Matrine improves diabetic cardiomyopathy through TGF-β-induced protein kinase RNA-like endoplasmic reticulum kinase signaling pathway
Abstract
Background
Matrine might play a vital role in cardiovascular diseases progression and treatment.
Objectives
We aimed to explore the protective effects and potential mechanism of matrine against diabetic cardiomyopathy (DCM) in rat model.
Method
A rat model of DCM was induced by streptozotocin, which were then divided into two groups and treated with matrine. Inflammatory cytokines were investigated in serum and myocardial cells after matrine administration. The effects of matrine on cardiac reactive oxygen species (ROS) generation, Malondialdehyde (MDA) levels, and Glutathione peroxidase (GPx), PPARγ1 activity were detected in myocardial cells. The protein kinase RNA-like endoplasmic reticulum kinase (PERK) signal pathway in endoplasmic reticulum stress was studied to elaborated protective effects of matrine in DCM rat by Western blot analysis. Fasting blood glucose and hemodynamic parameters were analyzed after treatment with matrine.
Results
Matrine-inhibited expression levels of inflammatory cytokines of tumor necrosis factor alpha (TNF-α) and interleukin 6. Matrine administration decreased ROS generation, MDA, and transforming growth factor beta levels, and Peroxisome proliferator-activated receptor beta (PPARβ) and Peroxisome proliferator-activated receptorγ 1 (PPARγ1) activity. Matrine administration also significantly inhibited PERK expression. Endogenic expression of PERK canceled matrine-induced apoptosis of myocardial cells. Notably, treatment with matrine significantly decreased nonfasting blood glucose levels and improved hemodynamic parameters of DCM rat.
Conclusions
Matrine may be a promising agent for the treatment of DCM.
1 INTRODUCTION
Morbidity and mortality of diabetes mellitus rapidly grows that makes it a prevailing disease in both developed and developing countries.1, 2 Diabetes mellitus is characterized by hyperglycemia and metabolic abnormalities, two of the most common causes of disability and death for patients suffered diabetes mellitus.3 The occurrences of hyperglycemia and metabolic syndrome may lead to the initiation and progression of diabetic cardiomyopathy (DCM) in patients with diabetes mellitus.2 DCM often caused by metabolic disorders and microvascular lesions in patients with diabetes mellitus, which is responsible for inflammation and apoptosis of myocardial tissue.4, 5 These metabolic imbalance induced by DCM may disturb metabolism of energy substrates and determine the myocardial homeostasis in patients with diabetes mellitus.6
Matrine (C15H24N2O) is an alkaloid extracted from traditional Chinese herb of Sophora alopecuroides L. Evidence have identified that matrine has shown a variety of pharmacological activities and potential therapeutic value in chronic liver and renal diseases, heart failure, and diabetes mellitus. Research has demonstrated many biological activities of matrine in regulation of inflammation, apoptosis, fibrosis, oxidative stress, and immunity. In recent year, matrine presents therapeutic benefits in cardiac fibrosis, injury, and dysfunction through inhibition of apoptosis in myocardial tissues in DCM rat.
Chronic inflammation is one of the most important causes of metabolic syndrome and development of vascular damage in DCM for patients suffering with diabetes mellitus.7 Although the mechanisms between inflammation and the development of DCM remain largely unknown, expression levels of inflammatory cytokines in cardiac tissue were indeed increased in patients with diabetic heart injury.8 A study has revealed that cardiac inflammation and abnormal Matrix metalloproteinase (MMP) activity could result in cardiac fibrosis in a streptozotocin-induced DCM rat model.9 In addition, expression levels of TNF-α, interleukin 6 (IL-6), and PARP-1 have been reported to increase during the DCM.10 These reports suggest that inhibition of inflammation may be contributed to the prevention of DCM induced by metabolism disorder of myocardial cells in patients suffering from diabetes mellitus.
DCM is characterized by focal fibrosis, cardiomyocyte hypertrophy, and necrosis.11 Apoptosis of myocardial cells has been closely related with myocardial necrosis/infarction in progression of the DCM in patients with diabetes mellitus.12 Endoplasmic reticulum (ER) stress is widely considered to be one of the major pathological processes underlying the cardiac apoptosis in pathogenesis of DCM via attenuating mitochondrion-dependent myocardial apoptosis.13 ER stress mediating apoptosis by the protein kinase RNA-like endoplasmic reticulum kinase (PERK) signal pathway has been well understood in published literatures.14 PERK downregulation can effectively inhibit CHOP expression and play a protective role against the aggravation of DCM.15
DCM is a specific myocardial disease in patients with diabetes. As the disease progresses, a series of lesions appear, including dysregulation of glucose, lipid, and energy metabolism in cardiomyocytes, then, cardiomyocytes undergo apoptosis and necrosis, and chronic hyperglycemia leads to myocardial fibrosis and scar formation, and eventually the myocardial structure changes. In this study, we explored the efficacy and potential mechanism of matrine in progression of DCM at the cellular level. The design analyzed the beneficial effects exerted by matrine on inhibition of inflammation and apoptosis of myocardial cells and improvement of cardiac dysfunction, as well as the underlying mechanisms of matrine involved in cytoprotective effects in the experimental DCM rat model. These data provide a novel insight into potential antiapoptosis treatment of matrine for DCM.
2 MATERIALS AND METHODS
2.1 Ethics statement
This study was carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of China. Experiments were verified by Dezhou People's Hospital (DZPH20140012). All surgery and euthanasia were conducted to minimize suffering under anesthesia.
2.2 Animals study
Male Sprague-Dawley rats (7~8 weeks old weighing approximately 160~180 g) from Animal Experimental Center of Wuhan University. All rats were housed in a temperature-controlled room (25 ± 1°C) with artificial 12/12 hours light/dark cycles, and free access to food and water. DCM rats were induced by streptozotocin (Sigma-Aldrich, St. Louis, MO) according to previously described study.16 Experimental rats were separated into two groups and received oral treatment of matrine (5 mg/kg) or phosphate buffered saline (PBS; 5 mg/kg) once a day. The total treatment was implemented once a day for 10 weeks. All rats were killed on day 70 for further analysis.
2.3 Cell culture and reagents
Myocardial cells were isolated from DCM rat and cultured in Dulbecco modified Eagle medium (Gibco, Waltham, MA) supplemented with 10% fetal bovine serum (Invitrogen, Waltham, MA). All cells were cultured in a 37°C humidified atmosphere of 5% CO2.
2.4 Enzyme-linked immunosorbent assay
In the protein detection assay, mouse IL-6 and the transforming growth factor beta (TGF-β) enzyme-linked immunosorbent assay (ELISA) kits (R&D, Minneapolis, MN) were used to determine serum levels of the inflammatory factors, respectively. The procedures were conducted according to the manufacturer's instructions. The final results were recorded at 450 nm on an ELISA plate reader.
2.5 Western blot analysis
Total liver cells were isolated from the rat with DCM and homogenized in a 1× radioimmunoprecipitation assay buffer on day 30. Subsequently, Western blot analysis was performed to analyze the purpose proteins expression. For Western blot analysis, goat anti-rat primary antibodies such as; TNF-α, IL-6, caspase-3, caspase-9, Bcl-2, P53, PERK, IER1, ATF6, ROS, MDA, TGF-β, CHOP, ATF-4, GRP78, and β-actin (Cell Signalling, Boston, MA) were added at a dilution of 1:200 after blocking (5% skimmed milk) for 1 hour at 37°C and then incubated with rabbit anti-goat second antibodies (Beyotime, Shanghai, China) for 24 hours at 4°C. The results were visualized by using the chemi-luminescence detection system.
2.6 Endogenous expression of TGF-β
Myocardial cells were cultured until 90% confluence and the media was then removed. Myocardial cells were transfected by lentivirus-TGF-β using Lipofectamine 2000 (Sigma-Aldrich). Protein expression levels of MDA, PERK, and GRP78 were analyzed in the TGF-β overexpression myocardial cells.
2.7 Histological, immunohistochemical, and immunofluorescence staining analyses
Cardiac tissues were isolated from the experimental rat and fixed in situ overnight in 10% buffered formalin. The cardiac tissues were cut midsagittally and being embedded in paraffin using standard protocols. Eosin and methylene blue were used to the area of myocardial infarction after treatment with matrine. Immunohistochemical staining was performed by an avidin-biotin-peroxidase technique. Paraffin-embedded liver tissue sections were prepared and epitope retrieval was performed for further analysis. The paraffin sections were subjected with hydrogen peroxide (3%) for 10~15 minutes, which subsequently were blocked by a regular blocking solution for 10~15 minutes 37°C. Finally, the sections were incubated in goat anti-rat anti-CHOP at 4°C for 12 hours after blocking. All sections were washed three times and incubated with secondary rabbit anti-goat antibodies for 1 hour at 37°C. For immunofluorescence, myocardial cells were stained with Hoechst and goat anti-rat PERK antibody. In addition, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was performed with Peroxidase Apoptosis Detection Kit (Chemicon International, Billerica, MA). All sections were observed by six-random views in the microscope.
2.8 Plasma brain natriuretic peptide assay
Blood samples were collected and centrifuged at 1500 rpm for 20 minutes. Supernatant plasma was collected and brain natriuretic peptide (BNP) concentration was analyzed by the Triage BNP Assay (Biosite, SanDiego, CA) according to the manufacturer's instructions.
2.9 Hemodynamic parameters
Experimental rats were anesthetized by isoflurane (0.03 mL/kg body weight). Hemodynamic parameters were analyzed in accordance with methods described in the previous report.17 The hemodynamic parameters including left ventricular systolic pressure (LVSP), maximum rate of left ventricular pressure decline (LVdP/dt min), maximum rate of left ventricular pressure increase (LVdP/dt max), and left ventricular end-diastolic pressure (LVEDP) were measured according to Mikro Tip catheter transducer connected with left ventricle through right carotid artery.
2.10 Statistical analysis
All data were presented as mean and SEM with three independent experiments. Statistical significance was analyzed using the two tailed Student t test between groups. Unpaired data was analyzed by the analysis of variance. *P < 0.05 and **P < 0.01 were considered statistically significant.
3 RESULTS
3.1 Effects of matrine administration on inflammation in serum and myocardial cells in DCM rat
To investigate the influence of matrine on TNF-α and IL-6 expression, we analyzed expression levels of TNF-α and IL-6 in both the serum and cardiac tissue in a streptozotocin-induced DCM model after 10-week matrine treatment. We observed that TNF-α protein level was decreased by matrine in both the serum and cardiac tissue of DCM rat than that of the control rat (Figure 1A and 1B). Consistently, the protein level of IL-6 was also decreased by matrine in both the serum and heart of experimental rat (Figure 1C and 1D).
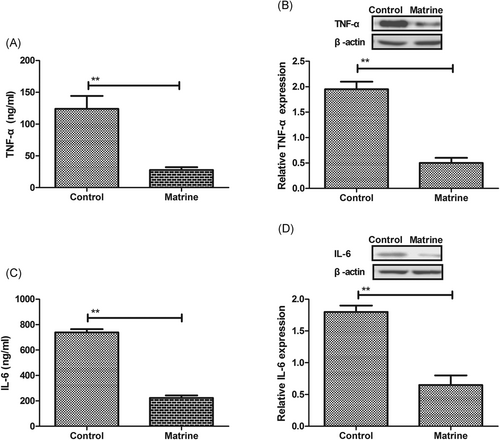
Inflammatory factors expression in serum and myocardial cells in diabetic cardiomyopathy rat, A,B, Expression levels of TNF-α in serum B, and myocardial cells C, in diabetic cardiomyopathy rat after treatment with matrine or PBS. C,D, Expression levels of IL-6 in serum C, and myocardial cells D, in diabetic cardiomyopathy rat after treatment with matrine or PBS. Error bars represent SD of three independent experiments; **P < 0.01. IL-6, interleukin 6; PBS, phosphate buffered saline; TNF-α, tumor necrosis factor alpha
3.2 Effects of matrine administration on myocardial cells apoptosis in DCM rat
By utilizing TUNEL staining, apoptosis rate of myocardial cells apoptosis in DCM rat was analyzed in matrine- or PBS-treated DCM rat. The apoptosis rate of myocardial cells was markedly decreased by matrine administration in DCM rat (Figure 2A). Western blot demonstrated that expression levels of proapoptosis protein caspase-3 and caspase-9 were downregulated by matrine administration in cardiac tissue (Figure 2B). However, expression levels of antiapoptosis protein Bcl-2 and P53 were downregulated by matrine administration in cardiac tissue compared with control (Figure 2C). It was also found that necrosis of microvascular lesions was improved by matrine administration compared with the control rat (Figure 2D).
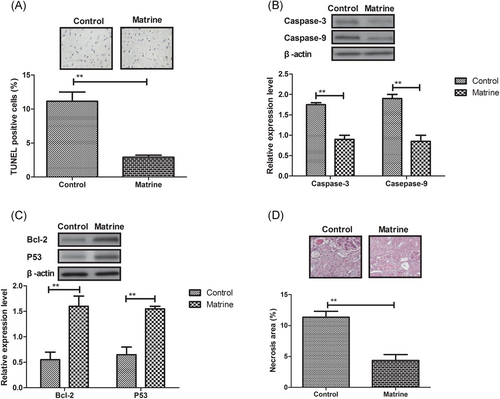
Effects of matrine administration on myocardial cells apoptosis in diabetic cardiomyopathy rat, A, Myocardial cells apoptosis in diabetic cardiomyopathy rat after treatment with matrine or PBS determined by TUNEL staining. B, Proapoptosis protein expression levels of caspase-3 and caspase-9 in myocardial cells determined by Western blot. C, Expression levels of antiapoptosis protein Bcl-2 and P53 in myocardial cells determined by Western blot. D, Necrosis of microvascular lesions in rats after treatment with matrine or PBS. Error bars represent SD of three independent experiments; **P < 0.01. PBS, phosphate buffered saline; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling
3.3 Effects of matrine administration on ER stress in myocardial cells in DCM rat
We further analyzed ER stress to underlying antiapoptosis effects of matrine in myocardial cells in DCM rat. Western blot demonstrated that the expression and phosphorylation levels of PERK were decreased by matrine in myocardial cells in DCM rat, whereas there was no indication of decreased ER stress sensors of IRE1 and ATF6 (Figure 3A). Immunofluorescence showed that GRP78 expression level was downregulated by matrine in cardiac tissue in DCM rat (Figure 3B). It was also observed that reactive oxygen species (ROS) generation, MDA, and TGF-β levels were decreased by matrine treatment in cardiac tissue in DCM rat (Figure 3C). In addition, the PPARβ and PPARγ1 activity was markedly decreased by matrine treatment in myocardial tissue compared with control (Figure 3D).
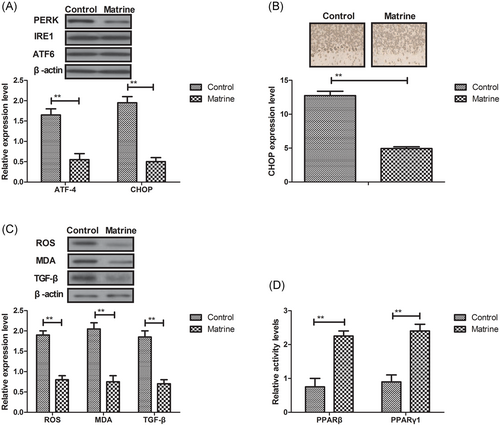
Effects of matrine treatment on ER stress in myocardial cells in diabetic cardiomyopathy rat. A, Expression levels of IRE1 and ATF6 in myocardial cells in diabetic cardiomyopathy rat after treatment with matrine or PBS determined by Western blot analysis. B, GRP78 expression level in myocardial cells in diabetic cardiomyopathy determined by immunofluorescence. C, MDA and TGF-β levels in diabetic cardiomyopathy after treatment with matrine or PBS determined by Western blot analysis. D, The PPARβ and PPARγ1 activity in myocardial cells after treatment with matrine or PBS. Error bars represent SD of three independent experiments; **P < 0.01 ER, endoplasmic reticulum; MDA, Malondialdehyde; PBS, phosphate buffered saline; PPARβ, Peroxisome proliferator-activated receptor beta; PPARγ1, Peroxisome proliferator-activated receptorγ 1
3.4 Matrine inhibits myocardial cells apoptosis through TGF-β-induced PERK signaling pathway
To further analyze the potential mechanism of matrine-medicated antiapoptosis effects, ER stress sensors in the PERK signaling pathway were investigated in myocardial cells. Compared with control, expression levels of ATF-4 and CHOP were downregulated by matrine administration in the PERK signaling pathway in myocardial cells (Figure 4A). It was found that the expression level of PERK was inhibited by matrine in myocardial cells (Figure 4B). Mechanistically, endogenous expression of TGF-β canceled matrine-inhibited PERK expression and phosphorylation levels in myocardial cells (Figure 4C). In vitro assay demonstrated that endogenous TGF-β expression also abolished decreasing MDA and GRP78 expression inhibited by matrine administration (Figure 4D).
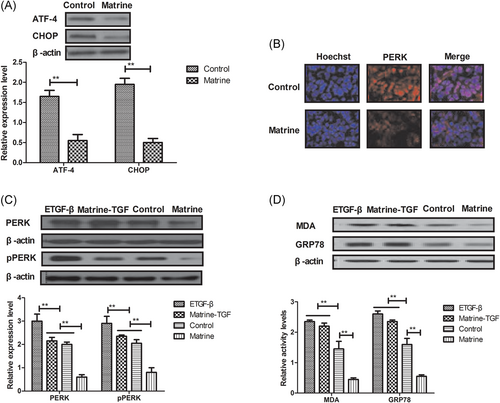
Matrine treatment inhibits myocardial cells apoptosis through the TGF-β-induced PERK signaling pathway, A, Expression levels of ATF-4 and CHOP in myocardial cells after treatment with matrine or PBS. B, Expression level of PERK in myocardial cells after treatment with matrine or PBS. C, Effects of TGF-β on expression and phosphorylation levels of PERK in myocardial cells. D, Expression levels of MDA and GRP78 in myocardial cells after endogenous TGF-β expression. Error bars represent SD of three independent experiments; **P < 0.01 MDA, Malondialdehyde; PBS, phosphate buffered saline; PERK, protein kinase RNA-like endoplasmic reticulum kinase
4 Effects of matrine administration on hemodynamic parameters and myocardial structure in DCM rat
Hemodynamic parameters and myocardial functions were analyzed in experimental rat after matrine administration. As shown in Figure 1A and 1B, results showed that matrine administration markedly improved LVdP/dt max, LVdP/dt min, and plasma BNP levels in DCM rat. Matrine administration also improved LVSP, LVEDP, and body weight in DCM rat compared with control (Figure 5C-E). In addition, arrhythmia vulnerability was improved by matrine administration compared with control in DCM rat (Figure 5F).
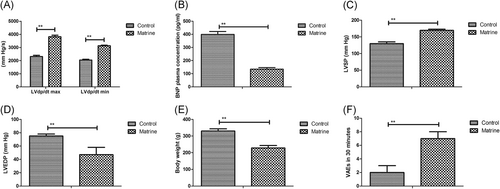
Effects of matrine treatment on hemodynamic parameters and myocardial structure in diabetic cardiomyopathy rat, A, Effects of matrine on LVdP/dt max and LVdP/dt min in diabetic cardiomyopathy rat after treatment with matrine or PBS. B, Effects of matrine on plasma BNP levels in diabetic cardiomyopathy rat. C,D, Effects of matrine on LVSP (C) and LVEDP (D) were examined in diabetic cardiomyopathy rat. E, Body weight was evaluated in diabetic cardiomyopathy rat. F, Arrhythmia vulnerability was improved by matrine in diabetic cardiomyopathy rat. Error bars represent SD of three independent experiments; **P < 0.01 BNP, brain natriuretic peptide; LVdP/dt max, maximum rate of left ventricular pressure increase; LVdP/dt min, maximum rate of left ventricular pressure decline; LVEDP, left ventricular end- diastolic pressure; LVSP, left ventricular systolic pressure; PBS, phosphate buffered saline
5 DISCUSSION
DCM is a kind of heart disease caused by metabolic disorders and microvascular lesions, and characterized by ventricular dysfunction, structural abnormalities, and focal necrosis in patients with diabetes mellitus.18 DCM is usually characterized by the presence of diastolic dysfunction and accompanies various metabolic disorders, which may precede the development of systolic dysfunction.19, 20 Research have suggested that inflammation and apoptosis are closely associated with the progression of DCM in patients suffering diabetes mellitus.21, 22 In this study, the DCM rat model was mimicked by intraperitoneal injection of streptozotocin which selectively damage islet beta cells. The rat model of DCM was used to analyze the efficacy of matrine for hemodynamic parameters and myocardial functions. Results have shown that matrine treatment significantly attenuates inflammation, and inhibits apoptosis in cardiac tissue in DCM rat. In accordance with previous reports, ER stress contributed to the apoptosis of myocardial cells in the progression of DCM.23, 24 It was found that matrine administration improves hemodynamic parameters and myocardial structure in DCM rat that is used to assess the efficacy of drugs for cardiac dysfunction.25
It was suggested that decreased inflammation and cardiomyocytes apoptosis in cardiac tissue take responsibility to the pathogenesis and severity of cardiovascular complications of DCM.26 A previous study has showed that inhibition of inflammation and apoptosis induced by high glucose in cardiomyocytes contributes to the functional recovery of DCM.27 Another report also elaborated the interplay of oxidative, ER stress, inflammation, apoptosis, cell death, and autophagy in DCM.28 In addition, Gupta et al29 have demonstrated that Genistein ameliorates cardiac inflammation and oxidative stress through inhibiting C-reactive protein and TNF-α expression levels in streptozotocin-induced DCM in rats. Furthermore, cardiac inflammation promotes endothelial cells loss and further leads to the development of DCM as well as evaluates the prognosis of diabetes mellitus.10, 30 In this study, our results have shown that matrine treatment could inhibit inflammation and apoptosis in cardiac tissue in DCM rat. In addition, evidence have shown that matrine pretreatment could improve the cardiac function in rats with DCM through inhibiting the ROS/TLR-4 signaling pathway.31 Our results revealed that matrine suppresses cardiomyocytes apoptosis through the TGF-β-induced PERK signal pathway.
Generally, an increase in ER stress is a response to cardiomyocytes apoptosis in multicellular eukaryotes, which has identified the three IRE1, PERK, and ATF6 ER signal pathway localized transmembrane and initiated subsequent responses.32 Jian et al33 revealed that attenuating ER stress by chemical chaperone 4-phenylbutyric acid protects H9c2 shows as a beneficial factor for inhibiting apoptosis of cardiomyocytes caused by ischemia/reperfusion injury. In addition, increasing of the PPARβ and PPARγ1 activity in cardiomyocytes prevents ER stress-associated inflammation and apoptosis through an AMPK-dependent mechanism.34 Also, research has suggested that TGF-β could improve the myocardial function and prevents apoptosis induced by anoxia-reoxygenation through the reduction of ER stress.35, 36 In this study, our results indicated that matrine treatment suppresses cardiomyocytes apoptosis through the TGF-β-induced PERK signal pathway, which led to an improvement of hemodynamic parameters and myocardial functions.
In conclusion, the current study investigates the effect and mechanism of matrine on cardiomyocytes inflammation and apoptosis and identifies matrine as a potential antiapoptosis agent in the progression of cardiovascular complications of DCM. Results in this analysis contribute to understanding the molecular mechanisms mediated by matrine treatment in inflammation and apoptosis processes in DCM rat. Our investigations demonstrated that matrine could serve as a potential anti-inflammation and antiapoptosis agent in the pathological processes of DCM through downregulation of the TGF-β-induced PERK signal pathway.
ACKNOWLEDGMENT
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.




