NUSAP1 regulates mouse oocyte meiotic maturation
Lina Yu, Na Kong, and Yuling Lin contributed equally to this work.
Abstract
The correct assembly of the spindle apparatus directly regulates the precise separation of chromosomes in mouse oocytes, which is crucial for obtaining high-quality oocytes capable of successful fertilization. The localization, assembly, migration, and disassembly of the spindle are regulated by a series of spindle-associated proteins, which exhibit unique expression level variations and specific localization in oocytes. Proteomic analysis revealed that among many representative spindle-associated proteins, the expression level of nucleolar and spindle-associated protein 1 (NUSAP1) significantly increased after meiotic resumption, with a magnitude of change higher than that of other proteins. However, the role of NUSAP1 during oocyte meiosis maturation has not been reported. Here, we report that NUSAP1 is distributed within the cell nucleus during the germinal vesicle (GV) oocytes with non-surrounded nucleolus stage and is not enriched in the nucleus during the GV-surrounded nucleolus stage. Interestingly, NUSAP1 forms distinct granular aggregates near the spindle poles during the prophase of the first meiotic division (Pro-MI), metaphase I, and anaphase I/telophase I stages. Nusap1 depletion leads to chromosome misalignment, increased aneuploidy, and abnormal spindle assembly, particularly a decrease in spindle pole width. Correspondingly, RNA-seq analysis revealed significant suppression of the “establishment of spindle orientation” signaling pathway. Additionally, the attenuation of F-actin in NUSAP1-deficient oocytes may affect the asymmetric division process. Gene ontology analysis of NUSAP1 interactomes, identified through mass spectrometry here, revealed significant enrichment for RNA binding. As an RNA-binding protein, NUSAP1 is likely involved in the regulation of messenger RNA homeostasis by influencing the dynamics of processing (P)-body components. Overall, our results demonstrate the critical importance of precise regulation of NUSAP1 expression levels and protein localization for maintaining mouse oocyte meiosis.
1 INTRODUCTION
The quality of oocytes determines female reproductive capacity. With an increase in hormone levels, immature oocytes that are arrested in prophase I of meiosis restart meiosis, marked by germinal vesicle breakdown (GVBD). Subsequently, the oocytes undergo the first meiotic division and are arrested metaphase II (MII), waiting for the arrival of the sperm. This process results in one larger MII-stage oocyte and one smaller first polar body (Pb1) through asymmetric division.1 After the resumption of meiosis in the oocyte, the establishment and migration of meiotic spindles within the cytoplasm, followed by their relocation toward the cortex, are essential prerequisites for achieving asymmetrical division.2 The formation, positioning, and migration of spindles are critical steps for proper chromosome separation. Spindle assembly defines the geometric shape of cell division and triggers the subsequent fate differentiation of daughter cells.3, 4 Spindle assembly plays a crucial role in ensuring accurate chromosome separation. It not only defines the geometric shape of cell division but also initiates the subsequent fate differentiation of daughter cells.
The role of the spindle in oocyte meiosis involves several processes, including spindle positioning, assembly, migration, and disassembly. Unlike in mitosis, mouse oocytes lack a centrosome but possess small microtubule (MT) organizing centers (MTOCs), which initially disperse in the cytoplasm and then cluster near the chromosomes to form a bipolar spindle. This suggests that the self-organization of these structures drives the assembly of an acentriolar spindle.5 Subsequently, MTs and actin begin to grow and diverge from the MTOCs. Recent findings have highlighted the importance of actin in addition to MTs for proper functioning of oocyte spindles.6, 7 The positioning of the spindle in mouse oocytes relies on a dynamic network of actin filaments, vesicles, and various myosin proteins that exert pulling or pushing forces.8, 9 The spindle does not migrate to a predetermined site but rather along its long axis toward the pole that is closer to the oocyte surface.10 As the spindle in MII oocytes is maintained, the spindle in Pb1 begins to disassemble. Although recent studies have explored the dynamic changes in messenger RNA (mRNA) and protein repositories within oocytes using single-cell transcriptomics or proteomics approaches,11, 12 there is still a lack of detailed investigation into the dynamic changes in key components involved in spindle assembly during MI/MII stage oocytes.
Nucleolar and spindle-associated protein 1 (NUSAP1) is a spindle-associated protein that possesses a DNA-binding SAP domain, a MT-binding domain, and a charged helical domain.13, 14 Initially, identified as selectively expressed in proliferating cells, particularly tumor cells,15-17 NUSAP1 has been identified as a clinical molecular marker for aggressive ovarian cancer.17 The potential molecular mechanisms of NUSAP1 in ovarian cancer have been investigated in relation to homologous recombination repair, mismatch repair, and immunology.17 Elevated expression of NUSAP1 has been shown to increase cell motility, invasion, and metastasis in prostate cancer and astrocytoma cells.18, 19 NUSAP1 has also been associated with chromosome instability and DNA damage response pathways by upregulating BRCA1 expression and recruitment to DNA damage foci in breast cancer, as well as through the activation of ataxia telangiectasia and Rad3-related protein (ATR) in glioblastoma.20, 21 While its role during mitosis in tumor cells has been studied, little is known about the function of NUSAP1 in mouse oocyte meiosis.
However, as a spindle-associated protein, we discovered that NUSAP1 exhibits unique expression patterns and functions during mouse oocyte meiosis. In this study, we first systematically compared the protein levels of spindle assembly related genes during the metaphase I (MI) and germinal vesicle (GV) oocytes stages and discovered that NUSAP1 had a much higher protein expression ratio (MI/GV) than other genes. Subsequently, we explored the function of NUSAP1 during mouse oocyte meiotic maturation. We found that NUSAP1 localized near the spindle poles during the prophase of the first meiotic division (Pro-MI), MI and anaphase I/telophase I (AI/TI) stages of oocytes. In the MII stage, NUSAP1 showed significant enrichment within Pb1. Knockdown (KD) of NUSAP1 resulted in a reduction in the GVBD rate and Pb1 extrusion rate. Morphological analysis of NUSAP1-KD oocytes revealed several defects during the MII stage, including abnormal spindle assembly, chromosome alignment, increased AI/TI arrest and aneuploidy rate. Furthermore, NUSAP1-KD oocytes in the MI stage exhibited significantly narrower spindle poles. Through mRNA sequencing, we identified dysregulation of the “establishment of spindle orientation” signaling pathway and confirmed abnormal expression of genes involved in spindle composition. Collectively, we provide detailed evidence demonstrating the involvement of NUSAP1 in regulating oocyte maturation.
2 MATERIALS AND METHODS
2.1 The screening of spindle-related proteins
Based on the importance of the spindle in oocyte meiotic division, we systematically compared the expression levels of spindle-related proteins during the MI and GV oocytes stages. The method was briefly described as follows. The proteomic data of oocytes at different stages was retrieved from the publicly available database by Li et al.,11 and re-ranked the fold change ratios of protein expressions during the MI/GV periods from high to low. Then, based on published literature in the PubMed database and GeneCards (https://www.genecards.org/), we screened proteins that are potentially associated with spindle and have positional relationships. Furthermore, the top 10 proteins with the highest fold change ratios were selected and NUSAP1 had a much higher protein expression ratio (MI/GV) than other genes. The list of protein profile can be found in Table S1.
2.2 Animal
Approval for the animal experiments conducted in this study was obtained from the Institutional Animal Care and Use Committee of Nanjing Drum Tower Hospital (SYXK 2019-0059). Three-week-old female ICR mice were obtained from the Nanjing Medical University Animal Center and housed in the Nanjing Drum Tower Hospital Animal Center. The mice were kept on a 12/12 h light/dark cycle.
2.3 Oocyte collection and culture
Fully grown oocytes arrested at the GV stage were obtained from the ovaries of 3-week-old female mice. Superovulation was induced by intraperitoneal injection of 5 IU of pregnant mare serum gonadotropin (PMSG) from Prospec, 46–48 h before oocyte collection. Cumulus-oocyte complexes were collected, and the surrounding cumulus cells were removed through repeated mouth pipetting.
The oocytes were then cultured in MEM-alpha supplemented with 3 mg/mL bovine serum albumin (BSA) from Sigma, and 5 mmol/L Milrinone from Calbiochem to prevent meiotic resumption. For in vitro maturation, the oocytes were incubated in MEM from Gibco, supplemented with 75 mg/mL penicillin G, 50 mg/mL streptomycin sulfate, 25 mg/mL pyruvate, 38 mg/mL ethylenediaminetetraacetic acid, and 3 mg/mL BSA. The incubation was carried out under mineral oil at 37°C in a 5% O2, 5% CO2, and 90% N2 incubator.
2.4 Antibodies
The following antibodies were employed in this study: a rabbit polyclonal antibody against NUSAP1 (Cat#: 12024-1-AP; dilution 1:200; Proteintech), DDX6 (Cat#: 14632-1-AP; dilution 1:1000; Proteintech), EDC4 (Cat#: 17737-1-AP; dilution 1:1000, Proteintech), γ-tubulin (Cat#: 15176-1-AP; dilution 1:1000; Proteintech), a mouse monoclonal antibody conjugated with FITC against α-tubulin (Cat#: F2168; dilution 1:500; Sigma), a mouse monoclonal antibody against β-actin (Cat#: AF0003; dilution 1:1000; Beyotime), rhodamine-phalloidin (Cat#: YP0063-50T; dilution 1:1000; US EVERBRIGHT®INC), and Alexa Fluor Plus 555 donkey anti-rabbit immunoglobulin G (Cat#: A31572; dilution 1:1000; Thermo Fisher Scientific).
2.5 Microinjection
Mouse oocytes were subjected to microinjection with approximately 10 pl of small interfering RNA (siRNA) (25 μM) targeting NUSAP1 using a FemtoJet microinjector (Eppendorf). A pool of three distinct siRNAs was specifically employed to target Nusap1, as well as control siRNAs (GenePharma), were obtained and diluted in RNase-free water. The 5′-3′ sequences of the Nusap1 siRNAs were as follows: GGACTTCTATTGCAGTTAT, GCCAGTATTTGATCTCAAA, and GGAGAAGTAAATGACTTAT. Following injection, the oocytes were cultured in a medium supplemented with 5 μM milrinone to maintain GV arrest for 20 h. Subsequently, the oocytes were transferred to fresh medium and cultured under mineral oil in a 37°C incubator with an atmosphere of 5% O2, 5% CO2, and 90% N2.
2.6 Immunostaining
Following fixation in 4% paraformaldehyde (PFA) for 30 min, mouse oocytes were blocked with a solution containing 5% BSA and 0.5% Triton X-100 in phosphate buffered saline (PBS) at room temperature for 1 h. Subsequent overnight incubation with primary antibodies at 4°C was performed. After three washes, secondary antibodies were applied to the oocytes for 1 h at room temperature. Chromosome staining using DAPI was carried out for 10 min. Finally, the oocytes were mounted on glass slides and subjected to examination using a Carl Zeiss LSM 780 laser scanning confocal microscope. The criteria utilized to distinguish between normal and abnormal spindle and chromosome morphology in mouse oocytes are as follows: A normal spindle typically exhibits an elliptical or ovoid shape with well-defined poles and spindle fibers. Abnormal spindles may manifest as irregular shapes, distortions, excessive enlargement, or reduction in size. Normal chromosomes should be uniformly aligned in the central region of the spindle fibers, forming a well-organized metaphase plate. Abnormal chromosome alignment may involve scattered distribution, clustering, or misalignment.
2.7 Western blotting
Eighty mouse oocytes were lysed in sample buffer and boiled at 100°C for 5 min for Western blot analysis. The denatured proteins were then separated by 7.5% sodium dodecyl sulphate-polyacrylamide gel electrophoresis and transferred onto a polyvinylidene fluoride membrane. After blocking in PBST (PBS with 5% low-fat dry milk and 0.1% Tween-20) for 1 h at room temperature, the membranes were incubated overnight at 4°C with rabbit anti-NUSAP1 antibody (1:1000; Proteintech) and mouse anti-β-actin antibody (1:1000; Beyotime). Following three PBST washes, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies in blocking buffer for 1 h. Subsequently, the protein bands were visualized using the ECL Plus Western blotting Detection System (GE Healthcare) after three additional PBST washes.
2.8 RT-PCR
The complementary DNA (cDNA) from the oocyte/embryos lysate was amplified using the Single Cell Sequence Specific Amplification kit (P621; Vazyme) following the manufacturer's instructions. A brief description is as follows: The amplification primers for different target genes were mixed to create an Assay Pool (with a final concentration of each primer at 0.1 μM). For the assembly of the assay pool, 1 μL of oocyte lysate and primers specific to the target genes were used. Reverse transcription was carried out with 11 cycles as recommended in the manual. This step generated cDNA for subsequent analysis of differential gene expression. To reduce errors between experimental groups, we used at least 10 oocytes/embryos for each sample group. The resulting cDNA was subsequently diluted 100 times, and 2 μL of the dilution was utilized for quantitative PCR on a LightCycler 480 II (Roche). The expression levels of the genes were determined using the 2−∆∆Ct method. The primer sequences employed are provided in Table S2.
2.9 Chromosome spreading
Chromosome spreading was conducted following a previously published protocol22 (29353819). To remove the zona pellucida of the oocytes, they were briefly exposed to Tyrode's buffer (pH 2.5) (T1788; Sigma) at 37°C for 30 s. After a 10-min recovery in fresh medium, the oocytes were fixed on a glass slide using a solution containing 1% PFA and 0.15% Triton X-100. Subsequently, the slides were air-dried, and the chromosomes were stained with Hoechst 33342. Finally, the slides were examined under a Carl Zeiss LSM 780 laser scanning confocal microscope.
2.10 RNA-Seq and gene enrichment analyses
After injection, GV-stage oocytes were cultured for 20 h in medium containing 5 μM milrinone to maintain GV arrest. Three sets of samples, each containing 10 control-siRNA and 10 NUSAP1-KD MI-stage oocytes, were collected for RNA-Seq analysis. Full-length cDNA amplification products were obtained using the Single Cell Full Length mRNA Amplification Kit (N712; Vazyme). Subsequently, the amplified cDNA was prepared into sequencing libraries specifically designed for the Illumina high-throughput sequencing platform using the TruePrep® DNA Library Prep Kit V2 for Illumina (TD503; Vazyme) following the manufacturer's instructions. The Illumina HiSeq X Ten platform was utilized to sequence the libraries, generating paired-end reads of 150 bp. Trim_galore (v0.6.4) was applied to remove adapter sequences and low-quality bases from the obtained reads. Trimmed read pairs were aligned to mouse genome(mm10) with STAR(v2.7.1a), and featureCounts (v2.0.0) was used to obtain read counts. The R package DESeq. 2 (v1.28.1) was used to analyze and normalize the differentially expressed genes. Gene enrichment analysis of the differentially expressed transcripts was performed using the DAVID (https://david.ncifcrf.gov/)23 and Metascape (https://metascape.org/) platforms.24
2.11 Immunoprecipitation (IP) coupled with mass spectrometry identification
Ovaries were collected from 3-week-old ICR female mice after injection of PMSG for 44–46 h. Ten ovaries were pooled as one group, and after sample lysis, IP of NUSAP1 was performed. The steps of IP were briefly described as follows: NUSAP1 antibody (Cat#: 12024-1-AP; Proteintech) was incubated with the ovarian lysate, followed by the addition of affinity beads to capture the antibody-antigen complex. The co-precipitated proteins were then analyzed by mass spectrometry (Thermo Scientific™ Q Exactive™ HF-X). Mass spectrometry data were searched using Proteome Discoverer 2.4, and the identified interacting proteins were listed in Table S3.
2.12 Statistical analysis
The experimental data are presented as the mean ± SEM, unless specified otherwise. Statistical analysis was conducted using the Student t test for comparing two groups and one-way analysis of variance followed by Tukey's HSD test for multiple group comparisons. Prism 5.0 software was utilized for the statistical analysis. The significance level was set at p ≤ 0.05.
3 RESULTS
3.1 Expression and localization of NUSAP1 during oocyte meiotic maturation
The spindle apparatus plays a vital role in the process of oocyte meiotic division, as its assembly and anchoring determine the proper segregation of chromosomes. By mining mass spectrometry data, we systematically compared the expression levels of spindle-related proteins during the GV, MI, and MII stages. Among spindle-related proteins, we observed that the protein expression of NUSAP1 during the MI stage was approximately fivefold higher than that during the GV stage, surpassing that of other spindle components11 (Figure 1A). This unique phenomenon surrounding NUSAP1 piqued our interest. We discovered that Nusap1 exhibited dominant expression in oocytes, with both its mRNA and protein expression levels significantly higher than those in granulosa cells (Figure 1B,C). Consistent with the proteomic data, NUSAP1 demonstrated a threefold increase in protein levels in MI-stage oocytes compared to GV-stage oocytes (Figure 1D). During the fully grown stage of oocytes (FGO), DNA transcription ceases, mRNA no longer increases, and degradation becomes prevalent.25 Correspondingly, as protein expression increased, the Nusap1 mRNA in oocytes rapidly decreased during the MI stage (Figure 1E). Furthermore, Nusap1 mRNA exhibits rapid degradation during the early stages of embryonic development following fertilization (Figure 1F).
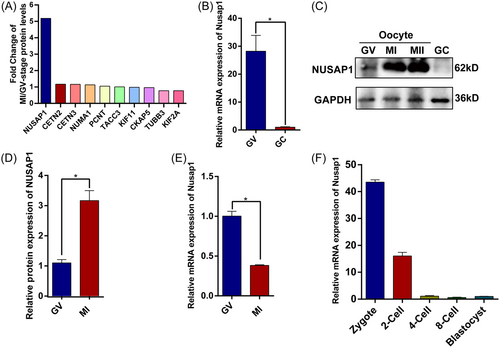
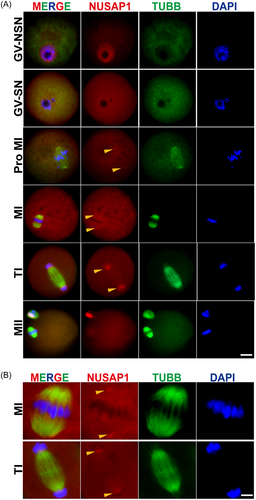
The abundant expression of Nusap1 prompted us to investigate its localization during oocyte meiotic maturation. Immunofluorescence staining of oocytes at various stages (GV, Pro-MI, MI, AI/TI, MII) revealed that NUSAP1 is distributed within the nuclear membrane of immature oocytes with a nonsurrounded nucleolus configuration (GV-NSN). Conversely, in mature oocytes with a surrounded nucleolus configuration (GV-SN), it does not accumulate within the nuclear membrane. This localization may be associated with the SAP-like domain of NUSAP1, allowing interaction with chromosomes.13 Interestingly, we observed that NUSAP1 is localized near the spindle pole during the Pro-MI, MI, and AI/TI stages, with protein condensation particles present on both sides of the spindle apparatus. However, during the MII stage of oocytes, NUSAP1 became significantly enriched within the polar body (Figure 2). These findings suggest that NUSAP1 plays a crucial role in the process of oocyte meiotic maturation.
3.1.1 Nusap1 depletion reduced the GVBD rate and polar body extrusion (PBE) rate of mouse oocytes
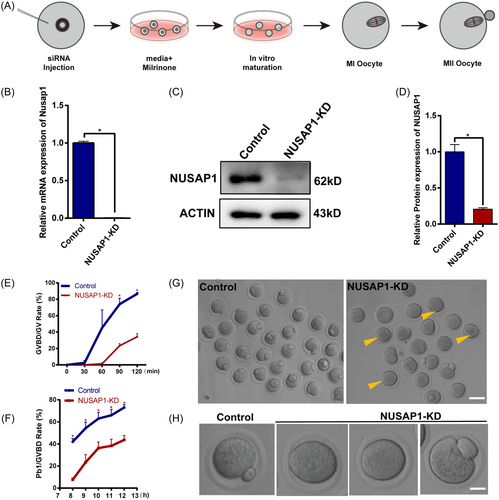
To investigate the role of NUSAP1 in the process of oocyte meiotic maturation, we utilized siRNA interference during the GV stage of oocytes to reduce Nusap1 expression (Figure 3A). Following microinjection, GV-stage oocytes were cultured in medium containing milrinone for 20 h to facilitate the degradation of Nusap1 mRNA. In NUSAP1-KD oocytes, the expression level of Nusap1 mRNA decreased by more than 90% (Figure 3B). Additionally, the expression level of NUSAP1 protein was reduced by over 50% in NUSAP1-KD oocytes (Figure 3C,D). Following NUSAP1 KD, the rate of GVBD was significantly delayed compared to that of the control group. After 120 min of maturation culture, less than 40% of NUSAP1-KD oocytes resumed meiosis, while the GVBD rate approached 90% in the control group (Figure 3E). The rate of PBE in NUSAP1-KD oocytes was also significantly lower than that in the control group (Figure 3F). The NUSAP1-KD oocytes exhibited various phenotypes, including arrest at the GV stage, failed extrusion of the first polar body, and higher percentage of enlarged polar bodies [NUSAP1-KD (15.0 ± 1.0%) vs. Control (6.7 ± 1.0%)] (Figure 3G,H). These findings strongly indicate that NUSAP1 serves as a critical regulatory molecule in oocyte meiotic maturation.
3.1.2 Nusap1 depletion leads to aberrant meiosis and increases aneuploidy in MII-stage oocytes
To further investigate the impact of NUSAP1 KD on oocyte maturation, we conducted immunofluorescence staining on oocytes cultured for 14 h in vitro. Consistent with the reduced rate of Pb1 observed in NUSAP1-KD oocytes, we found that almost no oocytes in the NUSAP1-KD group exhibited a normal MII morphology (Figure 4A). Upon Nusap1 depletion, approximately 25% of oocytes were arrested at the AI/TI stage, and approximately 25% exhibited disorganized chromosome alignment in the MI stage (Figure 4B,C). Even among the approximately 40% of oocytes that extruded Pb1, these oocytes displayed typical DNA lagging (Figure 4D). Due to the presence of elevated levels of chromosome misalignment in NSUAP1-KD oocytes, we performed karyotype analysis on MII-stage oocytes through chromosome spreading. Consistent with our expectations, there was a substantial increase in the proportion of aneuploidy in NUSAP1-KD oocytes (Figure 4E,F). These results suggest that NUSAP1 KD significantly affects chromosome arrangement.
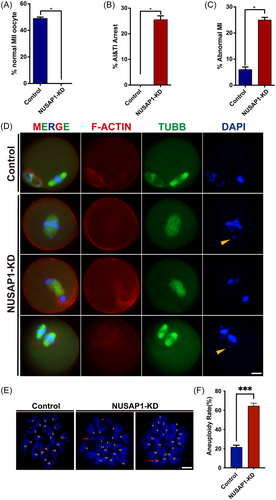
3.1.3 Nusap1 depletion disrupts spindle assembly during mouse oocyte meiosis
The unique localization of NUSAP1 near the spindle poles during oocyte maturation prompted us to investigate how Nusap1 depletion affects spindle assembly. After 8 h of in vitro maturation of oocytes, we labeled the spindles using tubulin antibodies. As expected, we observed a significant reduction in spindle pole width in NUSAP1-KD oocytes. Quantitative analysis revealed that the width of spindle poles in NUSAP1-KD oocytes was almost half that in the control group, where the abnormal spindle pole width was approximately 4 μm (Figure 5A,B).
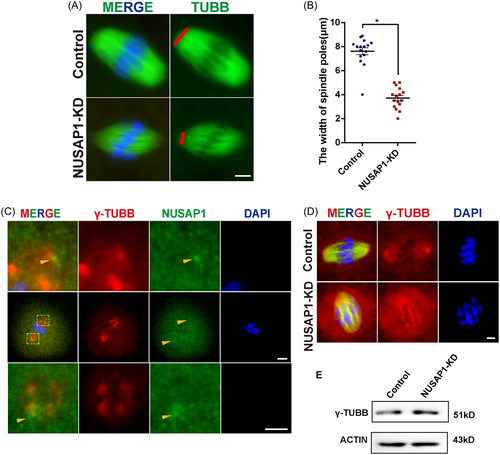
Due to the enrichment of NUSAP1 at the spindle poles (Figure 2) and the prominent phenotype observed in NUSAP1-KD oocytes, which primarily manifests as spindle pole defects, we further investigated the relationship between NUSAP1 and MTOC markers (such as γ-tubulin) in terms of spindle pole localization (Figure 5C). We found that although both NUSAP1 and γ-tubulin were enriched near the spindle poles, they did not exhibit co-localization. In NUSAP1-KD oocytes, although the protein expression levels of MTOC markers (such as γ-tubulin) remained unchanged, the distribution of γ-tubulin was affected, no longer displaying the characteristic crescent-shaped clustering (Figure 5D,E). These results indicate that the loss of NUSAP1 significantly affects spindle assembly during oocyte maturation.
3.1.4 NUSAP1 KD alters transcriptome dynamics in mouse oocytes
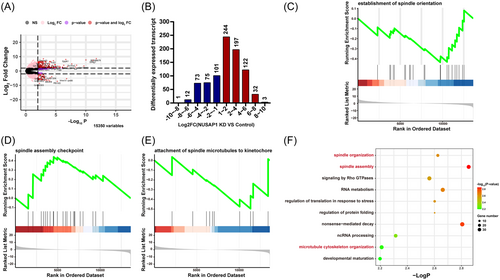
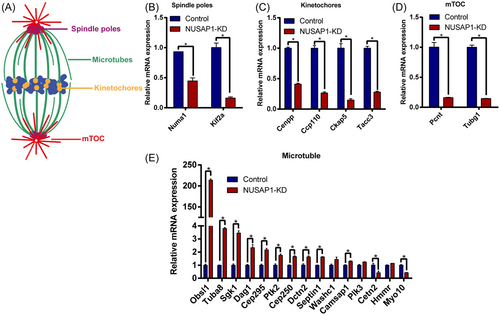
To explore the underlying mechanisms causing abnormal development in NUSAP1-KD oocytes, we conducted transcriptomic analysis on MI-stage NUSAP1-KD and control oocytes. Upon NUSAP1 KD, the volcano plot of the transcriptome analysis revealed drastic changes in mRNA within the oocytes (Figure 6A). A total of 860 transcripts showed significant alterations, with the number of upregulated transcripts being twice as high as that of downregulated transcripts. Among them, 598 transcripts were significantly upregulated, while 262 transcripts were significantly downregulated (Figure 6B). Consistent with the previously observed abnormal spindle assembly (Figure 5), further gene set enrichment analysis (GSEA) indicated significant inhibition of the “establishment of spindle orientation” signaling pathway. Additionally, the blockade of the “attachment of spindle microtubules to kinetochore” pathway and the activation of the “spindle assembly checkpoint” pathway suggested abnormal connections between the spindle and chromosomes (Figure 6C–E). Furthermore, Gene Ontology (GO) enrichment analysis bubble plots revealed significant differential changes in nearly 30 crucial transcripts associated with “spindle assembly” processes (Figure 6F). Based on the impact of NUSAP1 KD on spindle assembly, we categorized some important spindle-related genes into four groups: spindle pole-, MTOC-, kinetochore-, and MT-related genes. We further validated these findings through RT-PCR, which confirmed that the majority of spindle-related genes changed significantly (Figure 7). Among them, 13 spindle-related genes with differential changes were consistent with the dynamic trends (up- or downregulation) revealed by RNAseq (Figure 7). These findings suggest that NUSAP1 KD alters transcriptome dynamics in mouse oocytes.
3.1.5 NUSAP1 plays a critical role in actin assembly during oocyte meiosis
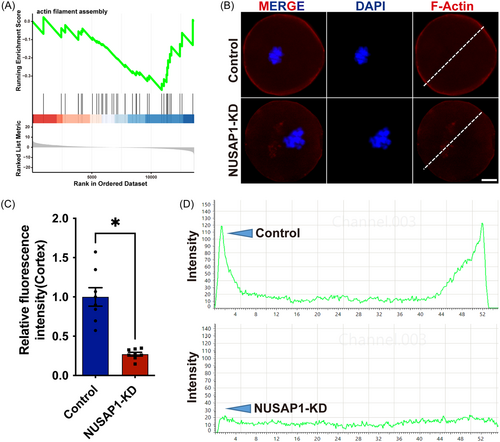
The localization of the spindle in mouse oocytes relies on dynamic changes in actin filaments.8 In mouse oocytes, the typical MT spindle is enveloped by actin filaments, known as spindle-actin. The interaction between spindle-actin, spindle MTs, and chromosomes is essential for meiotic division.7 When NUSAP1 was knocked down in mouse oocytes, the “actin filament assembly” signaling pathway was significantly inhibited (Figure 8A). The fluorescence signals of actin were greatly reduced in NUSAP1-KD oocytes. The distribution of actin in the cortical and cytoplasmic regions of oocytes is further magnified through line graphs. Compared to the control group, the fluorescence intensity of actin was significantly decreased in NUSAP1-KD oocytes (1 vs. 0.25 ± 0.12, p < 0.05) (Figure 8B–D). These findings suggest that NUSAP1 KD affects actin assembly during oocyte meiotic maturation.
3.2 The depletion of RNA-binding protein (RBP) NUSAP1 affected the dynamics of processing bodies—the key components involved in mRNA homeostatic regulation
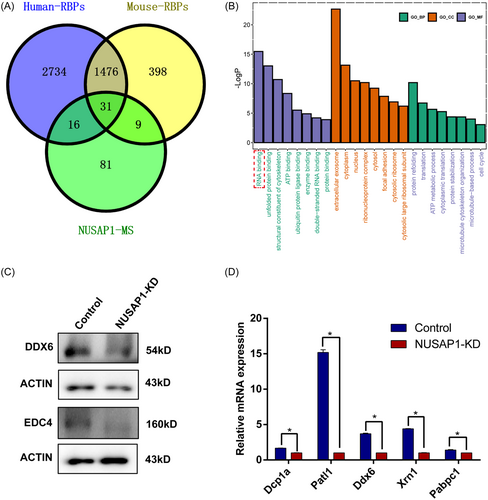
Given the impact of NUSAP1 functional loss on RNA stability, we further investigated the relationship between NUSAP1 and RNA stability. By examining publicly available human and mouse datasets of RBPs extracted from the EMBL RBPbase (https://rbpbase.shiny.embl.de/), we identified the RNA-binding capability of NUSAP126-29 (Figure 9A, Table S3). Moreover, through a comparative analysis of 137 NUSAP1-interacting proteins in ovary identified here and the available human and mouse RBP datasets, we identified 31 proteins with overlapping interactions.26-29 Within the NUSAP1 interactomes, RBPs accounted for 41% (56/137). GO analysis of the 56 common NUSAP1 interactomes revealed enrichment for RNA binding (Figure 9B). Processing bodies (P-bodies) represent a prominent category of cytoplasmic ribonucleoprotein (RNP) granules, where messenger RNAs (mRNAs) are intricately associated with proteins involved in various cellular processes, including translational repression, deadenylation, mRNA decapping, and 5′-3′ decay.30 Core proteins such as EDC4 and DDX6 are among these components.31, 32 Notably, significant differential changes were observed in the main constituents of processing (P)-bodies, such as DDX6 and EDC4, in NUSAP1-KD oocytes (Figure 9C,D). These findings suggest that the RBP NUSAP1 may directly or indirectly regulate mRNA homeostasis by affecting the dynamics of processing (P)-body components.
4 DISCUSSION
In this study, we investigated the dynamic expression, subcellular localization, and potential role of NUSAP1 during mouse oocyte meiotic maturation. NUSAP1 exhibits high expression levels in mouse oocytes. Consistent with its localization in the nucleolus during mitosis, we also observed NUSAP1 enrichment within the nuclear membrane of GV-NSN oocytes. Interestingly, as spindle formation commenced in mouse oocytes, NUSAP1 formed granular aggregates at both ends of the spindle. Deletion of NUSAP1 severely impaired the progression of meiosis, including a delayed rate of GVBD and PBE. Furthermore, NUSAP1 KD disrupted spindle assembly and chromosome alignment and increased aneuploidy.
Owing to recent advancements in mass spectrometry sensitivity, we conducted data mining and systematic comparisons of several known spindle assembly related genes.11, 33-35 At the mRNA expression level, consistent with these spindle-related genes, NUSAP1 mRNA levels exhibited dominant expression in oocytes compared to granulosa cells (Figure S1). Surprisingly, in terms of protein expression, we observed a significant increase in NUSAP1 levels during the MI and MII stages compared to the GV stage. Moreover, the fold increase in MI/GV protein levels of NUSAP1 far exceeded that of other spindle-related proteins, highlighting a distinctive feature of NUSAP1 expression (Figure 1). In the context of mitosis, NUSAP1 functions by stabilizing and cross-linking MTs, potentially tethering them to chromosomes.36, 37 In mouse oocyte meiosis, NUSAP1 localizes near the spindle poles (Figure 2). Furthermore, considering that NUSAP1 possesses a MT-binding domain,13, 14 we speculate that NUSAP1 plays a role in MT binding, determining their enrichment sites, and contributing to MT traction. Centrosome-derived MTOCs predominantly orchestrate mitotic spindle assembly and structure in animal cells. At each spindle pole, the centrosome nucleates, anchors, and organizes MTs, thus ensuring spindle bipolarity.38, 39 The major components of centrosomes include Pericentrin and γ-tubulin.40, 41 Upon NUSAP1 KD, both Pcnt and Tubg1, the genes encoding Pericentrin and γ-tubulin, respectively, exhibited significant downregulation (Figure 7). Indeed, the loss of NUSAP1 led to a discernible reduction in spindle pole width (Figure 5). RNA-seq data revealed suppression of the “establishment of spindle orientation” pathway. While our findings clearly indicate the influence of NUSAP1 on spindle pole formation, further investigation is needed to elucidate the interplay between NUSAP1 and factors such as pericentrin, γ-tubulin, and Cep192 in terms of pole composition.
The precise regulation of NUSAP1 protein levels is crucial for successful completion of cell division. The dynamic changes in NUSAP1 during the GV, MI, and MII stages prompt us to ponder the significance of its dynamic fluctuations. A highly conserved KEN box motif [KENxxx(DQEN)] has been identified in the C-terminus of NUSAP1.42 The anaphase-promoting complex/cyclosome (APC/C), a multisubunit E3 ubiquitin ligase, plays a central role in regulating cell cycle progression.43, 44 The KEN box can be specifically recognized by APC/C-Cdh1 and recruits ubiquitin ligases for degradation. When the KEN box of NUSAP1 is removed, APC/C-Cdh1 fails to degrade it.45 Additionally, the dynamic expression pattern of the NUSAP1 protein aligns well with known substrates of APC/C-Cdh1, such as cyclin B.46, 47 During mitosis, overexpression of NUSAP1 leads to the accumulation of cells with MT bundling and spindle deficiency.45 Moreover, the significant enrichment of NUSAP1 in Pb1 during MII-stage oocytes may also be recognized and guided for degradation by E3 ubiquitin ligases in a similar fashion to spindle-associated proteins (Figure 2). Moreover, studies have suggested that as mice age, there is a significant increase in the proportion of aneuploid oocytes and abnormal spindle assembly,48, 49 raising the question of whether NUSAP1 expression and aging are related. Although GSEA of RNA-seq data showed significant suppression of the “regulation of cell aging” signaling pathway in NUSAP1-KD oocytes, our results did not reveal a significant difference in NUSAP1 mRNA levels between young GV oocytes and aging oocytes (Figure S2). The crucial role of NUSAP1 in aging oocytes warrants further exploration.
The N-terminal region of the NUSAP1 protein contains a DNA-binding SAP domain. In HeLa cells, the SAP domain of NUSAP1 can bind to DNA in vitro and exhibits a preference for binding to dsDNA. Deletion of the SAP-like domain abolishes the binding of NUSAP1 to chromosome arms during mitosis.13 In interphase cells of mitosis, NUSAP1 localizes to the nucleus and interacts with chromatin through its SAP-like domain.13 Oocytes with ongoing transcriptional activity can be identified by the presence of decondensed chromatin, referred to as the NSN configuration, whereas those oocytes that have completed transcription exhibit a tightly condensed chromatin ring surrounding the nucleolus, known as the SN configuration.50 During the transition from NSN to SN, the chromatin in mouse GV-stage oocytes undergoes extensive remodeling and posttranslational modifications, crucial for transcriptional regulation and reshaping of the oocyte genome before meiotic resumption.51, 52 Unlike in mitosis, NUSAP1 is only found in the nucleus of GV-NSN oocytes. However, this localization disappears in GV-SN oocytes (Figure 2). Interestingly, there is a protein localization switch of NUSAP1 from NSN to SN, and its binding to chromatin suggests that NUSAP1 may be involved in oocyte transcriptional activity. However, further exploration is needed to understand how NUSAP1 regulates interphase DNA transcription in oocytes.
In fully grown GV oocytes, where transcription has been silenced, the loss of NUSAP1 function leads to abnormalities in RNA homeostasis. Through the utilization of the EMBL RBPbase and relevant published literature, it has been established that NUSAP1 is an RBP.26-29 Although mass spectrometry experiments here revealed enrichment for RNA binding in NUSAP1 interactomes, and NUSAP1 KD affected the changes in the major components of processing (P)-bodies (Figure 9), the specific regulation of spindle-related mRNAs by NUSAP1 remains an area for further exploration.
5 CONCLUSION
In summary, NUSAP1 emerges as a crucial gene essential for mouse oocyte meiotic maturation. Our data provide detailed evidence that precise regulation of NUSAP1 expression and localization influences spindle assembly, chromosome segregation, and the distribution of actin microfilaments in oocytes.
AUTHOR CONTRIBUTIONS
Guangyi Cao, Guijun Yan, Haixiang Sun, and Jie Mei: contributed to the conception and design of the study. Guangyi Cao, Lina Yu, Na Kong, Yuling Lin, Panpan Qiu, Qian Xu, Yang Zhang, and Zhen Xin: performed the research. Guangyi Cao, Lina Yu, and Guijun Yan: performed the statistical analysis. Guangyi Cao and Lina Yu: wrote the first draft of the manuscript. Guangyi Cao: wrote sections of the manuscript. All authors contributed to manuscript revision and read and approved the submitted version.
ACKNOWLEDGMENTS
This research is supported by grants from the National Natural Science Foundation of China (32000563, 82030040), State Key Laboratory of Reproductive Medicine and Offspring Health (SKLRM-2022D2), Clinical Trials from the Affiliated Drum Tower Hospital, Medical School of Nanjing University (2023-LCYJ-PY-35), Startup Funds from Nanjing Drum Tower Hospital (RC2022-019) and Guangdong Provincial Key Laboratory of Reproductive Medicine (2020B1212060029).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
All mouse experiments involved in this study were approved by the Institutional Animal Care and Use Committee of Nanjing Drum Tower Hospital (SYXK 2019-0059).
Open Research
DATA AVAILABILITY STATEMENT
The original contributions presented in the study are included in the article/supplementary materials, and further inquiries can be directed to the corresponding author. All data generated or analyzed during this study are included in this published article. Further inquiries can be directed to the corresponding author. The data that support the findings of this study are available from the corresponding author upon reasonable request.