Programmed cell death protein 4 deficiency suppresses foam cell formation by activating autophagy in advanced glycation end-product low-density lipoprotein–induced macrophages
Abstract
Background
Advanced glycation end-product is a modified form of low-density lipoprotein (AGE-LDL) and accelerates atherosclerosis through undefined mechanisms. Programmed cell death protein 4 (PDCD4), a transcriptional regulator, plays an important role in the regulation of autophagy. The aim of the present study was to investigate the role of PDCD4 involved in AGE-LDL–induced foam cell formation.
Methods
The characterization of AGE-LDL was measured by the thiobarbituric assay and agarose gel electrophoresis in vitro. RAW264.7, THP-1 cell line and primary peritoneal macrophages of mice were transfected with shPDCD4 plasmid AGE-LDL–induced foam cell formation was stained by Oil Red, and the levels of autophagy and apoptosis were determined by Western blot analysis. Autophagosome was observed with immunofluorescence microscopy. Mitochondrial membrane potential and autophagic flux were assessed by flow cytometry.
Results
AGE modification resulted in significant reduction of absorbance shown by thiobarbituric assay and augmentation of electrophoresis mobility. Further studies suggest that macrophages exposed AGE-LDL triggered autophagy in the early stage of foam cell formation. PDCD4 deficiency enhanced lipoautophagy but inhibited apoptosis and mitochondria dysfunction. Previous studies have been reported that autophagy is an adaptive response might prevent lesional macrophage apoptosis. In our study, we found PDCD4 deficiency attenuated apoptosis and AGE-LDL–induced foam cell formation relied on increased autophagy.
Conclusion
Our data revealed that PDCD4 deficiency can facilitate autophagy and benefit for AGE-LDL–induced foam cell formation.
1 INTRODUCTION
Diabetes mellitus aggravates the clinical prognosis of cardiovascular disease (CVD) through undefined mechanisms.1 As we known, hyperglycemia induces nonenzymatic glycation of proteins and lipids, leading to the accumulation of advanced glycation end-products (AGEs)2; in particular, an AGE-modified form of low-density lipoprotein (AGE-LDL) has been found to circulate in human plasma.3 AGE-LDL accelerates atherosclerosis progression via different pathways.4, 5 Even though AGE-LDL has proinflammatory properties and is considered being atherogenic, the underlying mechanism remains unclear.
Autophagy is an evolutionary conserved metabolic process in which denatured proteins and damaged organelles are degraded through the lysosomal system for the maintenance of intracellular homeostasis during various stress conditions.6 Mounting evidence have indicated that autophagy has a promising potential as diagnostic and prognostic indicators in various diseases containing atherosclerosis.7 Lipoautophagy, a type of autophagy that delivers lipid droplets (LDs) for degradation and regulates lipid metabolism, is involved in the process of atherosclerosis.8 Activation of autophagy by intercellular and/or extracellular stimuli protects macrophage cells against cell apoptosis.9, 10 Therefore, regulation of the level of macrophage autophagy would be a potential target for preventing the formation of atherosclerotic plaques.
Programmed cell death protein 4 (PDCD4) is regarded as a transcriptional regulator of gene expression and a tumor suppressor.11, 12 It inhibits cap-dependent translation through the interaction with the eukaryotic initiation factors (eIFs) 4A and eIF4G.13 PDCD4 has been reported as a negative regulator of autophagy by inhibiting ATG5 in both tumor and nontumor cells.14 Furthermore, PDCD4 deficiency attenuates oxidized-LDL (ox-LDL) induces foam cell formation and atherosclerosis in mice, which collectively demonstrated the important role of PDCD4 on the regulation of autophagy-dependent cholesterol efflux, foam cell formation, and atherosclerosis.15, 16
The aim of the present study was to investigate the role of PDCD4 involved in AGE-LDL–induced macrophage autophagy and cell apoptosis in macrophage cell line RAW264.7. We report here that the three aforementioned factors, PDCD4, autophagy, and apoptosis, form a unique regulatory streamline that controls cells destiny during AGE-LDL–induced foam cell formation. Studies suggest that AGE-LDL can facilitate cell autophagy in the early stage foam cell formation. Further studies suggest PDCD4 deficiency enhances cell autophagy but inhibits cell apoptosis. Previous studies have been reported that autophagy is an adaptive response might prevent lesional macrophage apoptosis. In our study, we also found PDCD4 deficiency attenuates apoptosis and foam cell formation relays on increased autophagy. This line is involved in autophagy-mediated protection of macrophage cells and may serve as a unique therapeutic target for treating atherosclerosis. These results indicate that PDCD4 is critical for atherosclerosis development induced by AGE-LDL and provides new insights into atherogenesis, and sheds light on the therapeutic target of the vascular disorders.
2 MATERIALS AND METHODS
2.1 Prepared AGE-LDL
LDL and ox-LDL were obtained from Yiyuan Biotechnology (Guangzhou, China). AGE-LDL was prepared by an established method described by Sima et al4 Glycated LDL was prepared by incubation of LDL with 0.2M glucose for 4 weeks at 37°C with antioxidants (1 mg/mL ethylenediaminetetraacetic acid [EDTA] and 1 μM butylated hydroxytoluene) under sterile conditions, and was extensively dialyzed before use. The free, nonglycated amino groups were determined using the trinitrobenzene sulfonic acid assay (TNBSA). The TNBSA (0.01% [wt/vol]) is a sensitive reagent for quantification of primary free amino groups in available lysine contents of proteins. This compound reacts with protein's free amino groups to form trinitrophenolic derivatives.17 The extent of LDL protein glycation was evaluated by measuring pentosidine formation spectrofluorometrically (excitation 335 nm and emission 385 nm). The protein concentration was measured by Modified Lowry Protein Assay Kit (Thermo Fisher Scientific, Rockford).18 Agarose gel (0.6%) electrophoresis was used for detecting the mobility of native LDL, AGE-LDL, and ox-LDL. After agarose gel electrophoresis, sudan black 10B was stained with lipoprotein.
2.2 Cell culture
Mice monocyte-macrophage leukemia-derived cell line RAW 264.7 and human monocytic cell line THP-1 were purchased from the Shanghai Cell Bank of the Chinese Academy of Science (Shanghai, China). The cell lines were grown in RPMI-1640 medium (Gibco, CA) supplemented with 10% fetal bovine serum (FBS). Human THP-1 monocytes were treated with 160 nM of Phorbol 12-myristate 13-acetate (PMA) for 2 days for macrophage transformation. Mouse primary peritoneal macrophages were isolated from C57BL/6 mice. Eight-week-old mice were killed and the peritoneal cavity was washed with 5 mL of ice-cold PBS to isolate primary peritoneal macrophages. Peritoneal fluid was filtered through a 70-μm pore nylon mesh and centrifuged at 1200 rpm for 10 minutes. The pellet was first treated with red blood cell lysis buffer and resuspended in Dulbecco's modification Eagle's medium supplemented with 10% (vol/vol) FBS, 100 μg/mL streptomycin and 100 U/mL penicillin.19
2.3 Foam cell formation and analysis
Cells were stimulated with ox-LDL and AGE-LDL for 24 hours, incubated with 3-methyladenine (MA) (Sigma-Aldrich, MI) to block autophagy. Cells were then stained with Oil Red O (Sigma-Aldrich, MI), rinsed in 60% isopropanol and water. Hematoxylin was used as a counterstain. Multiple images were then taken, and the percentage of stained cells was quantified. The three random independent locations were used for one experiment repeated, three independent experiments for the final calculation and comparison.
2.4 Cell transfection
Short hairpin RNA (shRNA) were purchased from Shanghai GenePharma Co, Ltd (Shanghai, China). The sequences were here: for shRNA scramble control (named shNC): GTTCTCCGAACGTGTCACGT; for shPDCD4: CTGGACAGGTGTATGATGTGG. The vector has two promoters to expression target shRNA and fluorescent protein independently. Exponentially growing RAW 264.7 cells and THP-1 cells were stably transfected with the plasmids as described before.20 Primary peritoneal macrophages were transciently transfected with the plasmids, using Lipofectamine 3000 (Invitrogen, Carlsbad) reagent according to the manufacturer's protocols. The efficiency of transfection was observed by fluorescence microscope and Western blot analysis.
2.5 Real-time quantitative polymerase chain reaction
Total RNA was extracted from cells using TRIzol reagent (Invitrogen, Carlsbad) PDCD4 expression was measured by SYBR green qRT-PCR assay (Takara, Dalian, China). Primers for PDCD4 were forward, 5′-GATTAACTGTGCCAACCAGTCCAAAG-3′ and reverse, 5′-CATCCACCTCCTCCACATCATACAC-3′. Primers for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were forward, 5′-AGCCTTCTCCATGGTGGTGAA-3′ and reverse, 5′-ATCACCATCTTCCAGGAGCGA-3′. Quantification real-time reverse-transcriptase polymerase chain reaction (RT-PCR) was performed on the iCycler System (Bio-Rad, Hercules, CA). The expression level of PDCD4 was normalized by the level of GAPDH. The PCR program was 95°C for 2 minutes, then 95°C for 15 seconds, 60°C for 30 seconds, and 72°C for 30 seconds for 40 cycles.
2.6 Western blots
Cells were lysed using mammalian protein extraction reagent containing protease inhibitors (Pierce, Thermo scientific, Rockford). The quantification of protein was detected by BCA assay (Pierce, Thermo scientific, Rockford). Samples (20 μg protein per lane) were loaded to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and subjected to electrophoresis. Proteins were transferred to polyvinylidene fluoride membranes and subsequently probed using the rabbit monoclonal antibodies against light chain 3 (LC3-II) (#3868), PDCD4, BAX, PARP(C), and caspase-3(C) (all 1:1000; Cell Signaling Technology, Danvers, MA), mouse monoclonal antibody against β-actin (sc-7210, 1:2000; Santa Cruz Biotechnology, Dallas), followed by secondary antibody conjugated (1:5000; EarthOx, San Francisco). Blots were developed with the enhanced chemiluminescence system according to the manufacturer's instructions (Pierce, Thermo scientific, Rockford).
2.7 Immunofluorescence
Cells were cultured on Glass Bottom Cell Culture Dishes (NEST Biotechnology, Wuxi, China) for detection by microscope (1.0 × 105 cells per 35-mm dish), and then subjected to treatments as indicated. Cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 for 15 minutes. After incubation for 2 hours with anti-LC3-II (1:300; Cell Signaling Technology, Danvers, MA), cells were washed with PBS and then incubated for 1 hour with DyLight 594-conjugated (1:500; EarthOx, San Francisco) secondary antibodies. Nuclei were stained by 4′,6-diamidino-2-phenylindole (DAPI; Beyotime, Shanghai, China) for 5 minutes. All slides were observed under a microscopy (Leica, Wetzlar, Germany).
2.8 Flow cytometry
Green fluorescent protein-LC3 (GFP-LC3) was used to monitor autophagic flux by flow cytometry. GFP-LC3 plasmid was cotransfect to the cells, after 24 hours, the cells were treatment with AGE-LDL. The cells are then washed, trypsinized, and resuspended in PBS and subjected to flow cytometric analysis. In the analysis, the GFP intensity of the treated cells is normalized to that of the control cells.21
Mitochondrial membrane potential was assessed using the fluorescent dye JC-1, which is a monomer molecule permeable to the mitochondrial membrane. JC-1 accumulates in functional mitochondria with high Δψ and forms aggregates that emit red fluorescence. But JC-1 does not accumulate in mitochondria with depolarized Δψ and remains in the cytoplasm as monomers. In mitochondria undergoing a transition from polarized to depolarized Δψ, JC-1 leaks out of the mitochondria into the cytoplasm as monomers resulting in a decrease of red fluorescence. Briefly, cells knockdown with PDCD4 and control were treated with 100 μg/mL AGE-LDL for 12 hours. Then, the cells were rinsed with PBS and stained with MitoScreen JC-1 (BD Pharmingen, Frank-lin Lakes, NJ), according to the manufacturer's instructions. Platelets were washed and resuspended into the MitoScreen buffer. Relative degrees of mitochondrial polarization were quantified by measuring the red-shifted JC-1 aggregates on FL-2 channel and the green-shifted monomers on FL-1 channel.
2.9 Statistical analysis
All data are expressed as mean ± SD and statistical significance was examined with one-way analysis of variance pairwise comparison using SPSS13.0 software (Chicago). P < 0.05 indicate statistical significance.
3 RESULTS
3.1 Characterization of AGE-LDL
In vivo, glycation of LDL is very complex involving the nonenzymatic reaction of glucose and its metabolites with the free amino groups of lysine.4, 6 The extent of LDL glycation was estimated using TNBSA, which upon coupling to free, nonglycated amino groups gives the highly chromogenic 2,4,6-trinitrophenyl (TNP) derivatives. We used TNBSA assays to detect the level of glycated epitopes. It showed that 36% reduction in free amino groups of apoB in AGE-LDL relative to n- or ox-LDL (0.28 ± 0.02 AU in AGE-LDL vs 0.45 ± 0.03 AU in n-LDL or 0.43 ± 0.05 AU in ox-LDL, respectively; P < 0.05) was consistent with irreversible protein glycation (Figure 1A). To assess the intact of LDL, we load the sample for the native PAGE gel electrophoresis dyed with coomassie brilliant blue (Figure 1B). In addition, agarose gel electrophoresis dyed by sudan black showed the electrophoresis mobility of AGE-LDL was faster than n- and ox-LDL, indicating loss of positive charges due to the glycation of Lys residues (Figure 1C). Native SDS-PAGE and agarose gel electrophoresis of LDL preparations appeared as a single band, ruling out the possibility of contamination with other proteins.
Characterization of AGE-LDL. A, Comparative evaluation of native LDL (n-LDL), copper-oxidized-LDL (ox-LDL), and AGE-LDL (obtained by incubation of n-LDL with 0.2 M glucose for 4 weeks at 37°C with antioxidants (1 mg/mL EDTA and 1 μM butylated hydroxytoluene) under sterile conditions, and extensively dialyzed before use). The glycated epitopes were measured by TNBSA. *P < 0.05, n = 5. B, Native PAGE gel electrophoresis for native LDL, AGE-LDL, and ox-LDL (dyed with coomassie brilliant blue). C, Representative agarose gel (0.6%) electrophoresis showing electrophoretic mobility of native LDL, AGE-LDL, and ox-LDL (sudan black 10B staining). AGE, advanced glycation end-product; EDTA, ethylenediaminetetraacetic acid; LDL, low-density lipoprotein; PAGE, polyacrylamide gel electrophoresis; TNBSA, trinitrobenzene sulfonic acid assay
3.2 AGE-LDL induces autophagy in mice macrophage cell line RAW264.7
Autophagy is a dynamic process, involving autophagosome formation, cargo sequestration, and eventual lysosomal fusion/degradation occurring in succession. LC3-II is a well-known marker of cell autophagy. To detect autophagy, we observed whether the levels of LC3-II was regulated by AGE-LDL. The results showed that both AGE-LDL and ox-LDL increased the expression of LC3-II (Figure 2A), AGE-LDL upregulated the levels of LC3-II in the concentrations of 50 and 100 μg/mL (Figure 2B). And the increased level of LC3-II determined during 2 to 12 hours indicated that the effect was time-independent (between 2 and 12 hours) (Figure 2C). For confirmation, the result in the primary nontransformed cells, primary peritoneal macrophages from C57BL/6 mice were used. We also detected the induction of autophagy in primary peritoneal macrophages by AGE-LDL (Figure 2D). Previous study reported that 9.3 mg/dL (93 μg/mL) glycated LDL apoB in the serum of diabetes versus 4.8 mg/dL (48 μg/mL) in controls. Therefore, we choose the 100 μg/mL (in the physiological range) as the optimal dose for the subsequent experiments.
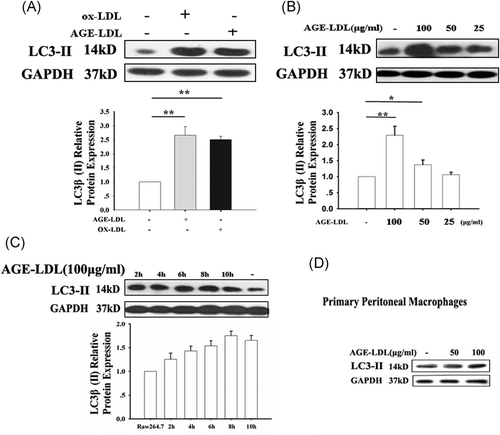
AGE-LDL–induced autophagy in RAW 264.7 and primary peritoneal macrophage cells. A, RAW 264.7 cells were plated in six-well plates with AGE-LDL or ox-LDL. After 12 hours, the cells were collected and subjected to Western blot analyses for LC3-II. B, RAW 264.7 cells were plated in 6-well plates treated with AGE-LDL, collected at different dose. C, Primary peritoneal macrophage cells were derived from nonstimulated C57BL/6 mice. Macrophages were plated in six-well plates treated with AGE-LDL (50 and 100 μg/mL). n = 5, *P < 0.05, **P < 0.01. AGE, advanced glycation end-product; LDL, low-density lipoprotein; ox-LDL, oxidized-LDL
3.3 Silence of PDCD4 facilitates AGE-LDL–induced autophagy
Previous studies have shown that PDCD4 deficiency clearly improved ox-LDL-impaired autophagy efflux, promoted autophagy-mediated lipid breakdown in murine macrophages and thus prevented macrophage conversion into foam cells.15, 16 Here, we detected the efficiency of two shPDCD4 (shPDCD4-1 and shPDCD4-2) by real-time RT-PCR and Western blot analysis. ShPDCD4-1 showed higher efficiency in knocking down of both (Figure 3A), so we used the shPDCD4-1 for the experiment after. Increases in the LC3-II levels were observed in PDCD4 deficiency cells compared with the control cells in the presence of AGE-LDL (Figure 3B). To observe the PDCD4 induced autophagic activity, we used a fluorescence microscope to detect the autophagosomes in cells. The autophagosome, a special vesicle with a double membrane layer, is an important marker of autophagy activation, which ultimately fuses with the lysosome to form the autolysosome, leading to the degradation of cellular structures.22-24 While control cells displayed a diffuse staining, PDCD4 deficiency cells exhibited a speckled fluorescent staining pattern, indicating the redistribution of LC3-II to autophagosomes in the cytoplasm (condensed spot, arrow in Figure 3C). In addition, pQCXIP-GFP-LC3 was used to monitor autophagic flux by flow cytometry in RAW 264.7 cells. pQCXIP-GFP-LC3 plasmid was cotransfect to the cells, after 24 hours, the cells were treatment with AGE-LDL. Flow cytometry detected that PDCD4 deficiency aggravated AGE-LDL–induced autophagic flux (represented by mean fluorescence intensity) in RAW 264.7 cells (Figure 3D).
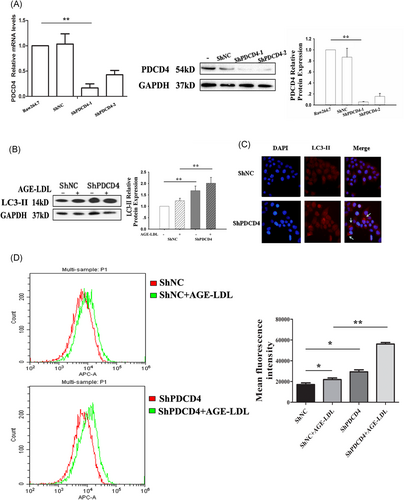
PDCD4 deficiency aggravated AGE-LDL–induced autophagy efflux in RAW 264.7 cells RAW 264.7 cells transfected with shNC and shPDCD4 were exposed to AGE-LDL (100 μg/mL), and cell samples were collected for real-time RT-PCR (A) and Western blot analysis (B). C, Autophagosomes (labeled by LC3-II) were detect by fluorescence microscope, indicated by arrows. D, pQCXIP-GFP-LC3 plasmid was cotransfect to RAW 264.7 cells, after 24 hours, the cells were treatment with AGE-LDL. Flow cytometry detected that PDCD4 deficiency aggravated AGE-LDL–induced autophagic flux (represented by mean fluorescence intensity) in RAW 264.7 cells. n = 4, *P < 0.05, **P < 0.01. AGE, advanced glycation end-product; LC3, light chain 3; LDL, low-density lipoprotein; PDCD4, programmed cell death protein 4; RT-PCR, reverse-transcriptase polymerase chain reaction
3.4 PDCD4 deficiency attenuates apoptosis that relies on increased autophagy
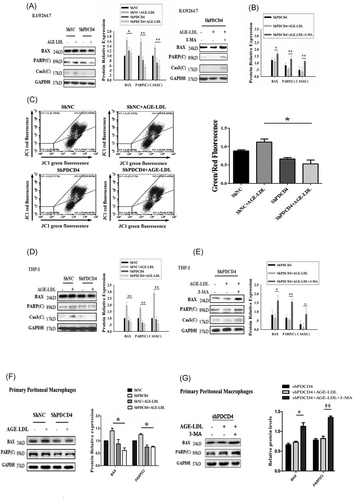
Autophagy and apoptosis are the two major pathways of cell death; the current knowledge has shown that there is a crosstalk between the components of these two pathways.25 Hence, we further investigated the roles of autophagy and apoptosis in PDCD4 deficiency cells. First, we detected the apoptosis induced by AGE-LDL in PDCD4 deficiency cells. Our results indicated that PDCD4 deficiency cells had decreased levels of BAX, cleaved poly(ADP-ribose) polymerase (PARP), and caspase-3 (Figure 4A), suggesting that the PDCD4 deficiency resulted in decreased apoptosis with the treatment of AGE-LDL. We wondered whether the mitochondria damage involved in the RAW 264.7 cells apoptosis. The mitochondria membrane potential was assessed by JC-1 staining and measured by flow cytometry. The results showed that JC-1 green fluorescence positive cells decreased, it indicated that PDCD4 deficiency attenuated cell mitochondria dysfunction (Figure 4C). Autophagy inhibitor 3-MA was utilized in the current study to examine whether autophagy plays a protective or promoting role in apoptosis. The apoptosis status of PDCD4 deficiency cells was examined again. Conversely, pharmacological treatment with 3-MA that inhibits autophagy resulted in restore of apoptosis proteins (BAX, PARP(C), and Cas3(C)), respectively (Figure 4B). To confirmation the effect of PDCD4 in the other independent system, we used human monocytic cell line-THP-1 and primary peritoneal macrophages to detect the role of PDCD4 deficiency involved in autophagy and apoptosis induced by AGE-LDL. The results showed that PDCD4 deficiency-activated autophagy is required for inhibition of apoptosis in THP-1 cells (Figure 4C and 4D) and primary peritoneal macrophages (Figure 4E and 4F). Taken together, the observation that PDCD4 deficiency inhibits apoptosis through activated autophagy in multiple cell types suggests that the effect of PDCD4 is universal. These results further suggested that the higher level of autophagy was a potential target for suppressing apoptosis.
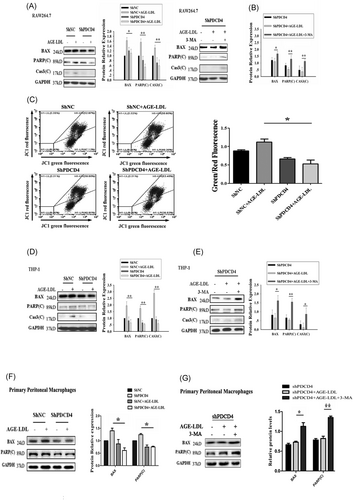
PDCD4 deficiency attenuates apoptosis that relies on increased autophagy. A, PDCD4 deficiency in RAW 264.7 cells leads to the down-regulation of BAX, PARP(C), and Cas3(C). RAW 264.7 cells transfected with shNC and shPDCD4 were plated in six-well plates with or without AGE-LDL. The cells were collected and subjected to Western blot analysis for BAX, PARP(C), Cas3(C), and GAPDH. B, The inhibition of autophagy abolishes the antiapoptotic effects by PDCD4 deficiency in RAW 264.7 cells. RAW 264.7 cells transfected with shPDCD4 were plated in six-well plates. The culture medium was replaced with the medium containing 5 mM 3-MA for 9 hours, and the cells were treated with AGE-LDL for another 12 hours. The cells were collected and subjected to Western blot analysis for BAX, PARP(C), Cas3(C), and GAPDH. C, RAW 264.7 cells knockdown with PDCD4 and control were treated with 100 μg/mL AGE-LDL for 12 hours. Then, the cells were rinsed with PBS and stained with JC-1. JC-1 green fluorescence positive cells decreased which indicated cell mitochondria dysfunction attenuation when PDCD4 deficiency. D, E, The repeat experiments used human monocytic cell line-THP-1. PDCD4 deficiency inhibits AGE-LDL–induced apoptosis in THP-1. PDCD4 deficiency cells had decreased levels of BAX, cleaved PARP, and cleaved Cas3 (D). PDCD4 deficiency attenuates apoptosis that relies on increased autophagy in THP-1 (E). F, G, The repeat experiments used primary peritoneal macrophage cells. PDCD4 deficiency cells had decreased levels of BAX and cleaved PARP, suggesting that the PDCD4 deficiency resulted in decreased apoptosis with the treatment of AGE-LDL (F). Moreover, Inhibition of autophagy activation abolishes PDCD4 deficiency-induced antiapoptotic effects in primary peritoneal macrophages (G). The data represent four independent experiments. n = 4, *P < 0.05, **P < 0.01. AGE, advanced glycation end-product; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; LDL, low-density lipoprotein; PARP, poly(ADP-ribose) polymerase; PDCD4, programmed cell death protein 4
3.5 PDCD4 deficiency suppress AGE-LDL–induced lipid accumulation
Macrophage with overloaded lipids will transform into foam cells. Next, we analyzed the role of PDCD4 in macrophage foam cell formation and established the association between autophagy and foam cell formation. As shown in Figure 5, PDCD4 deficiency caused the reduction of lipid accumulation in AGE-LDL–treated macrophages. However, PDCD4 deficiency-alleviated foam cell formation was restored by 3-MA. This result suggested that autophagy inhibitor exacerbated the antilipid accumulation effect of PDCD4 deficiency in RAW 264.7 cells.

PDCD4 deficiency suppressed macrophage conversion into foam cells in an autophagy-dependent manner Cells were stimulated by 100 μg/mL AGE-LDL for 24 hours or in the presence of 3-MA for 9 hours, and then cells were stained by Oil red O. Hematoxylin as counterstained to showed cell nuclear, lipid (red) deposited in the cytoplasm. Data came from three independent experiments. AGE, advanced glycation end-product; LDL, low-density lipoprotein
4 DISCUSSION
AGEs are a heterogeneous group of proteins and lipids formed by the Maillard reaction occurring in plasma and tissues. AGE-substances (products of advanced glycation) are formed not only from proteins (eg, Hb-AGE) but also from lipids (AGE-LDL), and DNA (AGE-DNA) and lead to an increased number of mutations. They are toxic and are accumulated in the vascular wall especially in diabetes.26 AGE-LDL has been found in human plasma27, 3. Tames et al28 reported that 9.3 mg/dL glycated LDL apoB in the serum of patients with diabetes versus 4.8 mg/dL in controls. Akanji et al29 reported that serum-glycated LDL level is increased in patients with hyperlipidemia without diabetes (6.12 mg/dL), and further increased in patients with both hyperlipidemia and diabetes (7.82 mg/dL) compared with 2.74 mg/dL in healthy subjects. So, the concentration that we used to incubate the cells (10 mg AGE-LDL protein/dL culture medium) is within the physiological range. A comparative study with LDL modified in vitro or isolated from diabetic patients indicated that both types of glycated LDL (artificial and naturally modified), modulate what of major microvascular. Subcutaneous immunization with AGE-LDL significantly inhibits atherosclerosis development in hyperlipidemic diabetic mice.5 In vivo, glycation of LDL entails nonenzymatic reaction of glucose and its metabolites with the free amino groups of lysine.4, 30 As shown in Figure 1A, using TNBSA assay, we observed an enormous reduction in the amount of free amino groups of apoB in AGE-LDL. Moreover, electrophoresis mobility of AGE-LDL was faster than n- and ox-LDL, implying that we prepared the AGE-LDL in vitro successfully (Figure 1C).
AGE-LDL plays a central role in the development of diabetic atherosclerosis.31, 32 It causes coagulopathy, stimulates cytokines, leads to inflammation and inactivates the synthesis of nitrogen oxide, which prevents relaxation of the vascular wall.4, 18, 32-34 AGE-LDL is taken up by macrophages and then contributes to the formation of a foam cell. In clinical, anti–AGE-LDL autoantibodies favor atherosclerosis progression in humans, but the underlying mechanism remains unknown.5
Macrophage cell plays a vital role in the development of atherosclerosis, as a major component in the lesion of atherosclerosis.35 Macrophage with overloaded lipids stored in LDs will transform into foam cells.36 Using ox-LDL, it has demonstrated that macrophage autophagy participates in plaque formation and progression. In early-stage of foam cells, the amount of autophagosomes gradually increased, and autophagic flux intensity accelerated.37 Similarly, in this study, we also found that AGE-LDL (50-100 μg/mL) worked as a trigger for macrophage cell autophagy (Figure 2) in the early stage.
Autophagy has a role in the hydrolysis of stored cholesterol droplets in macrophages, and thus facilitates cholesterol efflux. Using ox-LDL, many studies also demonstrated that treatment with autophagy activator markedly decrease intracellular lipid content and thus sabotage the formation of foam cells.37, 38 Macrophage autophagy contributes to the inhibition of foam cell formation by reducing ox-LDL ingestion by macrophages and increasing efferocytosis and cholesterol efflux in macrophages.39 It also inhibits the occurrence of plaque rupture and fall off by inhibiting apoptosis and inflammation in lesional plaques, which alleviates the severity of atherosclerosis.40 During the process of foam cell development, upregulating autophagy not only reduced intracellular LD accumulation, but also inhibited cell apoptosis through clearing dysfunctional mitochondria and lowering intracellular reactive oxygen species (ROS) level.35 Thus, increasing macrophage autophagy persistently would be an attractive strategy to reverse plaque lipid buildup.41, 42 But no study implies that the autophagy might be a target for attenuating foam cell formation induced by AGE-LDL.
In the previous studies, PDCD4, a tumor suppressor, has been proved negatively regulated autophagy in tumor cells and macrophage cells.14-16 So, in the present study, we characterized the role of PDCD4 played in AGE-LDL–induced autophagy. Several lines of evidence indicate that PDCD4 regulates the AGE-LDL–induced foam cell formation through autophagy. First, PDCD4 deficiency facilitates the macrophage autophagy, which is manifested by an increase in the number of autophagosomes and the levels of LC3-II (Figure 3). Second, it is commonsense that, in atherosclerosis, autophagy plays an important role in safeguarding cells against oxidative stress by reducing cell apoptosis and enhancing lesional stability.38 It has been reported that autophagy is an adaptive response that delays the eventual cell apoptosis and death.15, 16, 43 Moreover, autophagy prevents lesional macrophage apoptosis and defective efferocytosis and defective autophagy enhanced the total necrotic area in advanced atherosclerotic plaques and the damage caused by oxidative stress in LDL−/− mice.39 Here, PDCD4 deficiency results in decreased levels of apoptosis (BAX, cleaved caspase-3, and cleaved PARP) and mitochondria dysfunction in macrophages (Figure 4), which is conducive to the destiny of macrophages. Moreover, the decreased apoptosis in PDCD4 deficiency relies on autophagy. Third, blockage of autophagy by 3-MA aggravates macrophage conversion into foam cells (Figure 5). Autophagic flux is the process consisting of autophagosome the delivery of cell contents by autophagosome to the lysosome, and the fusion of two organelles for digestion. PDCD4-mediated autophagy is involved in the process of lipid hypolysis and efflux. The results highlight that PDCD4 deficiency attenuates foam cell formation and macrophage apoptosis by promoting AGE-LDL–induced autophagy.
Taken together, our data demonstrate that PDCD4 plays an essential role in protecting against diabetic atherosclerosis by reducing the formation of foam cell through an autophagy-dependent pathway. It provides new insights into the understanding of the molecular mechanism of PDCD4 involved in macrophage autophagy or apoptosis and the development of its therapeutic treatment of diabetic atherosclerosis by modulation of PDCD4 activities.
ACKNOWLEDGMENTS
The present study was supported by grants from The National Natural Sciences Foundation of China, Grant/Award Numbers: 81502637, 81702639; Natural Science Foundation of Hubei Provincial of China, Grant/Award Number: 2018CFB467, 2015CFB240; Natural Science Foundation of Hubei Provincial Department of Education, Grant/Award Numbers: Q20162114, D20142106, Q20142103; Principal Investigator Grant of Hubei University of Medicine, Grant/Award Number: HBMUPI201805; Faculty Development Grants from Hubei University of Medicine, Grant/Award Numbers: 2014QDJZR06, 2013QDJZR05, FDFR201609, 2015QDJZR09. Thanks, Guo Hao for valuable discussions and revised English grammar.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
LS wrote the manuscript, analyzed and interpreted the results. GGD conducted the experiments. WFY was a contributor in writing and editing the manuscript. LD, ZHY, KJ, LY, LF, LJ, CZY, TZM, BL, and ZJX provided experimental support. CX and ZW provided funding and intellectual input. All authors read and approved the final manuscript.
NOMENCLATURE
-
- AGE
-
- advanced glycation end-product
-
- AGE-LDL
-
- advanced glycation end-products modified form of low-density lipoprotein
-
- CVD
-
- cardiovascular disease
-
- eIF
-
- eukaryotic initiation factors
-
- LD
-
- lipid droplets
-
- LDL
-
- low-density lipoprotein
-
- ox-LDL
-
- oxidized low-density lipoprotein
-
- PDCD4
-
- programmed cell death protein 4
-
- ROS
-
- reactive oxygen species
-
- TNBSA
-
- trinitrobenzene sulfonic acid assay
-
- TNP
-
- trinitrophenyl