Daphnetin protects hippocampal neurons from oxygen-glucose deprivation–induced injury
Abstract
Daphnetin, a coumarin derivative extracted from Daphne odora var., was reported to possess a neuroprotective effect. Recently, it has been demonstrated that daphnetin attenuates ischemia/reperfusion (I/R) injury. However, the role of daphnetin in cerebral I/R injury and the potential mechanism have not been fully understood. The present study aimed to explore the regulatory roles of daphnetin on oxygen-glucose deprivation/reoxygenation (OGD/R)–induced cell injury in a model of hippocampal neurons. Our results demonstrated that daphnetin improved cell viability and reduced the lactate dehydrogenase leakage in OGD/R–stimulated hippocampal neurons. In addition, daphnetin inhibited oxidative stress and cell apoptosis in hippocampal neurons after OGD/R stimulation. Furthermore, daphnetin significantly enhanced the nuclear translocation of the nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1) expression in hippocampal neurons exposed to OGD/R. Knockdown of Nrf2 blocked the protective effect of daphnetin on OGD/R–induced hippocampal neurons. In conclusion, these findings demonstrated that daphnetin attenuated oxidative stress and neuronal apoptosis after OGD/R injury through the activation of the Nrf2/HO-1 signaling pathway in hippocampal neurons. Thus, daphnetin may be a novel therapeutic agent for cerebral I/R injury.
1 INTRODUCTION
Cerebral ischemia/reperfusion (I/R) is a medical condition in which there is insufficient blood flow in the brain followed by normal blood flow.1 Cerebral I/R can lead to global cerebral ischemia and neurological dysfunction. It is associated with substantial morbidity and mortality worldwide.1 Therefore, a better understanding of the molecular mechanism is helpful for the management of cerebral I/R injury. It is well accepted that oxidative stress is one of the main pathophysiological processes during I/R injury.2 Therapeutic strategies aimed at attenuating oxidative stress are required for the prevention and treatment of cerebral I/R injury.
Nuclear factor erythroid 2-related factor 2 (Nrf2) is an important transcription factor in the protection of cells against oxidative damage.3 Activation of Nrf2 activates the transcription of multiple antioxidant stress genes, such as nicotinamide adenine dinucleotide phosphate (NADPH) quinone oxidoreductase 1, glutamate-cysteine ligase, and heme oxygenase-1 (HO-1).4 Among these, the Nrf2/HO-1 pathway has been well studied to play an important neuroprotective role in cerebral I/R injury.5, 6 Previous studies have demonstrated that the Nrf2/HO-1 pathway is a potential target for alleviating the oxidative damage during cerebral I/R injury.5, 6
Daphnetin (7,8-dihydroxycoumarin) is a natural coumarin compound that possesses a variety of biological activities such as anticancer,7 antioxidative,8 anti-inflammatory,9 and neuroprotective effects.10 Daphnetin has been found to protect the liver against I/R injury via inhibiting the inflammation in an in vivo hepatic I/R model.11 In addition, it was reported that daphnetin exhibits neuroprotective and anti-inflammatory effects on cerebral I/R injury in mice.12 However, the mechanism of the protective effect of daphnetin on cerebral I/R injury has not been fully understood.
In this study, we investigated the effect of daphnetin on cerebral I/R injury using a cell ischemia model in vitro. We found that daphnetin protected hippocampal neurons from I/R injury via regulating the Nrf2/HO-1 pathway.
2 MATERIALS AND METHODS
2.1 Cell culture
Ten Sprague-Dawley rats (Charles River, Beijing Vital River Laboratory Animal Technology Co., Ltd., China) were used for the preparation of primary hippocampal neurons as described previously.13 Briefly, cerebral hippocampi were separated from brains and dissected in HBS. After mechanical dissociation and trypsinization, the cells were plated in dishes that were pretreated with 0.1% poly-d-lysine (Sigma-Aldrich, St. Louis, MO). After incubating for 2 hours, cells were cultured in neurobasal medium supplemented with 2% B-27, 1% penicillin-streptomycin, and 0.25% glumax for 7 days. Hippocampal neurons were then purified by density gradient centrifugation, and identified by neuron-specific enolase immunofluorescence (data not shown). All experiments were carried out according to the National Institute of Health Guide for the Care and Use of Laboratory Animals.
2.2 Oxygen-glucose deprivation/reperfusion model and treatment
After 7 days, the primary hippocampal neurons were subjected to oxygen-glucose deprivation (OGD), through incubating in serum and glucose-free medium with 5% CO2 and 95% N2 at 37°C for 3 hours. Then the cells were incubated with normal medium containing serum and glucose in normoxic conditions for 24 hours. For the daphnetin treatment group, cells were pretreated with 10, 20, 40 μM daphnetin (≥97%, Sigma) for 12 hours before OGD/reperfusion (OGD/R) stimulation. The experiment was repeated three times.
2.3 Cell transfection
Small interfering RNA targeting Nrf2 (si-Nrf2; Invitrogen, Thermo Fisher Scientific, Waltham, MA) that can silence the complementary target messenger RNA of Nrf2 was transfected into the hippocampal neurons using Lipofectamine 2000 (Invitrogen). After 24 hours, the transfection efficiency was tested using Western blot analysis. The experiment was repeated three times.
2.4 Cell viability assay
Approximately 5 × 103 cells/well hippocampal neurons were seeded in 96-well plates and incubated for 12 hours with or without the presence of daphnetin. Then, the cells were treated with OGD/R stimulation, and then cell viability was evaluated with the cell counting kit-8 (CCK-8) (Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer's instructions. Finally, the absorbance at 450 nm was measured with a microplate reader (Bio-Tek Instruments, Winooski, VT). The experiment was repeated three times.
2.5 Lactate dehydrogenase assay
The hippocampal neurons injury was assessed by detecting the lactate dehydrogenase (LDH) activity with a commercial LDH Cytotoxicity Assay Kit (Jiancheng Bioengineering Institute, Nanjing, China). Hippocampal neurons (5 × 103 cells/well) were plated in 96-well plates and subjected to OGD/R stimulation with or without the presence of daphnetin. After different treatments, 20 μL of cell supernatant was collected for the detection of LDH activity according to the manufacturer's instructions. The absorbance was measured spectrophotometrically at 450 nm using a microplate reader (Bio-Tek). The values are shown as percentages of the total LDH. The experiment was repeated three times.
2.6 Measurement of intracellular reactive oxygen species
Intracellular reactive oxygen species (ROS) was determined using a fluorescence probe 2′,7′-dichlorofluorescin-diacetate (DCFH-DA; Sigma). As described by the manufacture's protocol, in the presence of ROS, DCFH-DA can be oxidized to the 2′,7′-dichlorofluorescin, which is highly fluorescent. After treatment, hippocampal neurons were incubated with DCFH-DA in the dark for 30 minutes. Then, the fluorescence intensity of the cells was analyzed using a fluorospectrophotometer with the excitation/emission of 485/525 nm. The experiment was repeated three times.
2.7 Measurement of malondialdehyde, superoxide dismutase, and glutathione peroxidase
After treatments, the cell lysates of the hippocampal neurons were prepared using cell lysis buffer (Beyotime Biotechnology, Shanghai, China). The superoxide dismutase (SOD) activity was measured using a SOD assay kit with WST-8 method (Beyotime). The O2- produced by xanthine oxidase can react with WST-8 to produce formazan dye. The reaction can be blocked by SOD because it can react with the O2-; therefore, the production of formazan dye is correlated with the SOD activity. After incubation, the absorbance was measured at 450 nm. The data are expressed as the fold activity relative to the control. The measurement of malondialdehyde (MDA) level in cell lysates was measured using a lipid peroxidation MDA assay kit (Beyotime). MDA can react with thiobarbituric acid (TBA) in special conditions to produce MDA-TBA. The levels of MDA-TBA can be detected at 535 nm using a colorimetric method. The unit of MDA concentration is nmol/mg protein.
The glutathione peroxidase (GPx) activity in cell lysates was assessed by a reaction with the GPx detection working solution from the kit (0.25 mM NADPH, 2.1 mM reduced GSH, 0.5 unit/mL glutathione reductase, and 300 μM tert-butyl hydroperoxide), in which the reduction in NADPH absorbance was indicative of the GPx activity, detected at 340 nm after an initial delay of 15 seconds and monitored every 10 seconds for 1 minute at 25°C. The data are expressed as the fold activity relative to the control. The experiment was repeated three times.
2.8 Cell apoptosis assay
The cell apoptosis of hippocampal neurons was analyzed using an enzyme-linked immunosorbent assay (ELISA)–based cell death detection kit (Roche Molecular Biochemicals, Mannheim, Germany) on the principle of the quantification of apoptosis-specific DNA fragmentation. DNA fragmentation was measured by using a microplate reader to measure the absorbance at 405 nm. The experiment was repeated three times.
2.9 Western blot assay
The cytosolic and nuclear proteins were prepared using a celLytic nuclear extraction kit (Sigma). The protein concentration was measured using a BCA Protein Assay Kit (Thermo Fisher Scientific). After separation by 10% SDS-PAGE, the proteins were transferred to polyvinylidene difluoride membranes (Thermo Fisher Scientific). The membranes were blocked with 5% skim milk in tris-buffered saline containing 0.1% (v/v) Tween 20 (TBST) buffer for 1 hour at room temperature, and then incubated overnight at 4°C with the following antibodies: anti-bax (1:1000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA), anti-bcl-2 (1:1,000 dilution, Santa Cruz), anti-cleaved-caspase-3 (1:1000 dilution; Cell Signaling Technology, Boston, MA), anti-Nrf2 (1:500 dilution; Abcam, Cambridge, MA), anti-HO-1 (1:500 dilution; Abcam), anti-lamin B1 (1:1500 dilution; Abcam), and anti-GAPDH (1:1000 dilution; Santa Cruz). The membranes were incubated with horseradish peroxidase–conjugated secondary antibodies (1:3000 dilution; Cell Signaling Technology) at room temperature for 1 hour. An enhanced chemiluminescence detection system (Thermo Fisher Scientific) was used to visualize the protein bands. The intensity of the bands was analyzed using quantity one software (Bio-Rad, Hercules, CA). The experiment was repeated three times.
2.10 Statistical analysis
The quantitative data are shown as the mean ± SEM. A one-way analysis of variance followed by a Dunnett's post hoc test were applied for analyzing the multiple comparisons. The statistical analysis was performed using the SPSS 18.0 (SPSS, Chicago, IL), and the statistical significance was set at P < 0.05.
3 RESULTS
3.1 Daphnetin ameliorates OGD/R–induced neuron injury
We first evaluated the effect of daphnetin on the cell viability of hippocampal neurons. CCK-8 assay showed that daphnetin did not affect cell viability until the concentration up to 40 μM (Figure 1A). Thus, the concentrations of 10, 20, 40 μM were selected for the following study. Next, we investigated the role of daphnetin in the OGD/R–induced neuron injury. Figure 1B shows that daphnetin inhibited OGD/R–induced inhibition of hippocampal neurons viability in a dose-dependent manner. Figure 1C revealed that daphnetin dose-dependently reduced OGD/R–induced LDH release in hippocampal neurons.
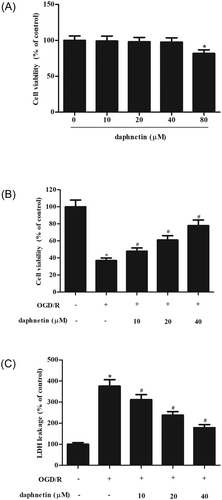
Daphnetin ameliorates OGD/R–induced cell injury in hippocampal neurons. A, Cell viability of hippocampal neurons after incubation with daphnetin (0, 10, 20, 40, and 80 μM) for 48 hours. B, Cell viability of hippocampal neurons subjected to OGD/R induction with or without the presence of daphnetin. C, LDH release of hippocampal neurons subjected to OGD/R induction with or without the presence of daphnetin. *P < 0.05 relative to control cells without OGD/R induction; #P < 0.05 relative to OGD/R–induced cells without daphnetin treatment. LDH, lactate dehydrogenase; OGD/R, oxygen-glucose deprivation/reoxygenation
3.2 Daphnetin represses OGD/R–induced oxidative stress in hippocampal neurons
The effect of daphnetin on OGD/R–induced oxidative stress was investigated by detecting the levels of ROS and MDA, as well as the activities of SOD and GPx. ROS and MDA production was significantly increased in the OGD/R–induced cells relative to the control cells. However, the induction caused by OGD/R was markedly inhibited by daphnetin in a dose-dependent manner (Figures 2A and 2B). The activities of SOD and GPx were lower than that in the control cells. Pretreatment with daphnetin increased the activities of SOD and GPx when compared with the OGD/R–induced cells (Figures 2C and 2D).

Daphnetin represses OGD/R–induced oxidative stress in hippocampal neurons. Hippocampal neurons were subjected to OGD/R induction with or without the presence of daphnetin. The oxidative stress was evaluated through detecting the levels of ROS and MDA, as well as the activities of SOD and GPx. A, ROS production. B, MDA production. C, SOD activity. D, GPx activity. *P < 0.05 relative to control cells without OGD/R induction; #P < 0.05 relative to OGD/R–induced cells without daphnetin treatment. GPx, glutathione peroxidase; MDA, measurement of malondialdehyde; OGD/R, oxygen-glucose deprivation/reoxygenation; ROS, reactive oxygen species; SOD, superoxide dismutase
3.3 Daphnetin suppresses OGD/R–induced cell apoptosis in hippocampal neurons
We next tested whether daphnetin affects the cell apoptosis of hippocampal neurons after OGD/R stimulation with an ELISA-based cell death detection kit. Figure 3A shows that the percentage of apoptotic cells was higher in the OGD/R–induced cells as compared to the normal cells. After daphnetin pretreatment, the percentage of apoptotic cells was decreased relative to the OGD/R-induced cells. Moreover, we detected the expressions of bax, bcl-2, and cleaved caspase-3 with Western blot analysis. Figure 3B shows that OGD/R induction caused significant increases in the expressions of bax and cleaved caspase-3, while it led to a reduction in the expression of bcl-2. Daphnetin pretreatment mitigated the changes in the expressions of bax, bcl-2, and cleaved caspase-3, which were caused by OGD/R induction.
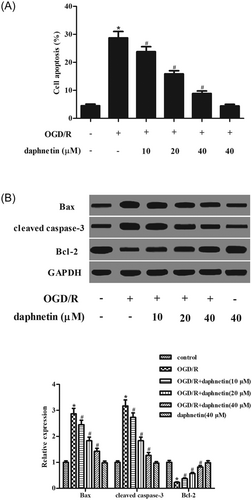
Daphnetin suppresses OGD/R–induced cell apoptosis in hippocampal neurons. A, The cell apoptosis of hippocampal neurons was analyzed by the ELISA-based cell death detection kit. B, The expressions of apoptotic related proteins including bax, bcl-2, and cleaved caspase-3 were measured by Western blot analysis. *P < 0.05 relative to control cells without OGD/R induction; #P < 0.05 relative to OGD/R–induced cells without daphnetin treatment. ELISA, enzyme-linked immunosorbent assay; OGD/R, oxygen-glucose deprivation/reoxygenation
3.4 Daphnetin induces the activation of Nrf2/HO-1 pathway in hippocampal neurons induced by OGD/R
To explore the effect of daphnetin on the activation of the Nrf2/HO-1 pathway, the expressions of Nrf2 in nuclear fraction, and the HO-1 in the whole cell lysates were measured by Western blot analysis. As indicated in Figure 4A, the expression of Nrf2 nucleus was increased in the OGD/R–induced hippocampal neurons, indicating that OGD/R slightly induced the nuclear translocation of Nrf2. The HO-1 expression in the whole cell lysates was increased in the OGD/R–induced hippocampal neurons compared with the control cells. However, daphnetin elevated the nuclear translocation of Nrf2 and HO-1 expression relative to the OGD/R–induced hippocampal neurons.
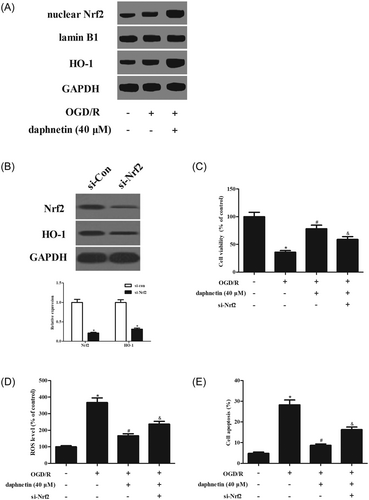
Daphnetin induces the activation of the Nrf2/HO-1 pathway in OGD/R OGD/R–induced hippocampal neurons. A, The expressions of Nrf2 and HO-1 were measured by Western blot analysis. B, Transfection efficiency was tested using Western blot analysis after transfection with si-Nrf2 or control siRNA (si-con). C, Cell viability. D, ROS production. E, Cell apoptosis. *P < 0.05 relative to control cells without OGD/R induction; #P < 0.05 relative to OGD/R–induced cells without daphnetin treatment; &P < 0.05 relative to OGD/R–induced cells transfected with si-con. HO-1, heme oxygenase-1; Nrf2, nuclear factor erythroid 2-related factor 2; OGD/R, oxygen-glucose deprivation/reoxygenation; ROS, reactive oxygen species; si-Nrf2, small interfering RNA targeting Nrf2
To further evaluate the role of the Nrf2/HO-1 pathway, the Nrf2 was silenced by si-Nrf2. Western blot analysis showed that the expressions of Nrf2 and HO-1 were dramatically decreased after transfection with si-Nrf2 (Figure 4B). Si-Nrf2 attenuated the inhibitory effects of daphnetin on cell viability, ROS production, and cell apoptosis (Figures 4C-E). The results indicated that the Nrf2/HO-1 pathway was implicated in the neuroprotective effects of daphnetin.
4 DISCUSSION
I/R injury is a pathological phenomenon in which the blood supply returns to normal levels after a period of ischemia.14 Ischemia leads to the lack of oxygen and nutrients, and the following restoration of circulation results in the cell injury or death through the oxidative-related pathway.14 Reperfusion of the ischemic tissues produces a large amount of reactive oxygen and nitrogen species, leading to redox signaling disruption and permanent mitochondrial lesion, and finally resulting in cell damage and apoptosis.15 Oxidative stress has been considered to be a major contributor to I/R injury. Accumulating evidence proves that inhibition of oxidative stress may contribute to the attenuation of I/R injury.16, 17 In the present study, we used an OGD/R model to simulate cerebral I/R injury in primary hippocampal neurons. As expected, OGD/R stimulation caused cytotoxicity, oxidative stress, and apoptosis in hippocampal neurons.
Daphnetin is a coumarin derivative extracted from Daphne odora var., which is a traditional Chinese medicine commonly used for diminishing inflammation. Daphnetin has been demonstrated to have strong anti-inflammatory and antioxidative properties. Yang et al10 reported that daphnetin prevents N-methyl-d-aspartate induced cell loss and apoptosis in primary cultured cortical neurons. Daphnetin significantly increases neurite outgrowth and promotes neuronal survival in primary cultured rat cortical neurons.18 Daphnetin attenuates hydrogen peroxide-induced cell apoptosis and improves cell morphology in neuronal-like rat pheochromocytoma PC12 cells.8 These findings suggest that daphnetin has neuroprotective effects. In addition, daphnetin has been proved to prevent cerebral ischemic injury in mice.10 In a middle cerebral artery occlusion and reperfusion (MCAO/R) model, daphnetin treatment improved neurological score and infarct size in mice.12 Daphnetin also inhibited the inflammation response and neural cells apoptosis in MCAO/R mice.12 In another in vivo study, daphnetin reduces infarct volume and improves neurological deficits in MCAO/R mice.19 Besides, daphnetin also reduces infarct volume in a hypoxia/ischemia neonatal rat model.19 Additional studies indicate that daphnetin executes its neuroprotective effect via elevating cellular antioxidative activity.19 In accordance with previous studies, in the current study, we found that daphnetin attenuated OGD/R–caused oxidative stress, cell damage, and apoptosis in hippocampal neurons, which suggested that daphnetin might ameliorate cerebral I/R injury.
Nrf2 is a basic leucine zipper transcription factor that controls the expressions of many proteins including antioxidant proteins, drug transporters, detoxifying enzymes, and numerous cytoprotective proteins.20 Nrf2 is normally bound to Kelch-like erythroid Cap “n” collar homologue (ECH)-associated protein 1 (Keap1), which is a cytoskeleton binding protein.3 Under specific conditions, the Nrf2-Keap1 complex is disrupted. Then the dissociative Nrf2 translocates to the nucleus, and binds to the promoter sequence “antioxidant responsive element (ARE),” thereby leading to the upregulation of target genes. The Nrf2/HO-1 pathway is a crucial pathway that is involved in oxidative stress.20 Daphnetin exposure suppresses tert-butyl hydroperoxide-induced oxidative damage and cell apoptosis.21 Daphnetin upregulated the Keap1-Nrf2/ARE signaling pathway, and the protective effects are mostly blocked by Nrf2 knockout.21 Mohamed et al22 report that daphnetin induces the nuclear translocation of Nrf2, thereby inducing the expression and activity of HO-1. These results suggest that daphnetin ameliorates oxidative injury via activating the Nrf2/HO-1 pathway.22 Our results showed that daphnetin enhanced the activation of the Nrf2/HO-1 pathway in OGD/R–induced hippocampal neurons. We further demonstrated that the Nrf2/HO-1 pathway was implicated in the neuroprotective effects of daphnetin. However, the molecular mechanism of activating the Nrf2/HO-1 pathway in response to daphnetin pretreatment remains unclear, and further investigation is needed.
In summary, we explored the role of daphnetin in an in vitro OGD/R model in primary hippocampal neurons. The results showed that daphnetin attenuated OGD/R–caused oxidative stress, cell damage, and apoptosis in hippocampal neurons. Daphnetin enhanced the activation of the Nrf2/HO-1 pathway in OGD/R–induced hippocampal neurons. Transfection with si-Nrf2 abolished the protective effect of daphnetin. These data indicated that daphnetin protected hippocampal neurons from OGD/R–induced cell damage through the activation of the Nrf2/HO-1 pathway.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.