, which will hopefully eventually inform the development of medicines to treat the neural symptoms of EV-A71 infection.
AUTHOR CONTRIBUTIONS
Jhao-Yin Lin carried out the experiments, performed the data analysis, and prepared the draft. Jing-Yi Lin analyzed the results and supervised the experiments. Rei-Lin Kuo supervised the experiment. Hsing-I Huang analyzed the results and supervised the experiments.
ACKNOWLEDGMENTS
This work was financially supported by the Research Center for Emerging Viral Infections from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan. This work was also funded by the Ministry of Science and Technology, Taiwan (111-2320-B-182-023, 112-2320-B-182-048-MY3), Chang Gung University, Taiwan (BMRPB33), and Chang Gung Memorial Hospital, Taiwan (CMRPD1M0931, CMRPD1M0932).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




