LncRNA NEAT1 promotes hypoxia-induced renal tubular epithelial apoptosis through downregulating miR-27a-3p
Xinxin Jiang and Daoting Li contributed equally to this work.
Abstract
Acute kidney injury (AKI) is a common kidney disorder that affects public health and the incidence of AKI. Sepsis, acute ischemia or hypoxia is the main reason for the occurrence of AKI. Recently, noncoding RNA that include microRNA and long noncoding RNA (lncRNAs) were reported to play important roles in AKI as well as have the potential to serve as a biomarker or therapeutic target for the development of the diagnostic and prognostic strategies of AKI. In the current study, we aimed to investigate the expression and biological function of lncRNA nuclear enriched abundant transcript 1 (NEAT1) in ischemia-induced AKI in patients' sample and in vitro. The expressions of NEAT1 and miR-27a-3p in ischemia/reperfusion-induced AKI patients were examined by quantitative reverse transcription polymerase chain reaction. Cell injury was induced by treatment of human kidney tubular cells (HK-2) with CoCl2. After treatment, the influences of NEAT1 and miR-27a-3p on the cell apoptosis in the CoCl2-stimulated HK-2 were tested by flow cytometry. The flow analysis results showed that the expression of NEAT1 was markedly higher in the ischemia-induced AKI patients compared with normal control. Moreover, repression the expression of NEAT1 decreased CoCl2-induced injury in HK-2. The expression of miR-27a-3p was negatively regulated by NEAT1. Inhibition the expression of NEAT1 attenuated overexpression of miR-27a-3p enhanced CoCl2-induced injury. In summary, an ischemia-induced injury may be enhanced by a high level of NEAT1 through targeting miR-27a-3p. Thus, NEAT1 has the potential to be explored as a biomarker for diagnosis and target for therapeutic strategies in ischemia-induced AKI.
1 INTRODUCTION
Acute renal failure which is also called acute kidney failure (AKF), is a life-threatening disease which is characterized with loss of or decreasing of function in kidney lead to the accumulation of nitrogenous waste, including blood urea nitrogen and creatinine.1, 2 The ischemia characterized by short of oxygen and nutrition supplies is the major cause for AKI.1, 2 The incidence of AKI is still increasing over the past decades. Even the recovered patients still have the 25% risk increase in the progression to chronic kidney disease and 50% increase in mortality after over 10 years of follow-up when compared with the healthy population.3, 4 AKI is characterized by tubular injury, as well as necrosis or apoptosis-induced cell death.5, 6 The regeneration of tubular epithelial cell has been reported to promote the progression of AKI repair and this process is regulated by the balance of cell proliferation and apoptosis.5, 6 Increased epithelial cell apoptosis suppressed the renal tubular cell regeneration and delayed the renal tubular cell recovery function in AKI.7, 8 Thus, inhibition of apoptosis can promote the regeneration and the recovery of AKI, which can be explored as a potential therapeutic strategy in the treatment of AKI patients in the future.
Long noncoding RNAs are endogenous noncoding RNAs that consist of over 200 nucleotides in length. Recent years, long noncoding RNAs were suggested to play critical roles in the regulation of different types of biological processes that include cell proliferation, apoptosis, cell cycle progression, differentiation and inflammation etc.9 In addition, long noncoding RNAs are highly regulated and specific. Accumulated studies proved that noncoding RNAs plays critical roles in the occurance and pathogenesis of AKI, especially ischemic induced AKI.10-12 However, the detailed biophysical and biological role of lncRNA in ischemic AKI is still largely unknown. Among these lncRNAs, nuclear enriched abundant transcript 1 (NEAT1) that is highly abundant lnc RNA was reported to be involved in different kinds of diseases.13, 14 NEAT1 is upregulated in many tissues in mice and is required for the development of mammary gland and lactation of mice, as well as required for the formation of paraspeckles.15, 16 Several groups have reported the expression of certain genes was regulated by upregulation of NEAT1 regulates through sequestering specific mRNAs and proteins into paraspeckles. The upregulation of NEAT1 also responded to different cellular stresses which include hypoxia, replication stress in cancer, viral infection et al.13, 17-21 However, its biologic role and function in AKI in still under investigation.
Here, in this study, we aimed to investigate the expression and biologic function of lncRNA NEAT1 in ischemia-induced AKI in vitro. First, we examined the expression of NEAT1 in ischemia/reperfusion-induced AKI tissue from the patients and CoCl2-induced human kidney 2 (HK-2) cells were detected. Then, we identified a binding target of NEAT1, miR-27a-3p. Moreover, we also detect the effect of NEAT1 on cell apoptosis of CoCl2-stimulated HK-2. In addition, we also explored the regulation of NEAT1 on miRNA, and miRNA targeted genes. These findings suggested that NEAT1 and related miRNA, as well as miRNA target, may have the potential to be explored as therapeutic targets and novel therapeutic strategies for the treatment of ischemic AKI.
2 MATERIALS AND METHODS
2.1 Patient samples
From March 2017 to May 2018, 18 patients with varying degrees of ischemia-induced AKI participated in this study. At the same time, 18 healthy volunteers were enrolled as controls. These patients were excluded from this study, including pregnant patients, as well as organ transplant recipients or bone marrow, corticosteroids, or acquired immune deficiency syndrome. Informed consent was signed by all participates before the start of the study. Our hospital Institute for the Protection of Human Ethics Committee proved the procedures used in this study.
2.2 Detection of the expression of NEAT1 in patient samples serum
The blood samples collected from AKI patients were centrifuged at 5000g for 3 minutes and then serum samples were collected. Total RNAs were extracted from serum using the miRNeasy Mini kit (Qiagen, Hilden, Germany) following the manufacturer's protocols. The expression of serum NEAT1 was determined on the StepOnePlus thermocycler (Applied Biosystems, Valencia, CA) by quantitative reverse transcription polymerase chain reaction (qRT-PCR) (RT2 SYBR Green qPCR MasterMix and SYBR primers, Qiagen). Then cDNA was abstained by reverse-transcribed of RNAs (RT2 PreAMP cDNA Synthesis Kit; Qiagen). relative quantification method was used to calculate the relative expression of NEAT1. Furthermore, Pearson's correlation coefficient analysis was conducted to analyze the correlation between creatinine clearance rate (CCr) levels and serum NEAT1 expression by using the SPSS 19.0 software (SPSS, Chicago, IL) to investigate the influences of NEAT1 on AKI induced by sepsis.
2.3 Lentiviral vector constructs preparation and infection
Synthesized NEAT1 short-hairpin RNA (sh-NEAT1) against was cloned into the vector (LV10-CMV-RFP-Puro, GenePharma, Shanghai, China) and scramble shRNA (sh-NC) was cloned into the vector and served as a negative control. 293T cells were used to pack the lenti-virus by transfection of vectors containing sh-NEAT1 or sh-NC, and then after 72 hours transfection, the virus particles were collected. The cells were then infected with virus particles by adding with 8 μg/mL Polybrene (Sigma, St Louis, MO), and then puromycin was used for selection up to 7 days.
In addition, for construction of NEAT1 overexpression plasmid, NEAT1 was amplified from the complementary DNA (cDNA) by PCR using PfuUltra II Fusion H DNA Polymerase (Stratagene; Agilent Technologies, Santa Barbara, CA), and then inserted into the HindIII and EcoRI sites of pcDNA3.1 vector (Invitrogen, Carlsbad, CA) with the name of pcDNA-NEAT1. miR-27a-3p mimic, mimic NC, miR-27a-3p inhibitor, and inhibitor NC were purchased from GenePharma (Shanghai, China). Cell transfection was determined by Lipofectamine 2000 (Life Technologies Corporation, Carlsbad, CA) following the manufacturer's instructions.
2.4 qRT-PCR
Total RNAs were isolated from the cells using TRIzol reagent following the manufacturer's instructions (Invitrogen), and then High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) was used to reverse transcribing of RNA into cDNA. TaqMan miRNA assays for detection of miR-27a-3p and U6 was performed by qPCR using TaqMan Universal Master Mix II. TaqMan gene expression assays for NEAT1 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was also performed by using TaqMan Universal Master Mix II.
2.5 Western-blot analysis
Cell lysates were prepared by using the radioimmunoprecipitation assay buffer containing 1 mM phenylmethylsulfonyl fluoride. A sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels was used to separate 40 µg of total proteins from each sample and then proteins were transferred to polyvinylidene fluoride membranes at 100 v for 90 minutes. The membranes were blocked with 5% nonfat milk in 1× Tris-buffered saline buffer for 1 hour and then incubated with primary antibodies against Bax (ab32503; Abcam, Cambridge, MA), cleaved caspase-3 (ab49822; Abcam), Bcl-2 (ab59348; Abcam), GAPDH (ab9485; Abcam), at 4°C for overnight. After a wash with 0.1% Tween–20 and Tris-buffered saline for four times with 5 minutes for each wash, anti-mouse or anti-goat secondary antibodies were then incubated with the membranes (goat anti-mouse IgG H&L (ab6789; Abcam), or goat anti-rabbit IgG H&L (ab205718; Abcam). The chemiluminescence reagents enhanced (Pierce, Rockford, IL) was used for the determines of specific bands for target proteins.
2.6 Luciferase reporter assay
NEAT1-mut (containing the mutant binding sequence) and NEAT1-wt (containing miR-27a-3p target binding sequence in NEAT1) dual-luciferase reporter plasmids were commercially purchased from Genescript (Nanjing, China). Cotransfection of miR-27a-3p or miR-NC and NEAT1-wt or NEAT1-mut into HEK 293T cells were performed. After 48 hours of cotransfection in HEK 293T cells, the luciferase activity was determined using a dual-luciferase reporter assay system (Promega, Madison, WI) according to the manufacturer's protocols.
2.7 RNA pull-down assay
Biotinylated (Sangon, Shanghai, China) probes of miR-27a-3p WT or miR-27a-3p MT were transfected into HK2 cells. After 48 hours of transfection, cells were harvested and cell lysates were collected. Dynabeads M-280 Streptavidin (Invitrogen) was used to incubate with the lysates of each sample. Beads were mixed with biotinylated miR-27a-3p following 10 minutes incubation and washed with washing buffer. Then qRT-PCR was performed to determine the bound RNAs.
2.8 RNA immunoprecipitation
The Ago2 antibody (2897; Cell Signaling, Danvers, MA) and Magna RIP RNA-Binding Protein IP Kit (Millipore, Bedford, MA) were used for RNA Immunoprecipitation experiments according to previous studies33 and the manufacturer's instructions. Then the expression of NEAT1 and miR-27a-3p were determined in the purified RNAs in the precipitates.
2.9 HK-2 cell culture and hypoxia reoxygeration model
Human renal proximal tubule cell line HK-2 (American Type Culture Collection, Manassas, VA) were cultured in Dulbecco's modified Eagle's media/F12 (DMEM-F12) at 37°C in a humidified air and 5% CO2. For the hypoxia-reoxygenation model establishment, HK-2 cells were cultured in DMEM and treated with CoCl2.
2.10 Apoptosis analysis
After 48 hours of transfection, cells were collected and then resuspended in FACs binding buffer containing annexin V and propidium iodide (PI) (Annexin V-FITC Apoptosis Detection Kit; eBioscience, Carlsbad, CA) and incubated for 20 minutes at room temperature in the dark. After staining, the cells were detected and analyzed by BD FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) equipped with Cell Quest Software (BD Biosciences). The annexin V/PI double staining cells were considered as apoptotic cells, PI-positive cells are considered as dead cells. The relative ratios of annexin V/PI double staining cells were counted for further comparisons. The experiments were repeated for at least three times with similar results.
2.11 Statistical analysis
Data were presented as means ± SD (standard deviation). Statistical difference between two groups was analyzed by the unpaired two-tailed Student t test, while statistical difference among more than two groups was analyzed by one-way analysis of variance followed by Dunnett's multiple comparison tests with Graph Pad Prism Software (version 5.00; Graph Pad Prism Software Inc, San Diego, CA). P < 0.05 was considered as statistical significance.
3 RESULTS
3.1 The expression of NEAT1 and miR-27a-3p in the ischemia-induced AKI patients and in HK-2 cells
The expression of NEAT1 and miR-27a-3p mRNA in the serum from patients with ischemia-induced AKI and healthy controls were detected by qPCR. The results indicated that the lower expression of miR-27a-3p was performed while upregulation NEAT1 was performed in AKI patients compared with healthy control (P < 0.01) (Figure 1A and 1B). CoCl2 has long been used as a hypoxia inducer to establish a cell AKI model. In our study, the results suggested that the level of NEAT1 in HK-2 cells treated with CoCl2 in a dose-dependent manner was also higher while the expression of miR-27a-3p was decreased as well as in dose-dependent manner (P < 0.05) (Figure 1C and 1D). These findings indicated that NEAT1 and miR-27a-3p might play a critical role in ischemia-induced AKI.
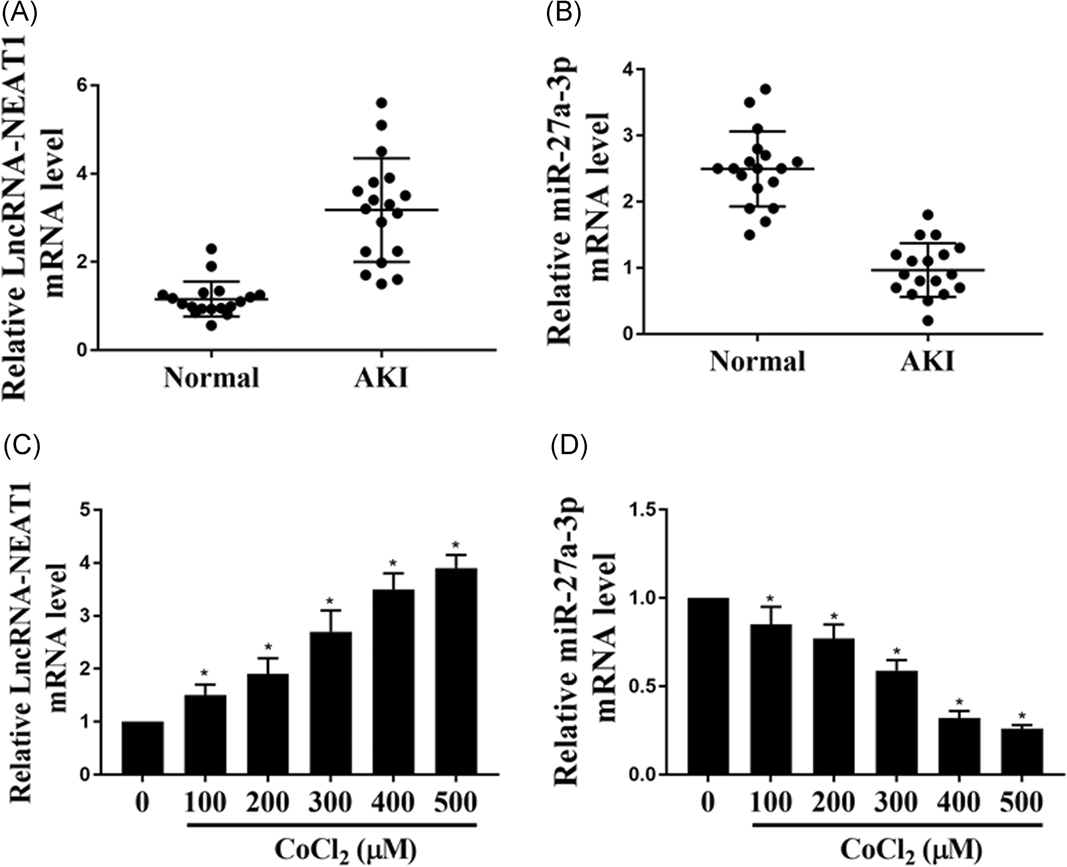
The expression of NEAT1 and miR-27a-3p in the ischemia-induced AKI patients and in HK-2 cells. A,B, The relative expression level of NEAT1 and miR-27a-3p mRNAs in ischemia-induced AKI patients and healthy volunteers (N = 18). C,D, The relative expression level of NEAT1 and miR-27a-3p mRNAs in HK-2 cells treated with different concentration of CoCl2 for 24 hours. The experiments were repeated for five times. AKI, a cute kidney injury; HK-2, human kidney 2; mRNA, messenger RNA; NEAT1, nuclear enriched abundant transcript 1; *P < 0.05
3.2 The suppression effect of NEAT1 on cell apoptosis in CoCl2-stimulated HK-2 cells
We next explored the function of NEAT1 in ischemia-induced AKI. Hypoxia reoxygenation model was established by treatment of HK-2 cells with CoCl2. Downregulation of NEAT1 was performed by transfection of sh-NEAT1 in the HK-2 cells and the downregulation efficacy was measured by qPCR (P < 0.05) (Figure 2A). Next, the effect of knockdown of NEAT1 on cell apoptosis in the CoCl2-stimulated HK-2 cells was determined. Western-blot analysis indicated that the protein level of proapoptotic protein Bax was decreased while the antiapoptotic protein Bcl-2 expression was markedly increased (P < 0.05) (Figure 2B). With the concept that a cell with high Bax/Bcl-2 ratio will be more sensitive to apoptosis, we analyzed the Bax/Bcl-2 ratio upon the downregulation of NEAT1. The Bax/Bcl-2 protein ratio was determined for each sample by dividing the relative expression of Bax by the relative expression level of Bcl-2. The results demonstrated that CoCl2 treatment significantly increased the Bax/Bcl-2 ratio (Figure 2B). Downregulation of NEAT1 remarkably inhibited the CoCl2 induced Bax/Bcl-2 ratio increase (Figure 2B). In addition, the expression of cleaved caspase 3 was also inhibited (P < 0.05) (Figure 2B). Flow cytometry was conducted to determine the effect of downregulation of NEAT1 on HK-2 cell apoptosis after the cells treated with CoCl2. The results showed that CoCl2 significantly increased the HK-2 cell apoptosis, while downregulation of NEAT1 markedly attenuated the HK-2 cell apoptosis (P < 0.05) (Figure 2C). These results suggested that downregulation of NEAT1 significantly suppresses the HK-2 cells apoptosis after treated with CoCl2 (P < 0.05).
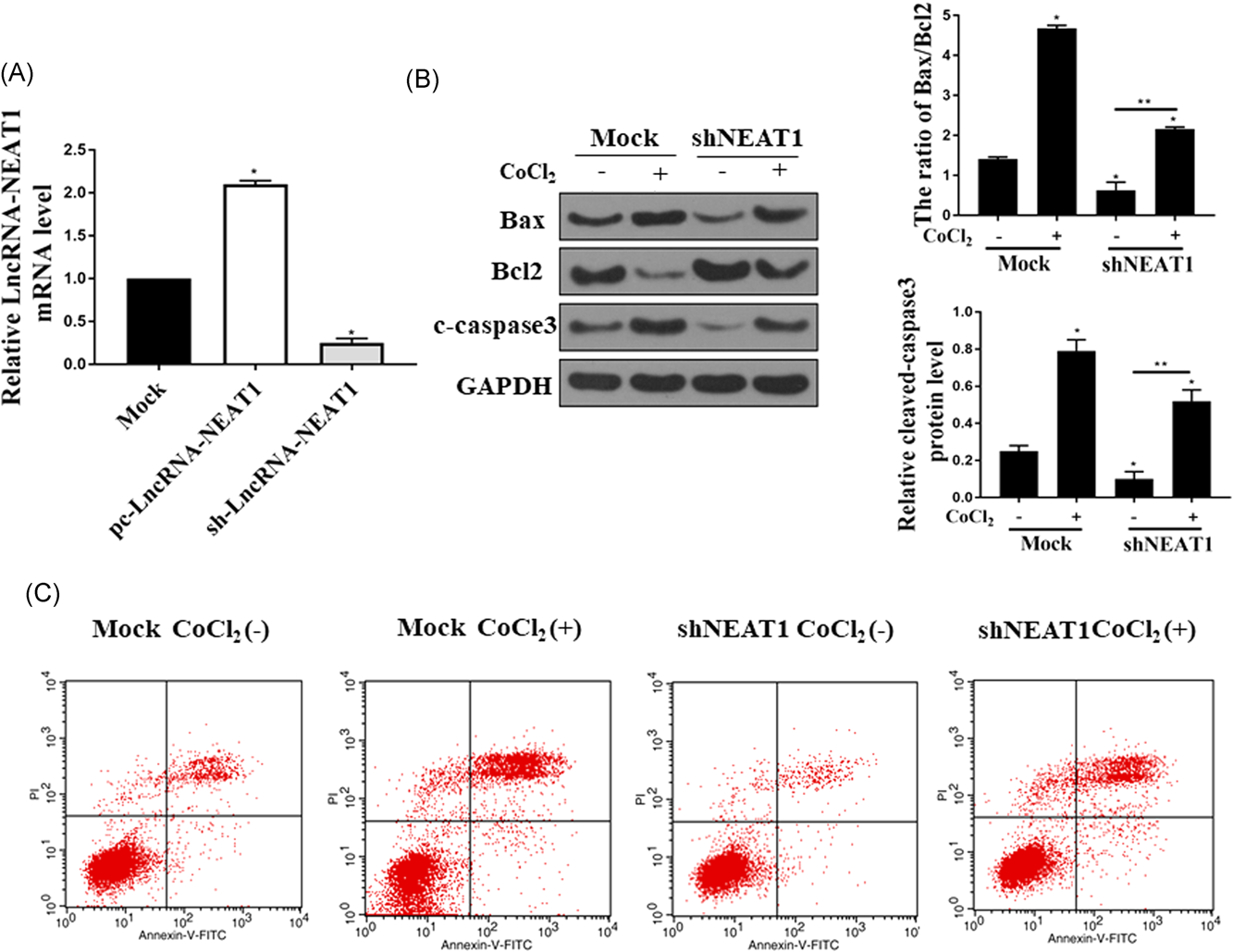
The suppression effect of NEAT1 on cell apoptosis in CoCl2-stimulated HK-2 cells. A, qPCR was performed to check the mRNA level of LncRNA-NEAT1 Mock, control sh-RNA and sh-NEAT1 groups. B, Western blot assay was conducted to determine the protein level of Bax, Bcl2, and cleaved-caspase3 in HK-2 cells treated with 300uM CoCl2 for 24 hours in both Mock and sh-NEAT1 groups. The Bax/Bcl-2 protein ratio was determined for each sample by dividing the relative expression of Bax by the relative expression level of Bcl-2. C. Cells treated with CoCl2 in both Mock and sh-NEAT1 groups and then double stained with annexin V and PI. The cell apoptosis was analyzed by Flow cytometry. The experiments were repeated for five times. Bcl-2, B-cell lymphoma 2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HK-2, human kidney 2; lncRNA, long non-coding RNA; mRNA, messenger RNA; NEAT1, nuclear enriched abundant transcript 1; PI, propidium iodide; qPCR, quantitative polymerase chain reaction; *P < 0.05; **P < 0.05
3.3 Effects of miR-27a-3p on the cell apoptosis in CoCl2-stimulated HK-2 cells
Since NEAT1 plays an antiapoptotic roll in HK-2 cells, we next investigated the role of miR-27a-3p in ischemia-induced AKI in CoCl2 induced hypoxia-reoxygenation model in HK-2 cells. miR-27a-3p expression level in HK-2 cells transfected with miR-27a-3p mimic and miR-27a-3p inhibitor was measured by RT-PCR. The expression of miR-27a-3p was significantly increased in HK-2 cells with transfection of its mimic while the expression level was downregulated with the transfection of its inhibitor (P < 0.05) (Figure 3A). Then, the effect of the expression level of miR-27a-3p on the cell apoptosis in the CoCl2-stimulated HK-2 was explored. Downregulation of miR-27a-3p dramatically induced the apoptosis by increased Bax/Bcl-2 ratio and annexin V/PI double positive cells, while overexpression of miR-27a-3p by transfection with its mimic ameliorated apoptosis in CoCl2-stimulated HK-2 (P < 0.05) (Figure 3B and 3C). Taken together, these data suggested that miR-27a-3p plays an antiapoptotic role in CoCl2 induced hypoxia-reoxygenation model.
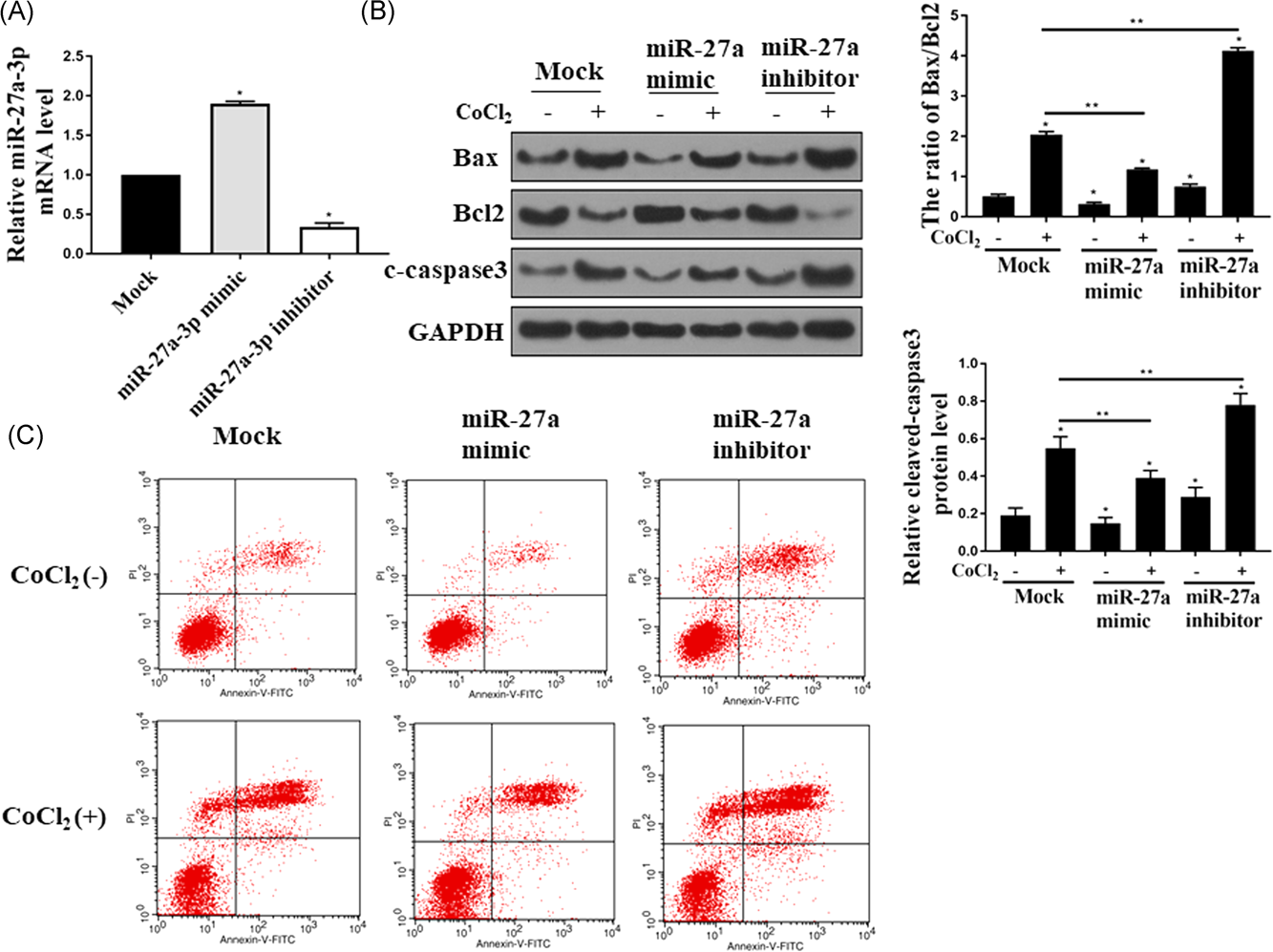
Effects of miR-27a-3p on the cell apoptosis in CoCl2-stimulated HK-2 cells. A, qPCR was performed to determine the mRNA level of miR-27a-3p in Mock, miR-27a-3p mimic and miR-27a-3p inhibitor groups. B, Western-blot assay was conducted to check the protein level of Bax, Bcl2, and cleaved-caspase3 in HK-2 cells treated with 300 uM CoCl2 for 24 hours in each group. C, HK-2 cells transfected with Mock, miR-27a-3p mimic or miR-27a-3p inhibitor with or without CoCl2 treatment were double stained with annexin V and PI and then flow cytometry was performed to examine the cell apoptosis. All the experiments were repeated for five times; Bcl-2, B-cell lymphoma 2; HK-2, human kidney 2; lncRNA, long non-coding RNA; mRNA, messenger RNA; NEAT1, nuclear enriched abundant transcript 1; PI, propidium iodide; qPCR, quantitative polymerase chain reaction; *P < 0.05; **P < 0.05
3.4 Reciprocal inhibition between miR-27a-3p and NEAT1 in human kidney cells
Next, the correlation of NEAT1 and miR-27a-3p was determined. As shown in Figure 4A, upregulation of NEAT1 decreased the expression of miR-27a-3p while downregulation of NEAT1 increased the expression of miR-27a-3p (P < 0.05). Bioinformatics websites such as Targetscan, Starbase, and microRNA.org, was used to search for the potential targets of NEAT1. Interestingly, a miR-27a-3p binding site was found in the NEAT1 transcript and NEAT1 was predicted to be the target gene of miR-27a-3p (Figure 4B). Next, the relation between miR-27a-3p with NEAT1 was verified by dual-luciferase reporter assay. The results in HEK293T cells overexpressed with miR-27a-3p indicated that the relative luciferase activity was decreased, and the foregoing inhibition was recovered by overexpression of its Mutant 9 P < 0.05) (Figure 4C). These results confirmed that NEAT1 was a direct target of miR-27a-3p. Moreover, the RNA pull-down assay results further confirmed that the biotinylated miR-27a-3p but not biotinylated miR-27a-3p-mut pulled down NEAT1 (Figure 4D), which contained the mutations in the predicted binding site between miR-27a-3p and NEAT1. These results demonstrated that miR-27a-3p was a direct target of NEAT1. In addition, the biotin-labeled NEAT1 probe was also pulled down by miR-27a-3p (Figure 4E).
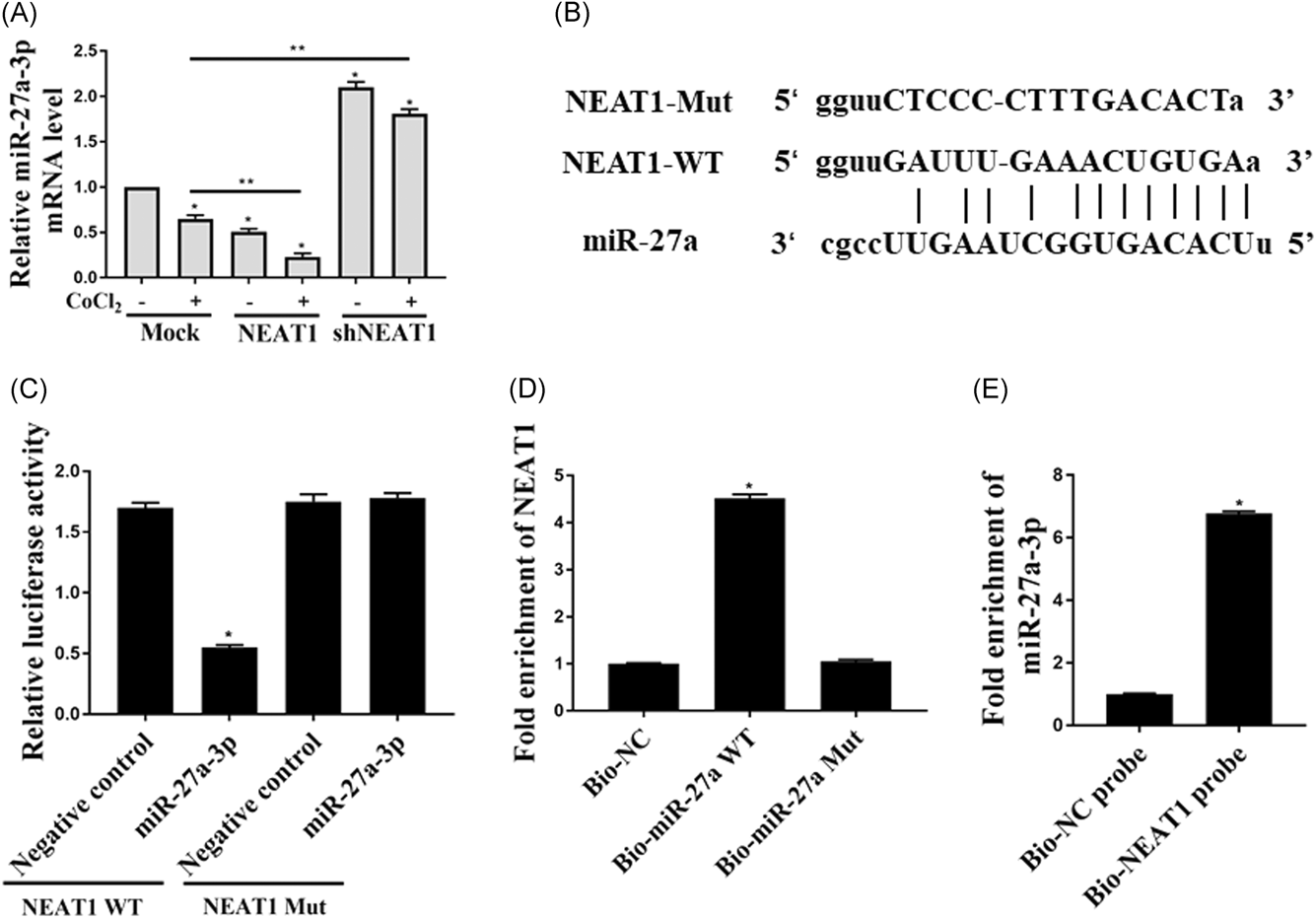
Reciprocal inhibition between miR-27a-3p and NEAT1 in human kidney cells. A, qPCR was performed to check the mRNA expression of miR-27a-3p expression in HEK293 cells transfected with NEAT1 or shNEAT1. B, The sequences of WT and mutant-type vectors and putative binding sites of miR-27a-3p within NEAT1 mRNA. C, The relative luciferase activities were determined by luciferase assay in the HEK-293 cells cotransfected with WT NEAT1 or NEAT1 mutant together with miR-27a-3p. D, The expression of NEAT1 mRNA was detected using qRT-PCR in the sample pulled down by biotinylated miR-27a-3p. E, The expression of miR-27a-3p was detected using qRT-PCR in the sample pulled down by biotinylated NEAT1. All the experiments were repeated for five times; mRNA, messenger RNA; NEAT1, nuclear enriched abundant transcript 1; qPCR, quantitative polymerase chain reaction; qRT-PCR, quantitative reverse transcription polymerase chain reaction; *P < 0.05
3.5 Upregulation of miR-27a-3p mostly reversed NEAT1-induced apoptosis in HK-2 cells
Although there was a reciprocal inhibition between miR-27a-3p and NEAT1, the regulatory influence of miR-27a-3p on NEAT1 remains unclear. miR-27a-3p mimic was transfected into HK-2 cells stably over-expressing NEAT1. Upregulated miR-27a-3p significantly reversed the overexpression of NEAT1 induced inhibition of HK-2 cell apoptosis by inhibition of the expression of Bcl2, promoting of the expression of Bax and increasing the expression of cleaved caspase3, as well as increasing the annexin-V positive/PI negative cells ratio and Bax/Bcl-2 ratio (P < 0.05) (Figure 5A and 5B). These results proved that miR-27a-3p and NEAT1 in ischemia cell model were negatively correlated and their biologic role in regulating cell apoptosis is also negatively correlated.

Upregulation of miR-27a-3p attenuates the suppression effect of NEAT1 on the apoptosis of HK-2 cells. A, HK-2 cells stably expression of NEAT1 were transfected with miR-NC or miR-72a-3p and then Western-blot assay was performed to determine the protein expression of Bax, Bcl2, and cleaved-caspase3. B, The apoptosis of the HK-2 cell in each group was double stained with annexin V and PI and then analyzed by flow cytometry. All the experiments were repeated for five times; Bcl-2, B-cell lymphoma 2; HK-2, human kidney 2; NC, negative control; NEAT1, nuclear enriched abundant transcript 1; PI, propidium iodide; *P < 0.05; **P < 0.05
4 DISCUSSION
Next-generation sequencing proved that a long noncoding transcript was expressed in most cells and in the past few years, much efforts revealed the biological role and function of long noncoding RNAs.22, 23 LncRNAs have been shown to play an important role in the regulation of gene expression and function.9, 22 Among these lncRNAs, NEAT1, which is a recently discovered architectural component of paraspeckles, has been proved to be abnormally expressed in different human disease, such as cancer, AKI.13, 14, 24, 25 However, the function of NEAT1 in AKI is still under investigation. Recently, one group showed that NEAT1 served a key role in sepsis-induced AKI through targeting of miR-204.14 They showed that NEAT1 upregulation enhanced the damage induced by LPS via activation of the NF-κB pathway and targeting of miR-204. Our studies suggested that NEAT1 was profoundly upregulated in ischemia-induced AKI patients compared with healthy control. Furthermore, the expression of NEAT1 was also enhanced in the HK-2 cells treated with CoCl2. In addition, downregulation of NEAT1 significantly mitigated the CoCl2-induced injury in HK-2 cells, which is consistent with the other groups' study which showed that upregulation of NEAT1 reduced the kidney injury.14, 26
Apoptosis is programmed cell death, which occurs in multicellular organisms. In AKI, apoptosis is also well recognized that induced by ischemia, toxic injury, radiation, and ureteral obstruction.7, 27 Although tubular apoptosis has long been reported in AKI, the mechanism involved in the regulation of tubular apoptosis by NEAT1 or miRNAs is still under investigation.28, 29 Bcl-2 family is notable for their regulation on apoptosis at the mitochondrion. This family consists of members that either promote or inhibit cell apoptosis with Bcl-2 serving as antiapoptotic factor and Bax as proapoptotic protein.30, 31 Upregulation of Bax induces the cytochrome C release from the mitochondrion to cytosol, resulting in the caspase cascade activation, especially caspase-3 and -9 cleavage.30, 31 Furthermore, the cells with high Bax/Bcl-2 ratio are susceptibility to apoptosis. In this study, our results indicated that downregulation of NEAT1 significantly suppressed the CoCl2-induced HK-2 cell apoptosis by reducing the cleaved caspase 3 expression and Bax/Bcl2 ratio. Altogether, the aberrant expression of NEAT1 might be associated with the development of AKI. Since there is no efficient therapy available once AKI occurs, our studies revealed that NEAT1 might be explored as the potential therapeutic target in the treatment of AKI in the future.
miR-27a-3p is a microRNA which located at chromosome 19 and is often frequently aberrantly expressed in different disease such as a tumor.32, 33 Recent studies reported that the expression of miR-27a-3p in patients with AKI was significantly lower,34, 35 which suggested that miR-27a-3p might be served as a biomarker of AKI. Moreover, another group reported that upregulation of miR-27a-3p attenuated the inflammatory damage of Blood-spinal cord barrier in the ischemia model and the results suggested its potential to be explored as a therapeutic target.36 In the current study, we found that miR-27a-3p was downregulated in both AKI patients and CoCl2 induced hypoxia-reoxygenation model in HK-2 cells. In addition, overexpression of miR-27a-3p significantly decreased the CoCl2-induced cell apoptosis while downregulation of miR-27a-3p increased the CoCl2 induced HK-2 cell apoptosis. These findings proved the function of miR-27a-3p in the development of AKI and miR-27s-3p has the potential to be explored as a biomarker for diagnosis and miR-27a-3p mimic has the potential to be investigated for the treatment of AKI in the future.
More interestingly, we also found that a miR-27a binding site was shown in NEAT1 transcript and NEAT1 was predicted to be a target gene of miR-27a-3p. Furthermore, downregulation of NEAT1 markedly increased the expression of miR-27a-3p and overexpression of miR-27a-3p attenuated the overexpression of NEAT1 induced HK-2 cell apoptosis increase after the cells treated with CoCl2. These results recovered that, NEAT1 promoted HK-2 cell apoptosis by regulation of the miR-27a-3p expression. This is the first results to prove the role and mechanism of how the aberrant expression of NEAT1 affect the development of AKI via regulation of miR-27a-3p. Thus, downregulation of NEAT1 or overexpression of miR-27a-3p has the potential to be explored as therapeutic strategies for the treatment of AKI clinically. NEAT1/ miR-27a-3p axis can be investigated as the biomarker for diagnosis and prognosis of AKI.
Therefore, NEAT1 which is a long noncoding RNA was significantly upregulated in AKI, while miR-27a-3p markedly downregulated in AKI and CoCl2 treated HK-2 cells. Downregulation of NEAT1 attenuated CoCl2-induced injury in HK-2 by reducing the cell apoptosis. NEAT1 negatively regulated the expression of miR-27a-3p. CoCl2-induced injury was alleviated by inhibition of NEAT1 through upregulation of miR-27a-3p. Thus, the ischemia-induced injury was aggravated by upregulation of NEAT1 through directly targeting of miR-27a-3p. NEAT1 together with miR-27a-3p may explore as a biomarker and therapeutic targets for the treatment of ischemia-induced AKI. In the future, more in vivo studies are needed to confirm the role of NEAT1/ miR-27a-3p axis using the in vivo model.
ACKNOWLEDGMENTS
We thank for the financial support from Zhejiang provincial natural science fund (LY17H050008) and Zhejiang provincial medical and health technology projects (2017KY227).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
JXX and LDT: manuscript writing, literature search, and study design; SW, SXG, and LYM: data analysis and statistical analysis.
ETHICS STATEMENT
This study was approved by the Ethics Committee of Zhejiang Provincial people's hospital. Informed consent was signed by all participates before the start of the study.