miR-145-5p inhibits tumor occurrence and metastasis through the NF-κB signaling pathway by targeting TLR4 in malignant melanoma
Abstract
Compelling evidence shows that deregulated microRNAs (miRNAs) are important regulators in the progression of melanoma. miR-145-5p has been suggested to exhibit antitumorigenic activity in melanoma. However, the molecular mechanism underlying the biological activity of miR-145-5p in melanoma remains to be further understood. Herein, quantitative real-time polymerase chain reaction was used to examine the miR-145-5p expression in malignant melanoma tissues and cells. The interaction between miR-145-5p and toll-like receptor 4 (TLR4) was explored by bioinformatics analyses, luciferase reporter assay, and Western blot. The effects of miR-145-5p or combined with TLR4 on cell proliferation, colony formation, migration, and invasion abilities were investigated by (4,5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide, colony formation, wound healing, and transwell assays, respectively. The melanoma xenograft tumor models were established to determine the biological activity of miR-145-5p in melanoma in vivo. In addition, the changes of the nuclear factor kappa B (NF-κB) pathway were analyzed by detecting the NF-κB activity and the NF-κB p65 protein level. We observed that the miR-145-5p expression was underexpressed in melanoma tissues and cells. miR-145-5p suppressed the TLR4 expression by binding to its 3′untranslated region in melanoma cells. Moreover, TLR4 overexpression abolished the inhibition of cell proliferation, colony formation, migration, and invasion abilities induced by miR-145-5p in melanoma cells. Meanwhile, miR-145-5p was confirmed to restrain melanoma tumor growth in vivo by targeting TLR4. Furthermore, miR-145-5p overexpression inactivated the NF-κB pathway in melanoma in vitro and in vivo, which was reversed by TLR4 overexpression. We concluded that miR-145-5p hindered the occurrence and metastasis of melanoma cells in vitro and in vivo by targeting TLR4 via inactivation of the NF-κB pathway.
1 INTRODUCTION
Malignant melanoma, deriving mainly from pigment-producing melanocytes, is a serious type of skin cancer, accompanied by the rapid growth and early metastasis.1, 2 It remains the most common cause of mortality from skin cancer, and the incidence rate of melanoma has been remarkably increased in recent years.3 Despite great advancements in melanoma therapy, the prognosis of patients with distant metastasis and at late stages of melanoma remains poor, with an estimated rate of 5-year survival <5%.4 According to the annual report, there were an estimated 87 110 cases diagnosed with melanoma and approximately 9730 deaths by the end of 2017 in the United States.5 Therefore, deciphering the mechanism at the basis of melanoma pathogenesis is crucial to identify new methods for the treatment of melanoma.
microRNAs (miRNAs), an abundant group of endogenous small RNA transcripts consisting of 18 to 22 nucleotides, could control target gene expression via base pairing with complementary sites in the 3′ untranslated regions (UTRs), thus causing mRNA degradation and/or translational suppression.6 Extensive research within the past decades have suggested that miRNAs are critical regulators in a broad range of physiological and pathological processes, such as cell proliferation, apoptosis, migration, invasion, as well as tumorigenesis.7 miRNAs have been well-documented to be often deregulated in diverse human malignancies including melanoma and are implicated in the pathogenesis and development of tumors.8 miR-145-5p, located on human chromosome 5 (5q32-33), is frequently downregulated in different types of human malignancies, such as gastric cancer,9 breast cancer,10 and ovarian cancer,11 and identified as a tumor suppressor. Also, many studies have suggested that miR-145-5p exhibits antitumorigenic activity in melanoma.12-15 However, the molecular basis by which miR-145-5p plays a tumor suppressive role in melanoma remains to be further understood.
Toll-like receptors (TLRs) are recognized as a class of evolutionarily conserved pattern recognition receptor proteins expressed on the cellular surface that plays crucial functions in the innate and adaptive immune responses against invading pathogens.16 Recent studies have strongly suggested that TLRs are associated with cancer initiation, development, and metastasis.17 As a member of the TLRs family, TLR4 has been extensively focused due to its involvement in the pathogenesis of many diseases, such as sepsis, autoimmunity, and even tumors.18 It has been proven that melanoma cells express TLR4 on the membrane and activation of TLR4 signaling contributes to melanoma progression.19, 20 Notably, previous studies have reported the direct binding between miR-145 and TLR4 in high glucose-treated retinal endothelial cells.21 However, whether miR-145-5p could target TLR4 in melanoma remains largely unknown.
Herein, we determined the expression profile of miR-145-5p in melanoma tissues and cells and explored the interaction between miR-145-5p and TLR4 in melanoma cells. We further analyzed the mechanism of the tumor suppressive roles of miR-145-5p in melanoma.
2 MATERIALS AND METHODS
2.1 Tissue specimens
A total of 55 pairs of melanoma tissue specimens and matched adjacent normal samples were obtained from malignant melanoma patients (including 37 melanoma patients with stage 1/2 and 18 melanoma patients with stage 3/4) who were subjected to surgical resection at the First Affiliated Hospital of Zhengzhou University between January 2010 and November 2017. All patients were diagnosed as malignant melanoma pathologically without cutaneous radiotherapy and/or chemotherapy before operation and staged using the current American Joint Committee on Cancer staging system for melanoma. All procedure in this study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University and the written informed consents for research purposes were obtained from all of the recipients enrolled.
2.2 Cell lines and culture
Human melanoma cell lines (A375, WM35, VMM5A, M14, A875, and HMCB), and normal melanocytes were purchased from the American Type Culture Collection (ATCC, Manassas, VA). Human embryonic kidney cell line 293T cells were preserved by our laboratory. Human melanoma cell lines and normal melanocytes were grown in RPMI-1640 (Hyclone, Logan, UT) containing 10% fetal bovine serum (FBS, Hyclone), 100 U/mL penicillin (Beyotime Biological Technology Co, Ltd, Shanghai, China), and 100 μg/mL streptomycin (Beyotime Biological Technology Co, Ltd) in a humidified incubator with 5% CO2 supply at 37°C. The 293T cells were cultured in the Dulbecco modified Eagle medium (Hyclone) supplemented with 10% FBS and 1% penicillin/streptomycin at 37°C in a CO2 incubator.
2.3 Cell transfection and infection
Referring to the previous method,22 293T cells were cotransfected with component packaging plasmids (pCMV-Δ8.2 and pCMV-VSV-G) as well as expression plasmids (pLenti-miR-145-5p, pLenti-sh-miR-145-5p, or pLenti-TLR4). After 48 hours, the packaged viruses were obtained and named LV-miR-145-5p, LV-sh-miR-145-5p, and LV-TLR4. The collected viruses (LV-miR-145-5p and LV-sh-miR-145-5p) were used to infect A375 and M14 cells to get stable cells. The viruses expressing TLR4 (LV-TLR4) infected the A375 and M14 cells infected with LV-miR-145-5p to gain the miR-145-5p+TLR4 A375 and M14 cells. The infected cells were used for the further experiments.
2.4 Quantitative real-time polymerase chain reaction
Total RNA was extracted from tissue samples or cultured cells by using TRIzol reagent (Invitrogen, Carlsbad, CA). The synthesis of complementary DNA was performed using PrimeScript RT reagent Kit (TaKaRa, Dalian, China) and specific stem-loop RT primers. For the detection of miR-145-5p expression, quantitative polymerase chain reaction (qPCR) was carried out using SYBR-Green Real-Time Master mix (Toyobo Life Science, Osaka, Japan) on an ABI StepOne Plus system (Applied Biosystems, Foster City, CA). The PCR reaction conditions were 95°C for 5 minutes, followed by 40 cycles of 95°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds. The fold changes in the miRNA expression level were calculated by relative quantification (2-ΔΔCt) method and U6 small nuclear RNA served as the normalization.
2.5 Western blot analyses
Protein samples (30 µg/lane) were fractionated on 10% sodium dodecyl sulfate-polyacrylamide gels and then transferred onto nitrocellulose membrane (Millipore, Billerica, MA). The membranes were then blocked with 5% skim milk powder at 37°C for 1 hour and probed with primary antibodies at 4°C overnight, including TLR4 (1:1000 dilution; Cell Signaling Technology, Danvers, MA), Ki67 (1:500 dilution; Cell Signaling Technology), matrix metalloproteinase (MMP)-2 (1:500 dilution; Cell Signaling Technology), MMP-7 (1:1000 dilution; Cell Signaling Technology), NF-κB p65 (1:1000 dilution; Cell Signaling Technology), and β-actin (1:500 dilution; Abcam, Cambridge, MA). After that, the membranes were incubated with horseradish peroxidase–conjugated secondary antibody (1:1000 dilution; Cell Signaling Technology) for 1 to 2 hours at room temperature. The protein brands were signaling by an ECL chemiluminescent kit (Millipore).
2.6 3-(4,5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assays
Cell proliferation was detected by (4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assays. A375 and M14 cells were seeded into 96-well plates at 2 × 103 cells/well. After incubation for 1, 2, and 3 days, MTT reagent (20 µL, Sigma-Aldrich, St. Louis, MO) was added into each well at a final concentration of 5 mg/mL and incubated for 4 hours at 37°C. After removing the culture medium, the formazan crystals were dissolved in 150 µL of dimethyl sulfoxide (Sigma-Aldrich). The optical density value at 490 nm was assessed using a microplate reader (Bio-Rad, Hercules, CA).
2.7 Colony formation assays
The transfected A375 and M14 cells were plated onto six-well plates at 500 density of cells/well and incubated for 2 to 3 weeks at 37°C to form colonies. The cells were then fixed with 4% paraformaldehyde, stained with 1% crystal violet solution for 10 minutes at room temperature, and counted under the microscope.
2.8 Wound healing assays
The transfected A375 and M14 cells were plated into six-well plates at 2 × 105 cells/well and cultured until cell confluence rate was about 70%-80%. The artificial wound was uniformly created by scratching the cell layers with a 200-μL sterile pipette tip. Then the cells were washed with phosphate-buffered saline three times to discard the detached cells and incubated in FBS-free for 24 hours. Then, the cells migrating at the front of the wound were imaged using a light microscope (Olympus Corporation, Tokyo, Japan) and cell migration ability was measured by calculating the wound width.
2.9 Cell invasion assays
Cell invasion assays were conducted by using Matrigel-coated Transwell chambers (Corning Incorporated, Corning, NY). In brief, 2 × 105 transfected A375 and M14 cells in 200 µL serum-free medium were plated into the upper chamber, and 500 µL of culture medium with 10% FBS was placed in the bottom chamber serving as a chemoattractant. After incubation at 37°C for 24 hours, the cells that had invaded to the bottom chamber were fixed and stained with 0.1% crystal violet for 30 minutes. The invaded cells were photographed and counted in 3 to 5 randomly fields under a microscope.
2.10 Luciferase reporter assays
The wild-type (WT) 3′UTR fragment of TLR4 holding the potential binding sites of miR-145-5p or its mutated sequence was synthesized and inserted into pmiR-GLO reporter vectors (Promega, Madison, WI), namely pmiR-GLO-WT-TLR4 or pmiR-GLO-Mut-TLR4. For luciferase reporter assays, 293T cells were cotransfected with luciferase reporter constructs, pRL-TK vector (Promega), along with miR-145-5p or miR-con (negative control (NC)) by using Lipofectamine 2000 (Invitrogen). After 48 hours of transfection, the cells were gathered and the firefly and Renilla luciferase activities were measured by using the Dual-Luciferase Reporter Assay System (Promega).
2.11 Xenograft tumor assays
All animal experiments were conducted with the approval of the Experimental Animal Ethics Committee of the First Affiliated Hospital of Zhengzhou University. Ten female athymic BALB/c nude mice (5-week-old) were bought from Vital River Laboratory Animal Technology Co, Ltd (Beijing, China). These mice were kept under pathogen-free conditions with a 12 hours light/dark cycle and allowed to have free access to food and water. A total of 2 × 105 A375 cells transfected with miR-145-5p or miR-con were subcutaneously inoculated into nude mice to construct tumor growth models. Tumor volume was determined with electronic calipers every 5 days for 30 days and calculated using the equation: π/6 × length × width2. All mice were killed at day 30 of inoculation and pictured, and the body weight of mice was detected. The xenograft tumor tissues were resected, photographed, weighed, and then used for the subsequent Western blot analyses.
2.12 NF-κB activity assays
The nuclear proteins were extracted from the transfected A375 or M14 cells, or tumor tissues using the Nuclear Extract Kit (Active Motif, Carlsbad, CA). The NF-κB (p65/p50) DNA binding activity in nuclear protein extracts of the treated cells or tissues was determined using TransAM NF-κB (p65/p50) transcription factor enzyme-linked immunosorbent assays (ELISA) kit (Active Motif).
2.13 Statistical analyses
All experimental results were shown as mean ± standard deviation. Statistical analysis was carried out with GraphPad Prism 5 software (Version 5.0, GraphPad Software, Inc, San Diego, CA) with the Student t test or one-way variance analysis. Differences were considered statistically significant when P values were less than 0.05.
3 RESULTS
3.1 miR-145-5p was underexpressed in melanoma tissues and cells
To determine the roles of miR-145-5p in melanoma, the expression level of miR-145-5p was determined in 55 pairs of melanoma tissues and matched adjacent normal samples. As displayed in Figure 1A, miR-145-5p expression was significantly downregulated in melanoma tissues compared with adjacent normal tissues. Moreover, the expression level of miR-145-5p was much lower in late-stage melanoma tissues (stage 3/4) than that in early-stage melanoma tissues (stage 1/2) (Figure 1B). Meanwhile, the low expression of miR-145-5p was validated in melanoma cells (A375, WM35, VMM5A, M14, A875, and HMCB) when compared with normal melanocytes, especially in A375 and M14 cells (Figure 1C). These data suggested that miR-145-5p was underexpressed in melanoma tissues and cells.
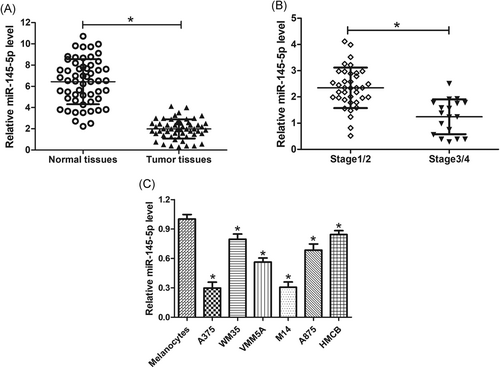
Expression profiles of miR-145-5p in melanoma tissues and cells. miR-145-5p levels were detected by qRT-PCR in 55 paired melanoma tissues and matched adjacent normal samples (A), 37 early-stage melanoma tissues and 18 late-stage melanoma tissues (B), and melanoma cell lines (A375, WM35, VMM5A, M14, A875, and HMCB), and normal melanocytes (C). *P < 0.05. qRT-PCR, quantitative real-time polymerase chain reaction
3.2 miR-145-5p suppressed TLR4 expression by binding to its 3′UTR in melanoma cells
To further figure out the mechanism underlying the functions of miR-145-5p in melanoma, bioinformatics online tools including TargetScan 7.0 and miRanda were used to confirm the functionally relevant targets of miR-145-5p. The 3′UTR of TLR4 mRNA contained the potential binding sites complementary to miR-145-5p, suggesting that TLR4 was a candidate target of miR-145-5p (Figure 2A). As demonstrated by luciferase reporter assays in 293T cells, the luciferase activity of pmiR-GLO-WT-TLR4 was dramatically suppressed after the introduction of miR-145-5p in 293T cells with respect to the control group (Figure 2B). By contrast, the luciferase activity of pmiR-GLO-Mut-TLR4 was not changed in 293T cells cotransfected with miR-145-5p (Figure 2B). To determine whether miR-145-5p modulated the TLR4 expression in melanoma cells, A375 and M14 cells were transfected with miR-145-5p, sh-miR-145-5p, or respective controls. Quantitative real-time polymerase chain reaction (qRT-PCR) results exhibited that the miR-145-5p expression was drastically upregulated after introduction with miR-145-5p and evidently decreased by sh-miR-145-5p transfection in A375 and M14 cells (Figure 2C). Further, Western blot analyses showed that the enforced expression of miR-145-5p greatly reduced the TLR4 expression, while inhibition of miR-145-5p distinctly elevated TLR4 expression in A375 and M14 cells (Figure 2D). Collectively, these data demonstrated that miR-145-5p suppressed the expression of TLR4 by binding to its 3′UTR.
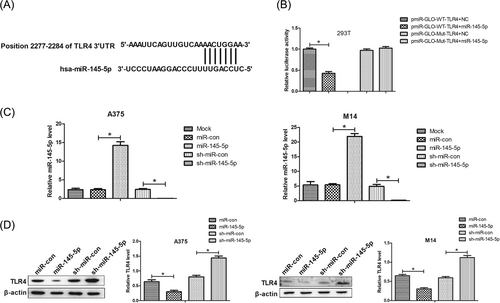
miR-145-5p suppressed TLR4 expression via binding to its 3′UTR in melanoma cells. A, 3′UTR of TLR4 contained the potential binding sites complementary to miR-145-5p. B, Luciferase reporter assays were carried out to determine luciferase activity after pmiR-GLO-WT-TLR4 or pmiR-GLO-Mut-TLR4 was introduced with miR-145-5p or NC into 293T cells. C, qRT-PCR analyses of the miR-145-5p expression in A375 and M14 cells after transfection with miR-145-5p, sh-miR-145-5p, or respective controls. D, Western blot analyses of the TLR4 expression in A375 and M14 cells introduced with miR-145-5p, sh-miR-145-5p, or respective controls. *P < 0.05. NC, negative control; qRT-PCR, quantitative real-time polymerase chain reaction; TLR4, toll-like receptor 4; UTR, untranslated region; WT, wild-type
3.3 miR-145-5p inhibited melanoma cell growth by downregulating TLR4
To clarify whether miR-145-5p played the tumor suppressor activity in melanoma cells via TLR4, we reintroduced TLR4 in A375 and M14 cells overexpressing miR-145-5p. MTT assays showed that overexpression of miR-145-5p significantly hindered cell proliferation of A375 and M14 cells compared with the miR-con group, which was markedly restored by reintroduction with TLR4 (Figure 3A). Moreover, the suppressive effect of miR-145-5p restoration on Ki67 expression in A375 and M14 cells was effectively reversed following enhanced expression of TLR4 (Figure 3B). In addition, colony formation assays uncovered that overexpressing TLR4 strikingly abolished the repression of colony-forming capacity induced by miR-145-5p in A375 and M14 cells (Figure 3C). Therefore, we concluded that miR-145-5p suppressed cell growth by targeting TLR4 in melanoma cells.
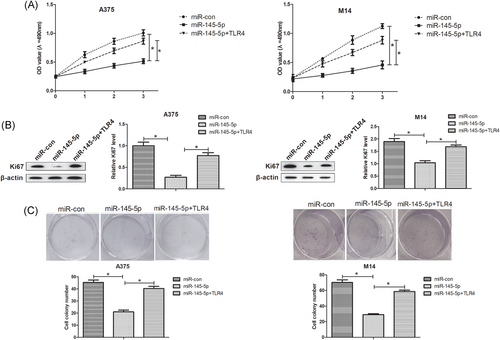
miR-145-5p inhibited melanoma cell growth by downregulating TLR4. A375 and M14 cells were introduced with miR-con, miR-145-5p, or miR-145-5p+TLR4. A, The transfected cell proliferation at 1, 2, and 3 days was estimated by MTT assays. B, Ki67 protein level was measured using Western blot analyses. C, Colony formation assays were applied to evaluate the colony-forming ability of the transfected cells after 2 to 3 weeks. *P < 0.05. MTT, (4,5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide; TLR4, toll-like receptor 4
3.4 miR-145-5p inhibited cell invasion and migration of melanoma cells by repressing TLR4
Next, the effects of miR-145-5p or combined with TLR4 on cell migratory and invasive capacities in A375 and M14 cells were assessed by wound healing and cell invasion assays, respectively. As compared with the miR-con group, overexpression of miR-145-5p prominently restrained migratory and invasive capacities of A375 and M14 cells, while reintroduction of TLR4 obviously alleviated the suppressive effects of miR-145-5p on cell migratory and invasive capacities (Figure 4A and 4B). MMPs including MMP-2 and MMP-7 are a family of extracellular matrix-degrading proteinases that promote cell invasion as well as metastasis.23 The results from Western blot analyses implicated that miR-145-5p overexpression-mediated repression on MMP-2 and MMP-7 protein levels in A375 and M14 cells was successfully relieved by ectopic expression of TLR4 (Figure 4C). Therefore, we concluded that TLR4 overexpression reversed the inhibitory effects of miR-145-5p on cell migration and invasion of melanoma cells.
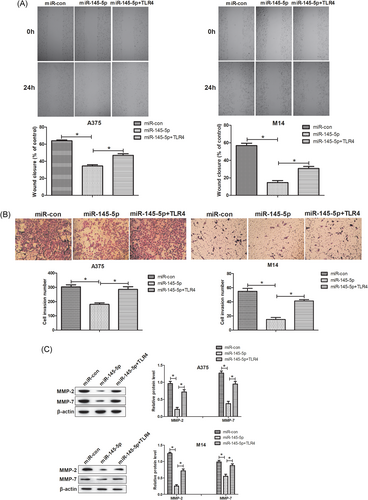
miR-145-5p supressed cell invasion and migration in melanoma cells by downregulation of TLR4. A375 and M14 cells were introduced with miR-con, miR-145-5p, or miR-145-5p+TLR4. A, Wound healing assays were conducted to detect the migration capacity of the transfected A375 and M14 cells. B, Cell invasion assays were applied to estimate cell invasion of transfected A375 and M14 cells. C, The protein levels of MMP-2 and MMP-7 in the treated A375 and M14 cells were determined via Western blot analyses. *P < 0.05. MMP, matrix metalloproteinase; TLR4, toll-like receptor 4
3.5 miR-145-5p inhibited melanoma cell growth in vivo by inhibiting TLR4
To further evaluate the effects of miR-145-5p on melanoma in vivo, miR-145-5p- or miR-con-transfected A375 cells were subcutaneously inoculating into nude mice. As shown in Figure 5A, tumor formed by miR-145-5p-overexpressing A375 cells grew more slowly when compared with the miR-con group. In addition, we found that miR-145-5p overexpression had no obvious effects on the body weight of melanoma xenograft mice but significantly reduced melanoma xenograft tumor weight and size (Figure 5B-5D). Moreover, the TLR4 expression was remarkably decreased in the miR-145-5p-treated group relative to the miR-con-introduced group (Figure 5E). Together, we concluded that miR-145-5p repressed melanoma xenograft tumor growth by targeting TLR4.
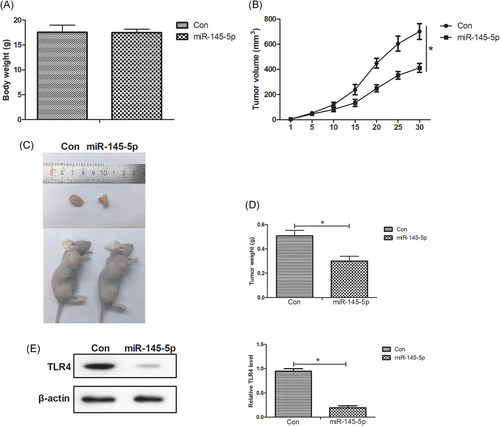
miR-145-5p repressed melanoma xenograft tumor growth by targeting TLR4. A375 cells transfected with miR-145-5p or miR-con were subcutaneously inoculated into nude mice to establish melanoma xenograft models. A, Tumor volume was valuated using electronic calipers every 5 days. B-D, After 30 days, the mice were killed by means of cervical dislocation and the body weight of mice was determined. Then, the xenograft tumor tissues were resected and weighed. E, The protein level of TLR4 in the excised xenograft tumor tissues was assessed by Western blot assays. *P < 0.05. TLR4, toll-like receptor 4
3.6 miR-145-5p inactivated the NF-κB pathway by targeting TLR4 in melanoma cells in vitro and in vivo
Since NF-κB is an essential downstream component of the TLR4 signal pathway, we further analyzed the effects of miR-145-5p or along with TLR4 on the NF-κB pathway in melanoma cells in vitro. The NF-κB activity in the nuclear protein extracts from transfected A375 and M14 cells was measured by ELISA. As illustrated in Figure 6A, the NF-κB activity was substantially declined in miR-145-5p-overexpressing A375 and M14 cells, which was recuperated after reintroduction with TLR4. Meanwhile, overexpression of miR-145-5p resulted in a substantial decline of the NF-κB p65 level in A375 and M14 cells, which was dramatically overturned by TLR4 overexpression (Figure 6B). Consistently with the in vitro results, the NF-κB activity in xenograft tumor was obviously decreased in the miR-145-5p-transfected group versus miR-con-introduced group (Figure 6C). Also, increased expression of miR-145-5p strikingly inhibited the NF-κB p65 level in xenograft tumor in comparison with the miR-con group (Figure 6D). Therefore, these results indicated that miR-145-5p inactivated the NF-κB pathway by targeting TLR4 in melanoma in vitro and in vivo.
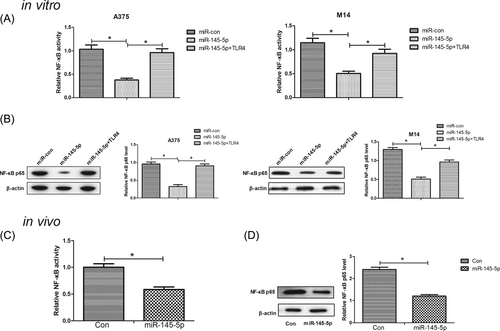
miR-145-5p inactivated the NF-κB pathway by repressing TLR4 in melanoma cells in vitro and in vivo. A, NF-κB activity was assessed via ELISA in A375 and M14 cells transfected with miR-145-5p, miR-con, or miR-145-5p+TLR4. B, The NF-κB p65 level was examined by Western blot assays in the transfected A375 and M14 cells. C, The NF-κB activity was measured by ELISA in the excised xenograft tumor tissues. D, The NF-κB p65 level was detected by Western blot in the excised xenograft tumor tissues. *P < 0.05. ELISA, enzyme-linked immunosorbent assays; NF-κB, nuclear factor kappa B; TLR4, toll-like receptor 4
4 DISCUSSION
According to recent evidence, aberrantly expressed miRNAs are implicated in various biological processes associated with melanoma pathogenesis and progression by targeting multiple genes. For example, enforced expression of miR-143-3p represses proliferation, migration, and invasion, and promoted apoptosis of melanoma cells partly through inhibition of cyclooxygenase-2.24 miR-331 plays tumor-suppressive roles in melanoma by directly targeting astrocyte-elevated gene-1 and regulating the PTEN/AKT signaling pathway.25 miR-370 represents a potential oncogenic miRNA in melanoma progression by downregulating pyruvate dehydrogenase B (PDHB).26 Therefore, focusing on melanoma-related miRNAs may contribute to the development of new therapeutic targets for melanoma. Herein, our study demonstrated that miR-145-5p was underexpressed in melanoma tissues and cells and functioned as a tumor suppressor. In addition, we also revealed the potential mechanisms involved in the effects of miR-145-5p in melanoma.
Recently, increasing studies have reported the tumor repressive role of miR-145-5p in various malignancies. For instance, miR-145-5p have been reported to be reduced in prostate cancer tissues and cells, and its overexpression inhibited prostate cancer progression by downregulating Fascin-1.27 Mei et al28 have demonstrated that the miR-145-5p expression is reduced in esophageal squamous cell carcinoma (ESCC) tissues and ectopic expression of miR-145-5p represses ESCC cell proliferation and metastasis by modulating the specificity protein 1 (Sp1)/NF-κB pathway.28 Jiang et al29 have found that in gastric cancer, miR-145-5p inhibited the cell invasion and migration through targeting N-cadherin and zinc-finger E-box binding homeobox 2 to suppress EMT.29 Notably, previous studies proved that miR-145-5p was significantly downregulated in melanoma tissues and cells, and miR-145-5p overexpression suppressed cell proliferation, invasion, and migration, induced apoptosis of melanoma cells in vitro, and inhibited tumor growth in vivo,13 which was in accordance with the present study. Therefore, our study confirmed that miR-145-5p played a tumor suppressive role in melanoma.
TLR4, which is well known as a critical signaling receptor for lipopolysaccharide (LPS) from Gram-negative bacteria, is closely associated with the regulation of autoimmune diseases, inflammatory diseases, as well as cancers.30 Some reports have shown that TLR4 is upregulated in many cancers, and promote carcinogenesis, metastasis, and cancer progression.31, 32 TLR4 has been reported to boost migration and invasion of lung cancer cells by inducing autophagy that can enhance the production of several cytokines that are necessary for promoting cell migration and invasion upon TLR activation.33 A previous report has shown that LPS can stimulate the TLR4/MD2 complex, which can activate the PI3K/AKT pathway and promote downstream b1 integrin function, thus enhancing the adhesiveness and metastatic capacity of colorectal cancer cells.32 In melanoma cells without LPS, knockdown of TLR4 or myeloid differentiation primary-response gene 88 (MyD88) has been reported to inhibit cell migration, which suggests TLR4 signaling may contribute to melanoma progression.20 In our study, we proved that miR-145-5p suppressed the TLR4 expression in melanoma cells by binding to its 3′UTR. Overexpression of miR-145-5p prominently restrained migratory and invasive capacities of A375 and M14 cells. Moreover, rescue experiments manifested that TLR4 abolished miR-145-5p-induced suppression on cell growth, migration, and invasion in melanoma cells, suggesting that miR-145-5p constrained the development of melanoma by repressing TLR4.
It has been widely recognized that LPS-induced activation of the TLR4 pathway results in the recruitment of MyD88, which initiates the activation of the NF-κB pathway and, thus, induces inflammatory response.34, 35 NF-κB, a key transcription factor that exists as a heterodimer composed of p50 and p65 subunits, is associated with tumor cell growth, metastasis, and apoptosis.36 The NF-κB pathway is aberrantly activated in diverse cancers and contributes to cancer progression in melanoma and several other malignancies.37, 38 Therefore, the NF-κB pathway has been considered a potential molecular therapeutic target for cancers.39 The NF-κB is an essential downstream component of the TLR4 signal pathway.35 MMPs are important effectors of migration and invasion, and NF-κB can induce expression of some MMPs.23, 40 Herein, we revealed that restoration of miR-145-5p suppressed the NF-κB pathway in melanoma in vitro and in vivo, as evidenced by the reduced NF-κB activity and NF-κB p65 expression. However, forced expression of TLR4 remarkably restored miR-145-5p-mediated repression of the NF-κB pathway in melanoma in vitro and in vivo. Moreover, our results showed that miR-145-5p overexpression–mediated repression on MMP-2 and MMP-7 protein levels in A375 and M14 cells was successfully relieved by ectopic expression of TLR4. According to the above findings, we concluded that miR-145-5p curbed the development of melanoma in vivo and in vitro by targeting TLR4 through inactivation of the NF-κB pathway.
In conclusion, our study found that the miR-145-5p expression was downregulated in melanoma tissues and cells. Moreover, miR-145-5p overexpression suppressed the occurrence and development of melanoma cells in vitro and in vivo by targeting TLR4 via inactivation of the NF-κB pathway, shedding light on the mechanism underlying the effects of miR-145-5p on melanoma progression. Therefore, this study suggests that miR-145-5p may be a promising therapeutic target for malignant melanoma therapy.
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interests.