Heparan sulfate proteoglycans as trastuzumab targets in anoikis-resistant endothelial cells
Abstract
Anoikis is a form of programmed cell death induced by loss of contact from neighboring cells or from their extracellular matrix (ECM). Many tumorigenic cells are anoikis resistant, facilitating cancer progression and metastasis. Trastuzumab is a monoclonal antibody used for the treatment of breast and gastric cell cancer, but its mechanism of action is not well elucidated and its target molecules not well defined. Heparan sulfate proteoglycans (HSPGs) and glycosaminoglycans (GAGs) play important roles in tumor development and in response of cancer cells to drugs. This study investigates the effect of trastuzumab on the expression of HSPGs and sulfated glycosaminoglycans (SGAGs) in anoikis-resistant endothelial cells. After trastuzumab treatment, endothelial cells resistant to anoikis show an increase in adhesion to fibronectin followed by a decrease in invasion, proliferation, and angiogenic capacity. In addition, a significant increase in the number of cells in the S phase of the cell cycle was also observed. In relation to HSPGs and SGAGs expression, we observed a decrease in syndecan-4 and perlecan expression, as well as in the heparan sulfate biosynthesis in anoikis-resistant endothelial cells after exposure to trastuzumab. Our results suggest that trastuzumab interacts with GAGs and proteoglycans of the cell surface and ECM and through this interaction controls cellular events in anoikis-resistant endothelial cells.
1 INTRODUCTION
Anoikis is a Greek word meaning “loss of home” used to define the programmed cell death induced by loss of contact from neighboring cells or from their extracellular matrix (ECM).1 This is a physiological process that regulates tissue homeostasis by mediating cell death in response to changes in cell–ECM interactions.2 Tumor cells use several strategies to escape anoikis because the resistance to anoikis is an important step in cell invasion and metastasis formation. During malignancies, anoikis-resistant cancer cells often traffic throughout the ECM.2, 3
The ECM is a major component of the cellular microenvironment and is a highly dynamic structure, composed of a set of macromolecules that modulate diverse functions of cell biology,4, 5 such as, glycosaminoglycans (GAGs) and proteoglycans. The ECM has an important function in the anoikis process and several ECM molecules critically contribute to the progress of cancer cell invasion.6 Recently we reported that the acquisition of anoikis resistance promotes matrix remodeling in endothelial cells causing changes in some integrant of ECM expression.7 This remodeling can compromise ECM organization and function and promoting diseases, such as cancer. The remodeled ECM mostly triggers cancer cell phenotype and aggressiveness.8
Cancer is the loss of tissue organization and aberrant behavior of the cellular components and is the second leading cause of death globally.4 Several strategies have described cancer treatment, such as chemotherapy, radiotherapy, and some drugs. Trastuzumab is a humanized recombinant monoclonal antibody targeting the extracellular domain IV of human epidermal growth factor receptor 2 (HER2) and is used to treat patients of breast cancer and gastric cancer with the overexpression of ErbB2 (HER2). However, many patients with HER2 overexpression do not respond to trastuzumab therapy and some patients acquire resistance within 1 year of the initial response. Unfortunately, trastuzumab is also associated with an increased risk of cardiotoxicity. Previous studies have shown that HER2 might not be the only molecular target of trastuzumab.9-12
Syndecan-4 and perlecan are important heparan sulfate proteoglycans (HSPGs). Syndecan-4 is a ubiquitous cell surface proteoglycan fundamental in cell adhesion13; this HSPG also mediates numerous cellular processes through signaling pathways that affect cellular proliferation and migration.14 In addition, perlecan supports the migratory and proliferative events necessary for proper angiogenic blood vessel development.15 However, changes in the expression of syndecan-4 and perlecan have been observed in tumor cells and in anoikis-resistant endothelial cells, indicating its involvement in cancer. The precise functions of these molecules in cancer therapy are not known.7, 16-18
Two major types of GAGs present in proteoglycans are heparan sulfate (HS) and chondroitin sulfate (CS).19 These linear polysaccharides are present in all animal cells and can act as a cofactor in many biological processes, such as cell proliferation, apoptosis, invasion, and differentiation, moreover, they interact with diverse protein ligands.20, 21 Experimental evidence suggests that HS might mediate the effect of trastuzumab in breast cancer cells.12
The incompressible mechanisms of trastuzumab action, the importance of GAGs to trastuzumab's effect in breast cancer cells and the role of HSPGs (syndecan-4 and perlecan) in tumorigenesis led us to investigate the expression of GAGs and HSPGs in anoikis-resistant endothelial cells after trastuzumab treatment. We also examined the effect of trastuzumab in cell events controlled by these molecules. The results found in other works of our group showed EC-derived cell lines are an appropriate cell model for studies of trastuzumab treatment and resistance.7, 17, 18 Our results suggest that trastuzumab controls several cell events in anoikis-resistant endothelial cells as well as changing the expression of GAGs and HSPGs.
2 MATERIALS AND METHODS
2.1 Chemicals and antibodies
2.1.1 Antibodies used in this study
Rat monoclonal antibody HSPG (perlecan) clone A7L6 and rabbit polyclonal antibody syndecan 4 (#ABT157) acquired from Merck Millipore (Danvers, MA). Mouse monoclonal antibody β-actin and horseradish peroxidase (HRP)-linked goat anti-rat immunoglobulin G (IgG) secondary antibody were purchased from Cell Signaling Technology, Inc (Danvers, MA), HRP-linked goat anti-mouse IgG (A4416) and HRP-linked goat anti-rabbit IgG (A6154) secondary antibodies obtained from Sigma-Aldrich (St Louis, MO). Trastuzumab (Herceptin, Genentech, San Francisco, CA) diluted with F12 medium.
2.2 Cell lines and treatment with trastuzumab
Endothelial cell line derived from rabbit aorta (EC),22 EC transfected with EJ-ras oncogene (EJ-ras EC)17 and EC resistant to anoikis (Adh1-EC and Adh2-EC)18 were maintained in F12 medium (Sigma-Aldrich) supplemented with 10% fetal calf serum (FCS; Athena, Campinas, Brazil) at 37°C, 2.5% CO2. Trastuzumab (Herceptin) was used on the same concentrations for all assays (20 µg/mL) for 24 hours in F12 medium on the presence or absence of FCS depending on the type of assay. This study was approved by the Ethics Committee of Federal University of São Paulo.
2.3 Cell viability assay
Cells (1 × 104 cells per well) were placed in 96-well plates. After treatment with trastuzumab (20 µg/mL for 24 hours), the cell viability was determined using the (3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) reagent (Thermo Fisher Scientific Inc, Rockford, IL) as described by the manufacturer. The assays were performed in triplicate.
2.4 Invasion assay
The invasion assays were performed on 24-well plates using polycarbonate transwell of 8-µm pore size (Corning Inc, Lowell, MA) coated with enhanced chemiluminescence cell attachment matrix (entactin, collagen IV, and laminin) (1 mg/mL; Merck Millipore). Briefly, the coated inserts were hydrated with warm F12 without serum in 2.5% CO2 at 37°C for 2 hours. A total of 5 × 104 endothelial cells in the presence or absence of trastuzumab (20 μg/mL for 24 hours) were seeded into the upper chamber containing F12 without serum, and the lower chamber contained F12 with 10% FCS. The cells were incubated at 37°C in 2.5% CO2 for 24 hours. Noninvading cells were removed from the upper membrane by scrubbing with a cotton swab. Invading cells were fixed with 4% formaldehyde at room temperature for 5 minutes and methanol for 20 minutes at 4°C, stained with 4′,6-diamidino-2-phenylindole (DAPI; 1:10 000) diluted in phosphate-buffered saline (PBS) containing 0.01% saponin for 30 minutes in the dark and visualized under a light microscope (Carl Zeiss, Jena, Germany). The number of stained cells was counted in 10 microscope fields (magnification, ×50). Each experiment was repeated three times.
2.5 Bromodeoxyuridine incorporation
For bromodeoxyuridine (BrdU) incorporation, serum-starved cultures in the presence or absence of trastuzumab (20 μg/mL for 24 hours) were incubated with BrdU 10 µM in the presence of 10% FCS for 4, 12, and 20 hours. BrdU uptake was determined essentially as recommended by the kit manufacturer (BrdU Cell Proliferation Assay, Merck Millipore). Each experiment was repeated three times.
2.6 Cell cycle assay
For the cytometric DNA analysis, quiescent cultures in the presence or absence of trastuzumab were stimulated with 10% FCS for 4, 12, and 20 hours. The cells were detached from the culture plate using trypsin and submitted to flow cytometric analysis as previously described23 using FlowJo software (Tree Star Inc, Ashland, OR). Each experiment was repeated three times.
2.7 Adhesion assays
Ninety-six-well tissue culture plates (Corning/Costar, New York, NY) were submitted to attach to substrate (5, 10, 20, or 30 µg/mL of fibronectin) for 2 hours at 37°C. The plates were washed with PBS and blocked with 1% bovine serum albumin (BSA) in PBS for 1 hour at 37°C. Endothelial cells (2 × 104 in F12-medium) in the presence or absence of trastuzumab (20 μg/mL for 24 hours) were added to the plates and submitted to attach to the substrate for 2 hours at 37°C. At the end of incubation, the unattached cells were removed by washing the plates with PBS. Attached cells were fixed in methanol for 20 minutes and stained with 0.8% crystal violet (Sigma-Aldrich) dissolved in 20% ethanol, and washed five times with PBS. The dye was then eluted with 50% ethanol 0.1M sodium citrate, pH 4.2, and measured for absorbance at 540 nm using a microplate reader EZ Read 400 (Biochrom, Holliston, MA). For control, the nonadhesive substrate was prepared by coating the wells with 1% BSA for 60 minutes at 37°C. The experiments were performed in triplicates for each dose and repeated three times in different days.
2.8 Angiogenesis assay
After treatment with trastuzumab (20 μg/mL for 24 hours), endothelial cells were detached with trypsin and seeded at a density of 1 × 104 viable cells per well on 96-well plates coated with Corning Matrigel Matrix (50 µL). After 8 hours of incubation at 37°C, tube formation was examined by light microscopy and the images were transferred to the computer for analysis. The images were processed using the ImageJ software (NIH, Bethesda, MD)to count the total number of pixels in three different fields and a mean value was determined for each sample. The control samples were defined as 100% (relative percentage). The experiments were performed in triplicate.
2.9 RNA isolation, reverse transcription polymerase chain reaction, and real-time polymerase chain reaction
Total RNA was isolated from the endothelial cells after treatment with trastuzumab (20 μg/mL for 24 hours) with RiboZol reagent (Amresco LLC,. Solon, OH) following the manufacturer's instructions. Complementary DNA (cDNA) was synthesized using All-in-One cDNA Synthesis SuperMix (Bimake, Houston, TX) and was analyzed using the Maxima SYBR Green qPCR Master Mix Kit (Thermo Fisher Scientific Inc) with an ABI 7500 Real-Time PCR instrument (Applied Biosystems, Foster, CA), according to the manufacturer's instruction, respectively. The primers used are as follows: perlecan, Fwd (5′-GCCTCGAGCACCTCTCGGAACT-3′) and Rev (5′-CTCAGGAGACGACCTGGGCAGT-3′); syndecan-4, Fwd (5′-AGCCTGGGCAGGTCGTGTCT-3′) and Rev (5′-GTGGTTCCCCTCACCGCAGC-3′); and RPL13A Fwd (5′-TTGAGGACCTCTGTGTATTTGTCAA-3′) and Rev (5′-CCTGGAGGAGAAGAGGAAAGAGA-3′). Polymerase chain reaction (PCR) was performed at 95°C for 15 seconds and 60°C for 60 seconds for 40 cycles. RPL13A was used as an internal standard to normalize messenger RNA (mRNA) levels for differences in sample concentration and loading. Fold changes in the expression of each target mRNA relative to RPL13A was calculated based on the threshold cycle (Ct) as , where ΔCt = Cttarget − CtRPL13A and Δ(ΔCt) = ΔCtAdh-EC − ΔCtEC. The post-amplification melting curve analysis was performed to confirm whether the nonspecific amplification was generated from primer-dimers. Quantitative PCR reactions were performed in triplicate and repeated three times.
2.10 Western blot analysis
Endothelial cells treated and untreated with trastuzumab (20 μg/mL for 24 hours) were lysed in buffer (50 mM Tris-HCl, pH 7.4, 1% Tween 20, and Halt Protease and Phosphatase Inhibitor Cocktail [Thermo Fisher Scientific Inc]) for 2 hours at 4°C. The homogenates were spun at 18 000g for 5 minutes and the supernatant was collected. Proteins were quantified using the BCA Protein Assay Kit (Thermo Fisher Scientific Inc). Equal amounts of protein extracts (20 µg) were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto a polyvinylidene fluoride membrane (Merck Millipore). After blocking, the membrane was incubated with the primary antibody (rabbit anti-syndecan-4 1:1000; rat anti-HS [perlecam] 1:500, and mouse anti-β-actin 1∶1000) diluted in blocking buffer overnight at 4°C and followed by incubation with secondary antibodies (1∶10 000) for 1 hour at room temperature. The samples were detected by enhanced chemiluminescence using the SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific Inc). An Alliance mini photo documentation system from UVITEC (Cambridge, UK) was used to scan the films and UVIBAND MAX v1503b software (UVITEC) was used to measure the amount of protein detected by each antibody. The experiments were performed in duplicate and repeated twice.
2.11 Immunocytochemistry
Cells were cultured on 13.0 mm diameter glass coverslips (5 × 103 cells per coverslip) in 24-well plates for 2 days followed by fixation with 2% formaldehyde in PBS (pH 7.4) for 20 minutes at room temperature. The cells treated and untreated with trastuzumab (20 μg/mL for 24 hours) were washed four times with PBS and once with PBS containing 0.1M glycine. They were then permeabilized with PBS containing 1% BSA and 0.01% saponin for 15 minutes at room temperature. After this step, the cells were washed with PBS and incubated with rat anti-perlecan (1:200) and rabbit syndecan-4 (1∶200) diluted in PBS or PBS containing 0.01% saponin for 1 hour at room temperature. After washing, the cells were incubated with goat anti-rabbit labeled with Hilyte Fluor 594 (1∶300) or goat anti-rat labeled with Alexa Fluor 488 (1:300) and DAPI (1:10 000) in PBS containing 0.01% saponin for 1 hour at room temperature. The cells were washed and mounted in Fluoromount-G (2∶1) diluted in PBS and examined using an inverted confocal laser-scanning microscope (Leica TCS SP8; Leica Microsystems, Wetzlar, DE). Each figure shown in the results section corresponds to the better of two experiments.
2.12 Extraction and identification of sulfated glycosaminoglycans
Sulfated glycosaminoglycans (SGAGs) synthesized by the cells in the presence or absence of trastuzumab (20 μg/mL for 24 hours) were metabolically labeled with [35S]-sulfate (150 µCi/mL) in F-12 medium for 18 hours at 37°C in 2.5% CO2 atmosphere. Afterward, the culture medium was removed, and the cells washed twice with F-12 medium and scrapped from the dish with 3.5M urea in 25 mM Tris-HCl pH 7.8. Both to the medium and the cell extract 100 µg of carrier HS, dermatan sulfate, and CS were added. The radioactive GAG free chains were prepared from the cell lysates and from the culture supernatants by incubation with 4 mg of maxatase (Biocon do Brasil Ltda, Rio de Janeiro, Brazil) for 4 hours at 60°C. The [35S]-SGAGs were identified and quantified by agarose gel electrophoresis, as previously described.24, 25 The radioactive compounds were located by exposure of the gels (after fixation, drying, and staining) to cyclone storage phosphor screen (Packard Institute, Meriden, CT). For quantification, the radioactive bands were scrapped off the agarose gels and counted in 5 mL of Ultima Gold (Packard Institute) in a liquid scintillation spectrometer (TRI-CARB 2100TR; Packard Institute). Protein was determined by the Coomassie blue assay. The experiments were performed in duplicate and repeated three times.
2.13 Statistical analysis
All data are expressed as the mean ± standard error of the mean. Statistical significance was assessed using analysis of variance. P < 0.05 were considered as statistically significant.
3 RESULTS
3.1 Trastuzumab reduces cell viability, invasion, and proliferation and induces changes in cell cycle in anoikis-resistant endothelial cell
To investigate the effect of trastuzumab in anoikis-resistant endothelial cells, we started this study with a viability assay (MTT), as described in Section 2. Previous studies have shown that the concentration of trastuzumab able to decrease cell viability is variable between different cells types and dependent on levels of HER2 and HS on the cell surface and in medium. The concentrations used are around 3 to 25 µg/mL for 12, 24, 48 or more hours.9, 12, 26-28 In our investigation, we observed that 20 µg/mL of trastuzumab for 24 hours is sufficient to reduce cell viability of anoikis-resistant endothelial cells (Adh-EC) by around 35% and it had no effect on parental cells (EC), as expected (Figure 1A). As anoikis-resistance endothelial cells exhibit characteristics of tumorigenic cells as high rate of proliferation, high invasive potential, and cell cycle dysregulation,18 we decided to analyze the effect of trastuzumab in these cellular events. The treatment with trastuzumab was able to decrease the cell invasion and proliferation rate of anoikis-resistant endothelial cells (Adh-EC) (Figure 1B and 1C). In addition, significant changes were observed in the distribution of cell cycle phases. Trastuzumab could induce the S-phase arrest in EC, EJ-ras EC, Adh1-EC, and Adh2-EC cells (Figure 2A and 2B). These data corroborated with the reduction of the proliferation rate observed at 12 and 20 hours, which correspond to phase S and G2/M phase of the cell cycle (Figure 1C).
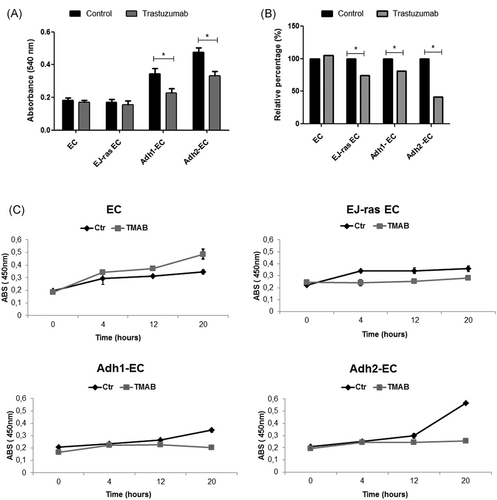
Cell viability, invasion capacity, and proliferation of EC-derived cell lines treated with trastuzumab. A, MTT assay was used to analyze cell viability of EC, EJ-ras EC, Adh1-EC, and Adh2-EC cells after trastuzumab treatment (20 µg/mL for 24 hours), as described in Section 2. B, Values of relative invasiveness of EC, EJ-ras EC, Adh1, and Adh2-EC normalized with control without trastuzumab (100%). Invasion activity of EC-derived cell lines treated with trastuzumab was analyzed by using as transwell coated with enhanced chemiluminescence cell attachment matrix as described in Section 2. C, Bromodeoxyuridine incorporation for 4, 12, and 20 hours. EC, EJ-ras, Adh1-EC, and Adh2-EC in the presence or absence of trastuzumab were serum-starved, as described in Section 2, and stimulated to proliferate by the addition of 10% fetal calf serum. The experiments were performed in triplicate and repeated three times. The bars represent the standard error (*P < 0.05). ABS, absorbance; Adh1-EC and Adh2-EC, anoikis-resistant endothelial cells; EC, parental endothelial cells; EJ-ras EC, EJ-ras transfected endothelial cells; MTT, (3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide
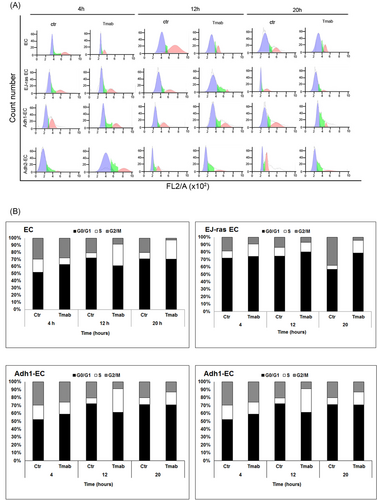
Effect of trastuzumab on cell cycle progression of EC, EJ-ras EC, Adh1-EC, and Adh2-EC cells. Cells were kept in 0,2 % FCS(fetal calf serum) for 24 hours and stimulated with 10% FCS. The cells were harvested for flow cytometric analysis of DNA content at various time periods (4, 12, and 20 hours) after stimulation. A, Histogram depicting cell cycle distribution. The histograms show the proportion of cells at different stages in the cell cycle (DNA content of propidium iodide-stained nuclei). B, Cell cycle distribution of EC, EJ-ras EC, Adh1-EC, and Adh2-EC cells. Results are represented as percentage of the cell population detected at the G0/G1, S, and G2/M phases of the cell cycle. The numbers represent an average of three different experiments with an error of 5%. Adh1-EC and Adh2-EC, anoikis-resistant endothelial cells; EC, parental endothelial cells; EJ-ras EC, EJ-ras transfected endothelial cells
3.2 Trastuzumab promotes increased adhesion and it has antiangiogenic effect in anoikis-resistant endothelial cells
To investigate the role of trastuzumab in angiogenesis of EC and EC-derived cell lineages, we analyzed the formation of tubes in Matrigel in the presence or absence of trastuzumab, as described in Section 2. Our results show that trastuzumab had a significant impact on vessel formation in EJ-ras EC, Adh1-EC, and Adh2-EC, causing a significant reduction of angiogenesis (48%, 25%, and 45%, respectively) (Figure 3A and 3B). Previous studies have reported that anoikis-resistant endothelial showed lower adhesiveness to fibronectin.18 Thus, to determine the effect of trastuzumab treatment on adhesion, endothelial cells treated or untreated with trastuzumab were submitted to attach to fibronectin (5, 10, 20, or 30 µg/mL) for 2 hours at 37°C. Figure 4 shows that trastuzumab treatment leads to an increase in the adherence to fibronectin of EJ-ras EC, Adh1-EC, and Adh2-EC. These results corroborated with the reduction of the invasiveness observed in these cell lines.
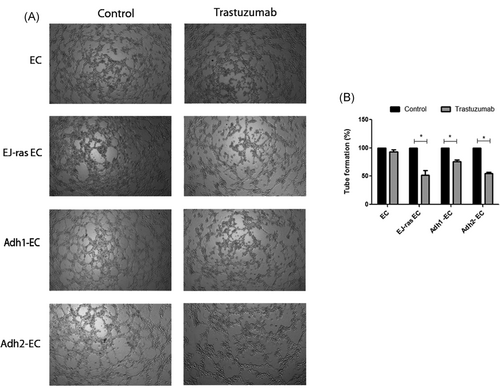
Antiangiogenic effect mediated by treatment with trastuzumab in EC, EJ-ras EC, Adh1-EC, and Adh2-EC cells. A, Representative photographs of tube-like structure. B, Quantitative analysis of total tube length. The control samples were defined as 100% and it was calculated for each samples the percentage of increase or decrease in the formation of tube relative to the control (relative percentage), as described in Section 2. The experiments were performed in triplicates. Adh1-EC and Adh2-EC, anoikis-resistant endothelial cells; EC, parental endothelial cells; EJ-ras EC, EJ-ras transfected endothelial cells
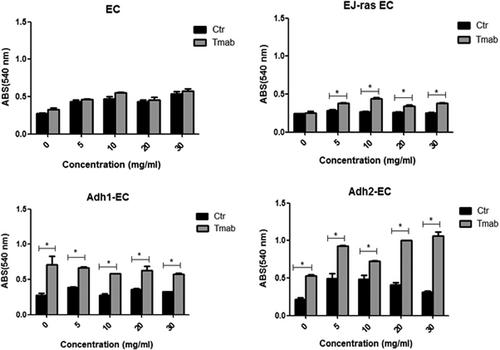
Adhesion rate of EC-derived cell lines after trastuzumab treatment. Assay adhesion of EC, EJ-ras EC, Adh1-EC, and Adh2-EC on fibronectin in the presence and absence of trastuzumab, as described in Section 2. The experiments were performed in triplicate and repeated three times. The bars represent the standard error (*P < 0.05). ABS, absorbance; Adh1-EC and Adh2-EC, anoikis-resistant endothelial cells; Ctr, control; EC, parental endothelial cells; EJ-ras EC, EJ-ras transfected endothelial cells
3.3 Expression of HSPGs in anoikis-resistant endothelial cells after treatment with trastuzumab
Recent work from our group has shown that anoikis-resistant endothelial cells and EJ-ras transfected cells display an increase in the expression of perlecan and syndecan-4.7, 17, 18 Thus, to investigate the effect of trastuzumab treatment in syndecan-4 and perlecan expression, endothelial cells treated or untreated with trastuzumab were submitted to qPCR, Western blot analysis, and immunofluorescence assays. We observed a decrease in the perlecan gene expression in endothelial cells resistant to anoikis and EJ-ras transfected cells, as shown in Figure 6A. Western blot analysis showed that protein expression levels of perlecan are decreased in Adh-EC and EJ-ras EC cells and increased in EC cells after treatment with trastuzumab. Similar data were observed using immunofluorescence assay (Figure 6B and 6C). Moreover, Figure 7A shows a significant decrease in the gene expression of syndecan-4 in EJ-ras EC and Adh-EC and an increase in EC after trastuzumab treatment. Similar data were observed in protein expression of syndecan-4 and in immunofluorescence assay.
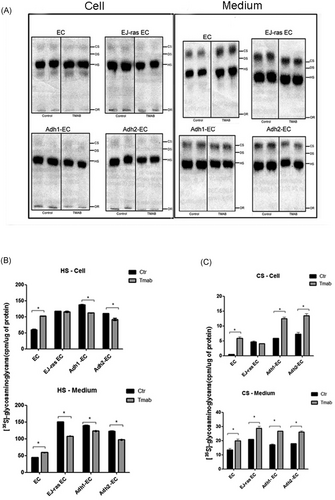
[35S] sulfated glycosaminoglycans synthesized by EC, EJ-ras EC, Adh1-EC, and Adh2-EC cells after trastuzumab treatment. A, Endothelial cells were exposed to [35S] sulfate for 18 hours. The radioactive glycosaminoglycans-free chains were prepared, from both cells and conditioned medium, by incubation with proteolytic enzyme. Aliquots were subjected to electrophoresis for 60 minutes 0.6% agarose (0.05M 1,3-diaminopropane-acetate buffer pH 9.0).22, 29 The radioactive compounds were located in the gels, as described in Section 2. B,C, Quantification of the experiment shown in A. For further details, see Section 2. The experiments were performed in duplicate and repeated three times. The bars represent the standard error (*P ≤ 0.05). Adh1-EC and Adh2-EC, anoikis-resistant endothelial cells. CS, chondroitin sulfate; DS, dermatan sulfate; EC: parental endothelial cells; EJ-ras EC, EJ-ras transfected endothelial cells; HS, heparan sulfate; OR, origin
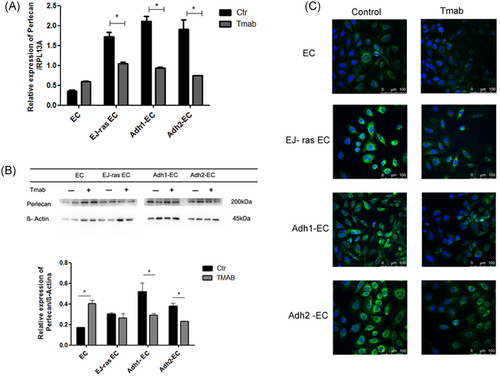
Gene and protein expression of perlecan in EC-derived cell lines. A, Expression of perlecan detected by quantitative polymerase chain reaction. RPL13A was used as a loading control. B, Protein expression of perlecam by Western blot analysis. β-Actin was used as a loading control. Histogram depicting perlecam protein levels normalized to β-actin. C, Immunofluorescent analysis of perlecan. Perlecan was stained with antiperlecan antibody followed by secondary anti-rat labeled with Alexa Fluor 488 (green). Nucleus stained with 4′,6-diamidino-2-phenylindole (blue). The images of immunofluorescence were obtained using an inverted confocal laser-scanning microscope (Leica TCS SP8; Leica Microsystems) and analyzed by Leica Application Suite Advanced Fluorescence software (Scale bar = 100 μM). A, Experiment was performed in triplicate. B,C, Experiments were performed in duplicate. The bars represent the standard error (*P < 0.05). Adh1-EC and Adh2-EC, anoikis-resistant endothelial cells; EC, parental endothelial cells; EJ-ras EC, EJ-ras transfected endothelial cells
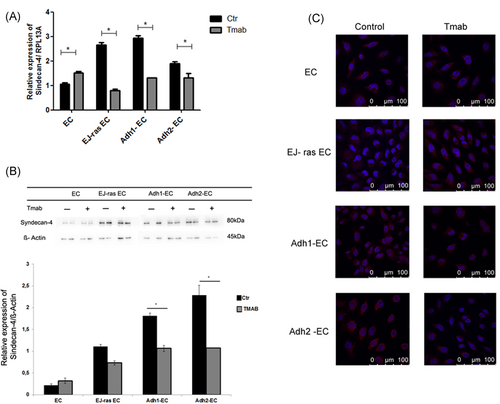
Gene and protein expression of syndecan-4 in EC-derived cell lines. A, Expression of syndecan-4 detected by quantitative polymerase chain reaction. β-Actin was used as a loading control. B, Protein expression of syndecan-4 by Western blot analysis. β-Actin was used as a loading control. Histogram depicting syndecan-4 protein levels normalized to β-actin. C, Immunofluorescent analysis of syndecan-4. Syndecan-4 was stained with antisyndecan-4 antibody followed by secondary (green). Nucleus stained with 4′,6-diamidino-2-phenylindole (blue). The images of immunofluorescence were obtained using an inverted confocal laser-scanning microscope (Leica TCS SP8; Leica Microsystems) and analyzed by Leica Application Suite Advanced Fluorescence software (Scale bar = 100 μM). A, The experiment was performed in triplicate. B,C, The experiments were performed in duplicate. The bars represent the standard error (*P < 0.05). Adh1-EC and Adh2-EC, anoikis-resistant endothelial cells; EC, parental endothelial cells; EJ-ras EC, EJ-ras transfected endothelial cells
3.4 Treatment with trastuzumab induces alterations on the synthesis of HS and CS in anoikis-resistant endothelial cells
The effect of trastuzumab in SGAGs synthesis is not well understood, but some studies determined that the profile of GAGs synthesized by breast and gastric cancer cell lines is different in relation to normal cells, In addition, a study performed on breast cancer cells demonstrated the profile of GAGs, as these tumor cells are altered after trastuzumab treatment.12 We have previously reported that HS and CS expression is increased in anoikis-resistant endothelial cells.18 To investigate the role of trastuzumab in the HS and CS synthesis, EC and EC-derived cell lineages treated or untreated with trastuzumab were exposed to [35S] sulfate for 18 hours and aliquots of the cell extracts and of the culture medium were treated with protease and subjected to electrophoresis, as described in Section 2. Figure 5A and 5B show that after trastuzumab treatment, EC cells present higher levels of HS in the cellular fraction (71%) and in the medium (32%). In contrast, after treatment with trastuzumab, a decrease in the HS expression in the cellular extract and in the medium was observed in Adh1-EC and Adh2-EC, respectively, 19% and 17% (Figure 5A and 5B). In addition, we observed an increase in the CS expression secreted to the medium and in the cellular fraction of all cells lines this (Figure 5A and 5C).
4 DISCUSSION
Cancer is a leading cause of death worldwide and metastasis is responsible for about 90% of cancer patient death. Hanahan and Weinberg described the hallmarks of cancer, that which encompass the fundamental biological capabilities acquired during the development of human cancers including sustained proliferation, evasion of growth suppression, dysregulated cellular metabolism, evasion of immune destruction, death resistance, replicative immortality, induced angiogenesis, and initiation of invasion and metastasis.30, 31
In relation to multistep of metastasis, the resistance to anoikis is a key hallmark of metastatic progression.32-34 We have previously demonstrated that endothelial cells submitted to stressful conditions by blocking adhesion to substrate (anoikis resistance) display several characteristics essentials of tumor cell, as morphological alterations, poor adhesion, high rates of proliferation and invasion and syndecan-4 upregulation. In addition, alterations in Ras/ERK and PI3K/Akt pathway, as high expression of Ras, ERK, PI3K (p110alfa), and Akt (Thr 308) and ECM remodeling also were observed.7, 18
Changes in ECM components influence the acquisition and maintenance of each of the cancer hallmarks.35 The ECM not only has multiple effects on the biological behaviors of tumor but also regulates the development and maintains tissue homeostasis and participates in multiple biological events. Due to these functions, ECM molecules may be potential targets in cancer therapy.16, 36
As detailed earlier, although trastuzumab is currently considered one of the most effective treatments in oncology, the mechanism of action is not fully understood.37, 38 Some studies suggest that the cell response is not only HER2 dependent, as initially described. These data have resulted in increasing the search of trastuzumab target molecules to understand the molecular mechanism of this drug.
Trastuzumab reduced the cell viability of several cancer cell lines in concentration and time variable, between 3 and 25 µg/Ml for 12, 24, and 48 hours.12, 39 Higher concentrations of trastuzumab is common to trigger resistance in some tumor cell, this can occur because the binding domains of trastuzumab were saturated or that Her-2 receptors were internalized and degraded or unable to recycle back to the surface for further trastuzumab binding.40 Similar to our study, it has been reported that trastuzumab, besides reducing cell viability, also modulates the proliferation, invasion, and angiogenesis of breast cancer cells.12, 26, 40, 41
Here we showed that trastuzumab reduced the cell viability of anoikis resistance in endothelial cell in 20 µg/mL for 24 hours. Furthermore, we have demonstrated that trastuzumab did not induce reduction of cell viability of parental cells (EC). Interestingly, trastuzumab had no effect in cell viability of transfected cells with EJ-ras EC but reduces the invasion and proliferation in these cells. Similar results were found in MCF7 (human breast adenocarcinoma cell line) cells,12 likely due to the fact this cells lineage presents different levels of HS secreted in relation to EC and Adh-EC.18 Trastuzumab effect in cell viability of anoikis-resistant endothelial cells was accompanied by modulation of invasion and adhesion with a concomitant reduction in proliferation and inhibition of the tube formation. These results are consistent with previous findings in breast cancer cells.12, 40
Tumors produce several molecules that facilitate this biological process, especially, proteoglycans and GAGs, which are involved in the pathobiology of all stages of cancer progression. The syndecan-4, a HSPG, can act in focal-adhesions of several cell types and also as a coreceptor of growth factors and proteins of the ECM by increasing the affinity of adhesion molecules to their specific receptors.42, 43 Some studies have shown that this HSPG may act as mechanotransducer in the progression of cancer.43-45 Syndecan-4 is highly expressed in anoikis-resistant endothelial cells.18 Here we showed a decrease of syndecan-4 expression followed by an increase in cell adhesion and consequently decrease in invasion and proliferation in anoikis resistant endothelial cells after trastuzumab treatment.
Perlecan is another HSPG also associated with neoplasms. Perlecan coordinates the formation of new vessels46 and changes in its expression is accompanied by alteration in angiogenesis.47 Our results show that trastuzumab treatment led to a decrease in perlecan levels followed by decrease in the angiogenesis of anoikis-resistant endothelial cells.
Recent work from our group has shown that anoikis-resistant endothelial cells display an increase in the expression of syndecan-4 and perlecan accompanied by an increase in the levels of HS and CS.7, 18 It is known that GAG structures are sensitive to drug treatment and some GAGs, for example, are used as targets in several therapies.48 Li et al demonstrate that the HS and CS at lung epithelial cells might facilitate bleomycin (anticancer drug) uptake. In addition, Suarez et al showed that the level of HS secreted to the medium is determinant to trastuzumab action.12, 49 Here we showed that anoikis resistant-endothelial cells and transfected with EJ-ras oncogene display have high level of HS in cell extract and medium and are sensitive to trastuzumab. The same is not observed in parental cells. On the other hand, we observed an increased in CS synthesis in all cell lines. CS plays a role in cell proliferation, cell motility, and metastasis. Some evidence suggests that CS in cell surface might play a metastasis promoting role, interacting with CD44 triggers and has an antimetastatic function, regulating breast cancer progression.50-52
In conclusion, our findings show that the antitumor effects of trastuzumab might be associated both with the amount of HS and CS present on the cell surface and medium and with the ability of trastuzumab to bind to syndecan-4 and perlecan. Furthermore, we demonstrated that the modulation in GAGs synthesis and in HSPGs expression by trastuzumab led to the reversal of the tumor phenotype in anoikis-resistant endothelial cells. This hypothesis is summarized in Figure 8.
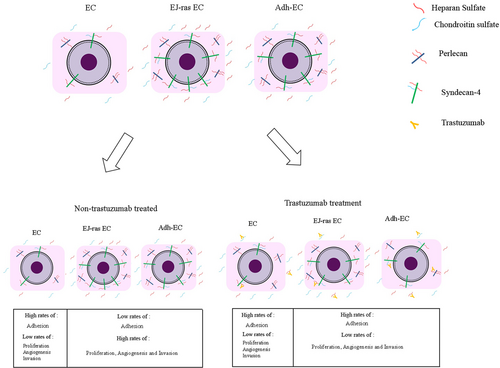
Scheme of proposed mechanism for action of trastuzumab in anoikis-resistant endothelial cells Adh1-EC and Adh2-EC, anoikis-resistant endothelial cells; EC, parental endothelial cells; EJ-ras EC, EJ-ras transfected endothelial cells
ACKNOWLEDGMENTS
Supported by grants from Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP); Equipamentos Multiusuários (EMU—FAPESP); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); Coordenação de Aperfeiçoamento de Pessoal do Ensino Superior (CAPES) and Financiadora de Estudos e Projetos (FINEP), Brazil.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.




