Investigation of follicular helper T cells, as a novel player, in preeclampsia
Abstract
Preeclampsia (PE) is characterized by hypertension and proteinuria. It occurs in an around 3% to 5% of all pregnancies worldwide. The fetus is kind of semiallograft to the maternal host; immune system components encounter fetal antigens and develop adverse immune responses. Recently, it has been observed that the immune system plays an important role in PE. In the current study, we have tried to investigate the role of follicular helper T (Tfh) cells in the pathogenesis of PE. Blood samples of 49 PE women and 50 healthy controls were collected. Peripheral blood mononuclear cells were isolated, cells were cultured, and then RNA was extracted. Autoantibody and secretory cytokine levels were analyzed by ELISA. Tfh frequency and transcription levels of the related molecules and cytokine were assessed by flow cytometry and real-time PCR, respectively. The frequency of circulating Tfh cell in PE women was significantly higher compared with the healthy pregnant woman (Tfh cells with CD4+ICOS +, P = 0.0064 and Tfh cells with CD4 +CXCR5 +, P = 0.029). Moreover, mRNA expression levels of CXCR5, BCL6, IL-21, and IL-6 ( P = 0.0006, P = 0.008, P = 0.0063, and P = 0.027, respectively) were upregulated in PE patients. Furthermore, IL-6 ( P = 0.0014) and IL-21 ( P = 0.0059) levels in both group were assayed and the results showed increased in patient group. We also measured autoantibody levels including antiphospholipid antibodies ( P = 0.0001), anticardiolipin antibodies ( P = 0.0004), anti-TPO ( P = 0.0008), anti-TG ( P = 0.001) in circulation of PE group, which were higher than the control group. This study provided insights into the involvement of Tfh cells in etiology and pathogenesis of PE, probably by developing autoantibodies.
1 INTRODUCTION
Preeclampsia (PE) is a relatively common multisystem human disorder, affecting about 3% to 5% of pregnancies, and it is considered to be a clinical illness only in the last stage.1, 2 It initiates in the placenta and causes different maternal and fetal problems. At worst stages, it may threaten maternal and perinatal survival. PE is characterized by hypertension with proteinuria related to the renal lesion, named as glomerular endotheliosis, in which endothelial fenestrations are lost and the endothelial cells of the glomerulus swell. Other consequences are liver dysfunction, neurological and hematological complications, placental abruption, intra-abdominal bleeding, pulmonary edema, acute renal failure, and ascites, all of which are severe clinical indexes related to PE. Studies have also revealed that immunological changes in the placental microenvironment probably play an important role in the initiation of PE.1, 3 The etiology of PE is still unknown, but there exist several hypotheses in this regard; the main pathogenesis of PE is low invasion of spiral arteries by endovascular cytotrophoblast cells, resulting in increased release of decidual cytokines, secretion of free radical species, and proteolytic enzymes, leading into endothelial cell dysfunction.4
Some epidemiological data and animal models have clarified the involvement of innate and adaptive immune response in PE. First, the systemic inflammation occurs and it induces endothelial disruption, indicating the initiation of PE. Activation of T cells and chronic presence of inflammation that attenuate regulatory T (Treg) cells function, cause impairments in tolerance during pregnancy.5 Helper T (Th) 1 and Th2 imbalance has also been indicated in PE. It is actually the predominant effect of Th1 and its cytokines that develop a proinflammatory state.6 Additionally, Th17/Treg imbalance with an increase in Th17 cells and a decrease in Treg cells has been reported in PE women.7, 8 Moreover, the levels of proinflammatory cytokines, adhesion molecules, and chemokines in the maternal circulation are unregulated.9, 10 A recent study about transcription factors revealed that T-bet (related to Th1) and RORc (related to Th17) was significantly increased in women with PE compared with healthy pregnant women. On the other hand, GATA-3 (related to Th2) and FoxP3 (related to Treg) were significantly decreased.11
As a novel and distinct lineage of CD4+ T cells, follicular helper T (Tfh) cells were first observed in human lymphoid tissues (tonsils). These cells express some special molecules and factors, including CXC chemokine receptor 5 (CXCR5), a surface receptor named inducible co-stimulatory molecule (ICOS), the master transcription factor B-cell lymphoma 6 (BCL6), CD40 ligand (CD40L), programmed death-1 (PD-1), and secretory cytokines, such as interleukin (IL)-21 and IL-6.12, 13 The main function of Tfh cells is defined as the generation of a T cell-dependent B cell response.14 In the absence of Tfh cells, protective antibody responses are decreased, as evidenced in patients with a genetic mutation in ICOS gene. Although Tfh cells are crucial for protective antibody responses.15, 16 Recent evidence showed that Tfh-cell dysfunction can lead to numerous immune diseases, like immunodeficiency, autoimmunity, and gestation problems.17, 18 Something else, there is still unclear and limited information about Tfh cells and pregnancy problems. A study showed that there is a linkage between Tfh and its cytokine (IL-21) in immunological infertility; it may happen during the generation of some antibodies. It was also observed that Tfh population in peripheral blood of immune infertile patients was significantly higher than healthy pregnant women.19 In a novel study in 2017, Xiaorui Luan et al20 revealed that Tfh significantly increased in recurrent spontaneous abortion (RSA) patients. Although some finding revealed that Tfh cells might have a role in the maintenance of a successful allogeneic pregnancy, Tfh cells role and characteristics during pregnancy have not been clearly reported yet.
Notably, Tfh cells play key roles in B cells differentiation into long-lived plasma cells and memory B cells.20 Considering this, we hypothesized that Tfh cells might affect various aspects of immune system in PE. Due to the fact that studies about Tfh and PE with respect to immune system are not clear and controversial, therefore, we conducted the current study to scrutinize the issue in a partial way.
2 MATERIALS AND METHODS
2.1 Patients and healthy controls
In total, 49 women with PE and 50 normal pregnant women at the gestational age, who were hospitalized in Alzahra Hospital of Tabriz University of Medical Sciences, were recruited in our study. The study was approved by the Research Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1396.724). An informed consent was taken from the participants former to the enrollment. Inclusion criteria were pregnancy with PE as complication, blood pressure ≥140/90 mm Hg observed after 20 weeks of gestation, proteinuria of ≥300 mg/24 or at least “++” by semiquantitative dipstick testing in the absence of urinary tract infection. Exclusion criteria were diabetes mellitus, gestational diabetes mellitus, chronic hypertension predating pregnancy, renal diseases, and any infectious and chronic diseases.21 Patients’ demographic data are summarized in Table 1.
| Parameters | Control (n = 50) | Preeclampsia (n = 49) | P value |
|---|---|---|---|
| Patient age, y | 28.9 ± 4.3 | 31.2 ± 3.9 | – |
| Gestational age, wk | 39.6 ± 1.2 | 37.8 ± 2.35 | – |
| Mean systolic pressure, mm Hg | 112 ± 12.4 | 149.6 ± 14.4 | 0.003 |
| Mean diastolic pressure, mm Hg | 71.2 ± 16.2 | 93.5 ± 21.7 | 0.008 |
| Proteinuria (dipstick) | – | 1+ -4+ | – |
| Birth weight of fetus (g) | 3325.3 ± 315.8 | 2579.3 ± 779.4 | 0.0005 |
| Antiphospholipid antibodies, n (%) | 0 (0) | 9 (18.36) | 0.0001 |
| Anticardiolipin antibodies, n (%) | 1 (2) | 8 (16.32) | 0.0004 |
| Anti-TPO | 0 (0) | 4 (8.16) | 0.0008 |
| Anti-TG | 0 (0) | 3 (6.12) | 0.001 |
| Total antibodies, IgG (mg/dL) | 1165 ± 593 | 1955 ± 1154 | 0.0001 |
| Total antibodies, IgM (mg/dL) | 144 ± 107 | 290 ± 141 | 0.0001 |
- Abbreviations: Anti-TG, antithyroglobulin; anti-TPO, antithyroid peroxidase.
2.2 Blood sample and cell isolation
Heparinized peripheral blood samples (10 mL) were obtained from pregnant women in sterile conditions. Blood samples were then diluted 1:1 in 10 mL phosphate-buffered saline (Sigma-Aldrich, Schnelldorf, Germany). Peripheral blood mononuclear cells (PBMCs) were separated freshly from the blood samples by standard Ficoll-Hypaque 1.077 g/mL (Biosera, Heathfield, East Sussex, UK) centrifugation at 450 g for 25 minutes. PBMCs were selected from the interlayer and then were washed with Roswell Park Memorial Institute (RPMI) 1640 medium (Sigma-Aldrich). Cells were immediately cultured in RPMI 1640 culture medium with 10% fetal calf serum (Sigma, St. Louis, MO) and phorbol myristate acetate (PMA; Sigma) in a concentration of 10 ng/mL for subsequence experiments. The cultured cells were used for RNA extraction and the supernatant was exploited for cytokine (IL-6 and IL-21) assessment using enzyme-linked immunosorbent assay (ELISA).
2.3 Gene expression analysis
Messenger RNA (mRNA) expressions of the CXCR5, BCL6, IL-6, and IL-21 genes were measured by quantitative real-time polymerase chain reaction (PCR) technique, with specific forward and reverse primers. Sequences of the primers are listed in Table 2. Total RNA was isolated with RNX-PLUS Solution (SinaClon, Tehran, Iran), and complementary DNA was synthesized using RevertAid Reverse Transcriptase Kit (Thermo Fisher, Waltham, MA). Reactions were performed using the LightCycler 2.0 Real-Time PCR System Machine (Roche Applied Science, Penzberg, Upper Bavaria, Germany) and SYBR Green qRT-PCR Master Mix method. The thermocycling was conducted as follows: holding at 95°C for 600 seconds, 40 cycles of denaturation at 95°C for 10 seconds, annealing and extending at 60°C for 20 seconds, then followed by 72°C for 20 seconds, 95°C for 10 seconds, 65°C for 60 seconds, and 97°C for 1 second. The PCR reactions were performed in a 15-μL reaction volume. Here, we used GAPDH as a housekeeping gene and endogenous control. Results were assayed by comparative Ct method and relative amounts of the target gene were calculated by formula. An electrophoresis analysis on 2% agarose gel and DNA sequencing by Biosystems (Seqlab, Göttingen, Germany) was performed to confirm the amplification.
| Gene | Primer | Sequence (5′ → 3′) |
|---|---|---|
| CXCR5 | Forward | CCCATAAGCTATAGACCCGAGGA |
| CXCR5 | Reverse | ACACTTCCCTGCCTAAGGTGA |
| BCL6 | Forward | CGACTTCACTTGCGCCAGA |
| BCL6 | Reverse | CTGGAGACGAAAGCATCAACAC |
| IL-6 | Forward | ACTCACCTCTTCAGAACGAATTG |
| IL-6 | Reverse | CCATCTTTGGAAGGTTCAGGTTG |
| IL- 21 | Forward | GGCAACATGGAGAGGATTG |
| IL-21 | Reverse | AAGCAGGAAAAAGCTGACCA |
| GAPDH | Forward | TGCACCACCAACTGCTTAGC |
| GAPDH | Reverse | GGCATGGACTGTGGTCATGAG |
- Abbreviations: BCL6, B-cell lymphoma 6; CXCR5, CXC chemokine receptor 5; IL, interleukin.
2.4 Determination of cytokines and antibodies
The supernatant of cultured PBMCs was used for IL-21 and IL-6 cytokines assay and serum concentration of anticardiolipin, antiphospholipid antibody (APA), antithyroglobulin antibodies (TG), and antithyroid peroxidase (TPO) antibody were determined by commercially available ELISA Kits (MyBiosource, Nivelles, Belgium). Isolated cells (at 1 × 106 cells/mL) were placed in RPMI 1640 culture medium with 10% fetal calf serum (Sigma). To stimulate cell proliferation, we added PMA (Sigma) in a concentration of 10 ng/mL of culture medium. PBMCs were incubated for 48 hours at 37°C in a humidified atmosphere with 5% CO2. Then, after 48 hours, we collected the supernatant for cytokines detection. All steps of ELISA assay were conducted according to the MyBioSource instructions.22
2.5 Flow cytometric analysis
We determined Tfh cells frequency by flow cytometry. Tfh cells population was evaluated by standard procedures, in which the cells were stained with fluorochrome-conjugated monoclonal antibody for CD4, CXCR5, and ICOS. Briefly, fluorochrome-conjugated monoclonal antibodies were added to 100 μL of whole blood and incubated 15 to 30 minutes in the dark at room temperature. Then 2 mL of 1× FACS Lysing Solution (Becton Dickinson, Franklin Lakes, NJ) was added and incubated for 10 minutes in the dark at room temperature. Subsequently, cells were washed with wash buffer and supernatant was removed. Follicular T-helper cells were detected using monoclonal antibodies against the surface antigens like anti-CD4-FITC (Becton Dickinson, Franklin Lakes) and anti-CXCR5-PE (Becton Dickinson, Franklin Lakes), and anti-ICOS-PE (Becton Dickinson, Franklin Lakes). Viable lymphocytes were gated based on their forward and side scatters profile. Results were analyzed using FACSCalibur flow cytometer with FLowJo software (Becton Dickinson, Mountain View, CA).
2.6 Statistical analyses
Statistical analysis was performed using SPSS PC statistics (version 19.0; SPSS Inc, Armonk, NY). Descriptive statistics for continuous data are expressed as the mean ± SD. Unpaired t-test was applied to compare the results of immunologic factors between control and PE group. P < 0.05 was considered to be statistically significant. For drawing the graphs, the GraphPad Prism version 7.00 for Windows (GraphPad Software, La Jolla, CA; www.graphpad.com) was used.
3 RESULTS
3.1 The expression of Tfh related genes and cytokines CXCR5/BCL6/IL-21/IL-6 levels in PE
Real-time PCR was used to determine whether the mRNA expression levels of CXCR5/BCL6/IL-21/IL-6 were changed in PE patients compared with healthy group (Figure 1). CXCR5 mRNA expression levels, respectively, were increased in PE women to 2.345 ± 1.097 (P = 0.0006) and expression level of BCL6 mRNA was higher 2.281 ± 1.427 (P = 0.008). mRNA expression level of Tfh-related cytokines (IL-21, IL-6) were also increased significantly in PE women compared with healthy controls (1.911 ± 0.9663, P = 0.0063 and 1.695 ± 0.9351, P = 0.027, respectively).
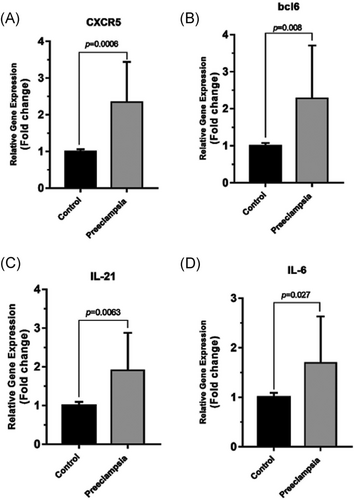
The mRNA expression levels of follicular helper T cells-related factors, CXCR5, BCL6, IL-21, and IL6 were measured by quantitative real-time polymerase reaction in the healthy control group (normal pregnant women) and PE women. A, B, The mRNA expression levels of CXCR5 and BCL6 were significantly higher in PE women compared with healthy control group (P = 0.0006, P = 0.008, respectively). C, D, mRNA expression levels of IL-21 and IL6 were increased significantly in PE women compared with healthy control group (P = 0.0063, P = 0.027, respectively). The results were normalized to GAPDH. Results are given as mean ± SD (healthy control group, n = 50; PE women, n = 49). P < 0.05 considered statistically significant. BCL6, B-cell lymphoma 6; CXCR5, CXC chemokine receptor 5; IL, interleukin; mRNA, messenger RNA; PE, preeclampsia
3.2 Tfh cytokines levels (IL-21, IL-6)
Evaluation of associated cytokines of Tfh in the stimulated supernatant of PBMCs isolated from the PE and control groups indicated that IL-6 (9.690210 ± 6.553 pg/mL vs 18.51 ± 9.346 pg/mL; P = 0.0014) and IL-21 (72.00 ± 50.57 pg/mL vs 129.0 ± 71.15 pg/mL; P = 0.0059) secretion were higher significantly in PE women compared with the healthy control (Figure 2).
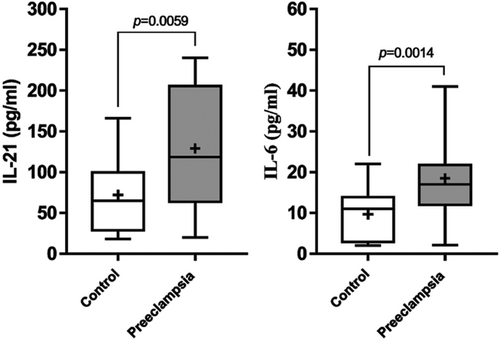
The secretion levels of cytokines evaluated using enzyme-linked immunosorbent assay in healthy control group (normal pregnant women) and PE women. The secretion levels of IL-21 and IL-6 in healthy control group were significantly in lower levels in compared with PE women (P = 0.0059, P = 0.0014, respectively; healthy control group: n = 50; PE women: n = 49). P < 0.05 is considered statistically significant. PE, preeclampsia
3.3 Autoantibody levels
Table 1 shows the results of women who were tested for anticardiolipin, antiphospholipid, anti-TPO, and anti-TG antibodies. The percentage of these antibodies was ranged from 0% to 1% in the healthy group of pregnant women, while it was significantly increased for all of the autoantibodies, APA (P = 0.0001), anticardiolipin antibodies (P = 0.0004), anti-TPO (P = 0.0008), anti-TG (P = 0.001) in PE group. We also evaluated total IgG (P = 0.0001) and IgM (P = 0.0001) in the PE and control group. The total antibody was upregulated in patient group.
3.4 The frequency of circulating Tfh cells
The frequency of Tfh cells with CD4+CXCR5+/ICOS+ phenotype from peripheral blood of PE women was measured by flow cytometry technique. Results revealed a significant increase in Tfh cells frequency in PE group compared with the control group. The frequency of Tfh cells with CD4+ICOS+ was significantly higher in PE patients compared with the control group (8.071 ± 4.070% vs 6.075 ± 2.928%, P = 0.0064). Furthermore, the frequency of CD4+CXCR5+ was also increased in PE patients compared with the control group (2.691 ± 1.973% vs 1.982 ± 1.104%, P = 0.029). Our findings demonstrated increased proportion of the Tfh cells in the PE patient (Figure 3).
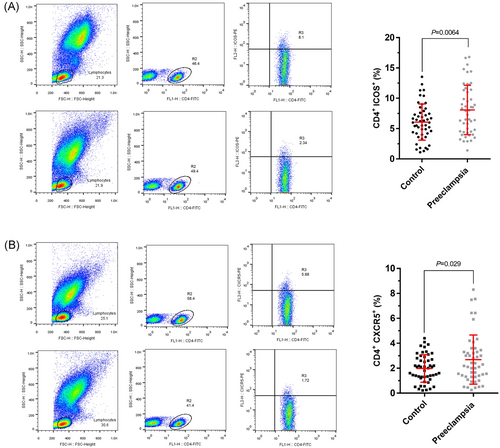
The frequency of Tfh cells in healthy control group (normal pregnant women) and PE women. Lymphocytes are gated on the forward scatter of living cells and then centered on CD4+ T cells, ICOS+ and CXCR5+ cells. A, The percentage of CD4+ICOS+ (Tfh) cells among the total CD4+ T cell population is indicated. Representative dot plots demonstrating the analyzing method used for enumeration of CD4+, ICOS+ (Tfh) cells is shown. There was a significant increase in the frequency of CD4+ICOS+ (Tfh) cells (P = 0.0064) in PE women compared with healthy control group. B, Representative results from the flow cytometry analysis of the samples obtained from PE women and healthy control subjects. The percentage of CD4+CXCR5+ (Tfh) cells among the total CD4+ T cell population is indicated. A representative dot plot demonstrating the analyzing method used for enumeration of CD4+, CXCR5+ (Tfh) cells is shown. There was a noticeable increase in the frequency of CD4+CXCR5+ (Tfh) cells in PE women compared with healthy control group (P = 0.029). Results are given as mean ± SD of three independent experiments (healthy control group: n = 50, PE women: n = 49). P < 0.05 is considered statistically significant. ICOS, inducible co-stimulatory molecule; PE, preeclampsia; Tfh, follicular helper T cell
4 DISCUSSION
Even though most pregnant women deliver the healthy baby today, but still there exist human pregnancy-specific disorders like PE. As mentioned earlier, PE happens in almost 3% to 5% of pregnancy worldwide and its mortality is observed in both fetus and mother.5
The past decades of studies demonstrated the etiological reasons for PE, especially immunological factors. In this study, we decided to investigate the Tfh cell as an important immune cell in PE. Here, for the first time we investigated Tfh cells frequency in PE women, and we showed that the Tfh cells in peripheral blood of PE women were significantly increased compared to healthy pregnant women.
Since the fetus is considered as a semiallograft to the maternal immune system. The maternal immune-competent cells do not communicate with the fetus directly, they encounter fetal antigens. At the first step, antigen-presenting cells recognize fetal antigens and then release some specific transcription factors and cytokines, leading to the activation of the naive CD4+ Th cells. These cell then differentiate into effector cells, such as Th1, Th2, Th17, Treg cells, and Tfh cells.23 Th1 cells are characterized by the secretion of cytokines, such as IL-2, interferon-γ, and tumor necrosis factor, while Th2 cells release IL-4, IL-5, IL-10, and IL-13. The findings revealed the imbalance in Th1/Th2 ratio in PE women. It means that promoted Th1 mediated immunity via subsided Th2 mediated immunity may be involved in PE pathogenesis.24 Moreover, Th17 cells produce IL-17, which is associated with inflammatory state. Upregulation of Th17 cells leads to chronic inflammatory diseases or probably induces allergic disorders. In PE studies, the predominant effect of Th17 cells and the reduction of Treg cells have clearly been depicted.8 These findings demonstrated the great role of immunological factors in development of PE, and our data deliver first proof that the circulating Tfh cells were increased in PE patients, presenting that Tfh cells may be involved in the pathogenesis of PE.
Tfh cells express some specific molecules and secrete some cytokines. Tfh are CD4+BCL6+ICOS+CXCR5+ and release IL-21 and IL-6. Tfh approximately 15% of CD4+ T cells in germinal center (GC) express BCL6 protein, which is also expressed on B cells in GC and inhibits differentiation of B cells to plasma cells. Bcl-6-deficient mice show impaired antibody maturation and produced antibody with limited affinity.15 BCL6 expression is regulated by IL-6 and IL-21.25 Tfh cells also secrete IL-6 that plays a role in inflammation process. It was demonstrated that systemic inflammation was related to the pathogenesis of PE. CXCR5 is another important factor of Tfh cell that was evaluated in this study. It is a chemokine receptor that helps Tfh to enter the GC through binding to CXCL13.
Here in addition to evaluating Tfh cells frequency, we measured related Tfh cells genes and cytokines. In accordance to increase in Tfh cells frequency in PE women compared with healthy pregnant women, an increase in mRNA expression levels of CXCR5, BCL6, IL-21, and IL-6 were found in patient group. Also higher levels of IL-21 and IL-6 were secreted in supernatant of cultured PBMCs of PE patients. So, according to this data we can assume that Tfh cells are associated with the pathogenesis of PE.
The nature of Tfh cells in lymphoid organs, mainly in rat models, has been largely studied during the past decades. Despite significant advances about biology and functions of these cells, blood circulating CXCR5+CD4+ T cells in humans has yet to be characterized in large part.26 Earlier studies have revealed that defective mechanisms of antibody production, including the probable disorder in Tfh cells function, can play an important role in various pathological conditions. These cells were found be upregulated in autoimmune diseases, including systemic lupus erythematosus, Sjogren’s syndrome, rheumatoid arthritis, autoimmune thyroid diseases, and also transplant rejection.26
Studies about transplantation also revealed some findings about Tfh cells effects. Antibody-mediated responses are the main reason for transplant rejection and failed allografts. For antibody production, the interaction between antigen-specific B cells and Tfh cells is crucial. Tfh cells stimulate B cells through secreting IL-21. It was observed that neutralization of IL-21 pathway contributed to the long-term transplantation procedure via inhabitation of B-cell function.27 Animal data revealed that Tfh cells and B cells migrate to the allograft and generate alloantibody in lymphoid organ around the allograft. In addition, some data supported the same reaction within human allografts.28
Using allogeneic-normal-pregnant mouse model, the effect of Tfh-like cells in the uterus and placenta was indicated.29 Moreover, memory Tfh cells were increased in women with RSA.20 Normal pregnant women showed upregulation of Tfh cells, suggesting that these cells might have a role in pregnancy.
The increase of Tfh cells and the subsequent generation of more antibody than regular pregnancy lead to the disruption of the natural process of pregnancy. Additionally, IL-21, which is an important cytokine of Tfh cell, is an essential factor that contributes to generating antibody in GC. Overall, dysregulation in humoral immune system may lead to pregnancy disorder. Previous study demonstrated the presence of autoantibodies in several pregnancy conditions such as PE.
We are interested in the relationship between the circulating Tfh cells and autoantibodies in PE. It has been observed that these antibodies effect on PE progression through various mechanisms. APA aggregates platelet and prevents normal process of clotting.30 Antithyroid antibodies like TG antibodies and TPO might be a marker of maternal immunological changes that induce T cells activity, which in turn may be responsible for pregnancy-related disorders.31 The results about evaluating of autoantibodies showed that the increased level of APA, anti-TPO, anti-TG, and anticardiolipin antibody correlated positively with the frequency of circulating Tfh cells. Also further studies are still needed to assess whether these antibodies actually play a pathogenic role in PE, and if they do, how these antibodies can lead to this disorder. According to the above-mentioned evidence and our results, it can be implied that Tfh cells might interact with B cells and facilitated autoantibody production in PE.
In conclusion, our data indicated, for the first time, that Tfh cells may participate in the pathogenesis of PE. Moreover, increased number of these cells might be associated with activated B cell, which has an effect on the maternal immune system. However, there are several reasons for PE to be developed. This study suggested that Tfh cells may be involved in the pathogenesis of PE and that their regulation could be an appropriate strategy to make better outcomes for both mother and fetus. Nonetheless, more investigation about these cells and their effects on PE are still necessary to clarify the molecular mechanism of their involvement in etiopathogenesis of PE. Generally, although total Tfh cells in blood are not indicative of disease activity, but increased number of the cells might be related to higher autoantibody levels and higher disease activity.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
AUTHOR’S CONTRIBUTION
Contributed to study design, wrote the paper, helped with data collection, and performed statistical analyses: HH; helped with data collection and performed statistical analyses, SE-F: was responsible for manuscript revision and helped with data collection: MA; contributed to cell collection and helped with immunological tests: LA-M, SD, AAM; participated in the final edition of the manuscript: FJ-N; responsible for subject selection, monitoring, and inpatient and outpatient care: SD, FM; contributed to flow cytometric analysis: MT, SR; designed and supervised the project: MY. All authors discussed the results and contributed to the final manuscript.