Berbamine ameliorates isoproterenol-induced myocardial infarction by inhibiting mitochondrial dysfunction and apoptosis in rats
Abstract
Berbamine (BBM), a bisbenzylisoquinoline alkaloid from roots, bark, and stem of Berberis plant such as Berberis aristata has a wide range of pharmacological activities. However, the evidence for the cardioprotective effect of BBM is inadequate and the molecular mechanism of BBM remains unclear. This study investigated the underlying molecular mechanism of BBM-mediated cardioprotection on isoproterenol (ISO)-induced mitochondrial dysfunction and apoptosis in rats. The assays of mitochondria antioxidant status, mitochondrial marker enzymes, and electron microscopic analysis of mitochondria revealed BBM significantly prevented the mitochondrial dysfunction induced by ISO. The ISO-induced elevation of mitochondrial oxidative stress was also curbed by BBM. Furthermore, pretreatment with BBM protected the heart tissue from ISO-induced apoptosis as evident from decreased terminal dUTP nickend-labeling positive cells and decreased expression of Bax, cytochrome c, cleaved caspase-9, and caspase-3, and poly (ADP-ribose) polymerase and increased expression of Bcl-2 in ISO-induced rats. These current findings suggest that BBM exerts a significant cardioprotective effect on ISO-induced myocardial infarction in rats.
1 INTRODUCTION
Myocardial infarction (MI) remains a serious health problem in both developing and developed countries, thus affects a high proportion of the population worldwide. It makes a major contribution toward the mortality statistics.1 As an acute condition of necrosis of the myocardium, MI occurs when the blood supply to a part of the heart is interrupted, causing damage to heart tissue.2 Even though various treatment strategies have been developed to minimize the risk of MI, but there is no effective remedy for MI. Thus, there is a need to find a new drug to improve the MI.
The pathophysiological consequence of heart disease thus involves mitochondrial dysfunction associated with the excessive generation of oxidative stress. Mitochondria are the principal source of energy generating processes within the cell. It is highly susceptible to free radical generation inside the cells. The mitochondrial electron transport chain (ETC) generates reactive oxygen species (ROS) and reactive nitrogen species in certain conditions such as ischemia, drug, or chemical-induced cell damage.3 Thus, ROS generation in large amounts leads to structural changes and myocardial necrosis of the heart tissue. Punithavathi and Prince4 reported that the lack of oxygen supply during infarction caused damage to the mitochondrial ETC, which results in accumulation of toxic metabolites, adenosine triphosphate (ATP) depletion, intracellular Ca2+ overload, mitochondrial membrane depolarization, mitochondrial swelling, and finally cell death.
Apoptosis, a type of cell death is triggered by several physiological regulators on exposure to various stimuli. A study conducted by Saraste et al5 demonstrated that cardiomyocytes underwent apoptosis during hypoxia, MI, heart failure, and ischemia/reperfusion (I/R). Accumulating evidence reported that mitochondria plays an important role in the execution of cell apoptosis. The sequence of the mitochondrial apoptotic pathway can be triggered by Bcl-2 family proteins causing mitochondrial permeability transition, mitochondrial depolarization resulting in the release of cytochrome c from mitochondrial membrane. These events provoke caspase activation which results in cell death.6 Isoproterenol (ISO) is a synthetic catecholamine and β-adrenergic agonist, which affects the myocardium resulting in infarction following free radical generation. The previous study conducted by Mitra et al7 showed that ISO-induced cardiotoxicity in rats was mediated by apoptosis. It has been reported that oxidative stress, mitochondrial dysfunction, and apoptosis are the main pathologic events that lead to myocardial damage.8, 9
Berberis aristata (Daruharidra) is commonly known as the Indian Barberry and tree turmeric, is widely used in traditional medicine to treat malaria, bleeding, fever, skin and eye infections, diarrhea, and hepatitis for a long time. It is a spinous shrub found growing in the sub-Himalayan regions and Nilgiri Hills of southern India.10, 11 Berbamine (BBM), a major bisbenzylisoquinoline alkaloid has been isolated from the stem, bark, and roots of B. aristata (Berberidaceae).12, 13 BBM has been reported to have several medicinal properties such as antiarrhythmic, antitumor, antipyretic, and anti-inflammatory effects. Further, cardioprotective, antihypertensive, and immunomodulatory properties of BBM have also been reported.12, 14, 15 According to Zheng et al,15 I/R injury was protected by BBM in rats through modulation of autophagy.
Therefore, the current investigation was undertaken to demonstrate the effect of BBM on ISO-induced mitochondrial dysfunction and apoptosis by assessing ROS generation, the involvement of mitochondrial antioxidant status and expression of apoptotic markers in rats. ISO-induced cardiotoxicity is also evaluated by DNA fragmentation. Moreover, the protective effect of BBM on ISO-induced mitochondrial damage was analyzed through ultra structural studies. The current study demonstrates that BBM pretreatment maintains mitochondrial integrity through the restoration of mitochondrial antioxidant status, upregulation of antiapoptotic protein such as Bcl-2 and downregulation of apoptotic proteins such as Bax, cytochrome c, cleaved caspase-9, cleaved caspase-3, and poly (ADP-ribose) polymerase (PARP) in ISO-treated rats. This is the first study that demonstrated the cardioprotective effect of BBM in ISO-induced MI in rats.
2 MATERIALS AND METHODS
2.1 Chemicals
BBM dihydrochloride, ISO, DiOC6, and dihydro dichlorofluorescein diacetate (DCF-DA) were obtained from Sigma-Aldrich (St Louis, MO) and all other analytical grade chemicals were obtained from Hi-Media Laboratories (Mumbai, India). Primary monoclonal antibodies for caspase-9, caspase-3, and cytochrome c were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). All other primary antibodies used were obtained from Cell Signaling Technology. The nitrocellulose membrane was purchased from GE Healthcare (Mumbai, India). Western blot development kit (diaminobenzidine [DAB]/H2O2 system) was purchased from Genei (Bangalore, India).
2.2 Animals
The whole experiment was carried out using male Wistar rats. Rats were procured from Kerala Agricultural University (Mannuthy, India), weighing 180 ± 10 g each. Rats were allowed to have water ad libitum and pelleted rat chow (Sai Durga Private Limited, Bangalore, India). Animals were maintained at a temperature of 28 ± 2°C, with a normal 12-hours light/dark cycle. The guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals and regulations were strictly followed throughout the experiment. The experiment was approved by the Institutional Animals Ethics Committee (IAEC), PSG Institute of Medical Sciences and Research (ethical approval number: 209/2013/IAEC), India.
2.3 MI induction in rats
The experimental MI was induced in rats by administration of ISO (85 mg/kg body weight/day) subcutaneously for 2 days at the 24-hours interval.16
2.4 Pilot study for dose fixation of test BBM
A pilot study was conducted with four different doses of BBM (15, 20, 25, and 30 mg/kg body weight/day) in ISO-treated rats. The optimum dose of BBM was determined by estimating the activity of serum creatine kinase and cardiac lipid peroxidation (LPO). BBM at 25 mg/kg body weight/day was effective in the recovery of biochemical alterations (data not shown).
2.5 Experimental design
After a week of acclimatization, rats were divided into four groups, each containing six rats. Group I (control) rats received standard diet for a period of 7 days and were given normal saline through subcutaneous injection for 2 days at the 24-hours interval. In group II animals, MI was induced with ISO administration (85 mg/kg body weight/day; dissolved in physiological saline) subcutaneously (on eighth and ninth day) for 2 days at the 24-hours interval. In group III, animals were injected with BBM (25 mg/kg body weight/day; dissolved in physiological saline) intraperitoneally for 7 days. In group IV, the animals were pretreated with BBM (25 mg/kg body weight/day) for 7 days followed by ISO administration at the dose mentioned above.
At the end of the experimental period, that is, 12 hours after the last injection of ISO, rats were anesthetized using ketamine (80 mg/kg body weight; intraperitoneal) and then killed by cervical decapitation. Blood (3 mL) was collected and serum was separated and used for biochemical assays. The dissected heart tissue was immediately washed in ice-cold saline. They were stained free of blood and tissue fluids. The heart tissue was weighed and preserved at −80°C for future works. For morphological studies, a small amount of tissue section was fixed in suitable fixatives.
2.6 Isolation of heart mitochondria
According to the method of Takasawa et al,17 we isolated the mitochondria from rat heart tissue. The heart tissue was homogenized in ice-cold 50 mM Tris-hydrochloric acid buffer (Tris-HCl; pH 7.4) containing 0.25M sucrose and then centrifuged for 20 minutes at 700g. The supernatant was centrifuged for 15 minutes at 9000g. Then, the pellets were washed with 10 mM Tris-HCl buffer (pH 7.8) containing 0.25M sucrose and finally suspended in the same buffer and stored at −20°C for further biochemical analysis.
2.7 Biochemical assays
2.7.1 Estimation of oxidative stress markers
Mitochondrial ROS production was measured by the method of Shinomol18 using the fluorescent dye DCF-DA. The level of thiobarbituric acid reactive substances in the heart mitochondrial fraction was estimated by the method of Ohkawa et al.19
2.7.2 Estimation of mitochondrial enzymatic and nonenzymatic antioxidant
The ISO-induced oxidative stress was assayed by estimating the activities of mitochondrial antioxidants such as superoxide dismutase (SOD),20 catalase (CAT),21 glutathione peroxidase (GPx),22 and glutathione-S-transferase (GST).23 The level of reduced glutathione (GSH) was estimated by the method of Moron et al.24
2.7.3 Estimation of heart mitochondrial marker enzymes
Effect of BBM on ISO-induced mitochondrial damage was investigated by analyzing the activities of mitochondrial enzymes such as isocitrate dehydrogenase (ICDH),25 α-ketoglutarate dehydrogenase (α-KGDH),26 succinate dehydrogenase (SDH),27 and malate dehydrogenase (MDH)28 were assayed. Also, the NADH dehydrogenase activity was assayed using the method of Minakami et al29 and cytochrome c oxidase by Pearl et al30 method.
2.7.4 Estimation of ATP and Ca2+ in heart mitochondrial fraction
Mitochondrial ATP concentration was quantified using hexokinase-glucose 6 phosphate dehydrogenase followed by NADP+ reduction fluorometrically by the method of Williamson and Corkey.31 The level of mitochondrial Ca2+ was measured by the o-cresolphthalein complexone (OCPC) method using a reagent kit (Agappe Diagnostic Limited, Kochi, Kerala, India).
2.8 Transmission electron microscopic study on heart mitochondria
The heart tissue was cut into small pieces and immediately fixed in 2.5% glutaraldehyde in 0.1M cacodylate buffer, pH 7.4, at 4°C for 12 hours. After fixation, the heart tissues were processed with 1% cacodylate-buffered osmium tetroxide, dehydrated with differently graded ethanol series and embedded in an epoxy resin. The ultrathin sections were placed on copper grids and stained with 2% uranyl acetate and 0.2% lead acetate. Then, the sections were examined using a transmission electron microscope (TEM).
2.9 Scanning electron microscopic study
For scanning electron microscopic (SEM) study, heart mitochondrial suspension was centrifuged and pellet was taken for further analysis. The pellet was fixed in 2.5% glutaraldehyde overnight. Once washed with phosphate-buffered saline (PBS), the pellet was dehydrated with a graded concentration of ethanol (50%, 70%, 80%, 90%, and 100%) for 10 minutes each. The pellet was immersed in pure tetra-butyl alcohol and stored in 4°C refrigerator until the tetra-butyl alcohol solidified. The frozen samples were dried by placing them in a vacuum bottle. Mitochondrial morphology was evaluated by SEM (Sigma SE2, Carl Zeiss, New York, NY).
2.10 Terminal deoxynucleotidyl transferase-mediated dUTP nickend-labeling assay
The assay is based on DNA end labeling using terminal deoxynucleotidyl transferase dUTP nickend labeling (TUNEL), which is subsequently detected using in situ cell death detection kit. The heart sections were incubated with proteinase K for 15 minutes at room temperature and then washed with PBS. The heart tissue was treated with 0.3% H2O2 at room temperature for 5 minutes and then incubated with TdT at 37°C for 1 hour. Tissues were then incubated for 10 minutes with streptavidin horseradish peroxidase (HRP). DAB solution was used for coloration. Finally, the specimens were counter stained with hematoxylin, washed with PBS. Apoptosis was expressed as a percentage of the total cells counted.
2.11 DNA fragmentation
To check the DNA fragmentation in heart tissue, agarose gel electrophoresis was done according to the method of Hebert et al.32 The heart tissue was homogenized using 5 mL of lysis buffer (50 mM Tris-HCl, pH 8.0, 10 mM NaCl, 10 mM EDTA, 100 mg/mL proteinase K, and 0.5% sodium dodecyl sulfate [SDS]) and incubated for 1 hour at 50°C. A volume of 10 mL of 100 mg/mL ribonuclease A (RNase A) was added to the mixture and incubated for an additional 1 hour at 50°C. Tissue samples were treated with 1 mL phenol followed by extraction with chloroform/isoamyl alcohol. The aqueous phase was treated with 25 to 50 µL of 3M sodium acetate (pH 5.2) and 1 volume of ethanol, shaken gently, and left at −20°C overnight. The precipitate was collected by centrifugation at 12 000g for 20 minutes. The pellet was rinsed with 1 mL of 70% ethanol and spin for 10 minutes. The supernatant was discarded and the pellet was air dried at room temperature and later dissolved in 0.5 mL of double distilled water. DNA was precipitated in cold ethanol at −20°C and finally dissolved in 0.5 mL of buffer. DNA sample was loaded in 1.0% agarose gel containing 0.5 mg/mL ethidiumbromide, electrophoresed at 80 V and visualized under ultraviolet transilluminator.
2.12 Western blot analysis
Cell lysis buffer (CelLytic MT Cell Lysis Reagent; Sigma-Aldrich) with 1% protease inhibitor cocktail was used to extract the whole heart tissue protein. The concentration of protein was estimated using Bradford reagent (Sigma-Aldrich) and BSA was used as the standard. A quanitity of 50 µg of total protein was taken for the study and separated on a 10% SDS-PAGE followed by protein transfer to a nitrocellulose membrane. The membranes were blocked using 5% skimmed milk in Tris-buffered saline-0.05% Tween 20 (TBST) solution at room temperature for 1 hour and incubated overnight with primary antibody at 4°C with gentle shaking. The membranes were washed with TBST solution three times and incubated with HRP-conjugated secondary antibody for 1 hour. The membrane was developed using DAB/H2O2 color development system. Glyceraldehyde 3-phosphate dehydrogenase was used as an internal control. Image J software (NIH) was used for densitometry analysis of immunoblots.
2.13 Statistical analysis
Data analyses were performed using SPSS 17. All data were expressed as a mean ± SEM. Statistical analysis was performed using one-way analysis of variance followed by the Tukey multiple comparison tests. Differences between treatment groups were considered statistically significant at P value of less than 0.001.
3 RESULTS
3.1 Effect of BBM on ISO-induced mitochondrial dysfunction
3.1.1 BBM treatment reduces ROS and LPO in the heart mitochondria
In the current study, treatment of rats with ISO showed a significantly (P < 0.001) increased level of mitochondrial ROS and elevated level of mitochondrial LPO when compared with the control. The value of ROS was expressed as relative fluorescence compared with the control value, which was taken as 100%. The levels of mitochondrial ROS and LPO were significantly (P < 0.001) decreased in BBM pretreated followed by ISO administered rats (Figure 1A and 1B).
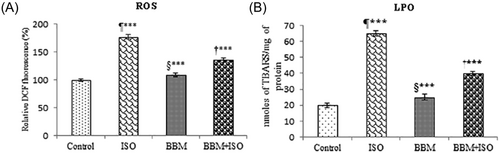
Effect of BBM on ISO-induced mitochondrial oxidative damage in rats. A, Generation of ROS. B, Level of TBARS. Values are expressed as mean ± SEM (n = 6). One way analysis of variance followed by the Tukey multiple comparison tests. ¶***P < 0.001 compared with control group. †***P < 0.001 compared with isoproterenol group. §***Nonsignificant compared with control. BBM, berbamine; BBM + ISO, berbamine pretreated ISO-induced rats; ISO, isoproterenol; LPO, lipid peroxidation; ROS, reactive oxygen species; TBARS, thiobarbituric acid reactive substances
3.1.2 BBM modulates mitochondrial antioxidant status in ISO-induced rats
To evaluate the role of BBM on the mitochondrial antioxidant status, level of nonenzymic antioxidant GSH and activities of antioxidant enzymes, SOD, CAT, GPx, and GST were measured in the various treatment groups. The level of GSH and the activities of mitochondrial antioxidant enzymes SOD, CAT, GPx, and GST were significantly (P < 0.001) decreased in ISO-treated rats when compared with control rats. Pretreatment with BBM followed by ISO administration significantly (P < 0.001) increased the activities antioxidant enzyme when compared with ISO alone treated rats (Table 1). These results revealed the antioxidant property of BBM.
| Antioxidants | Control | Isoproterenol | Berbamine | Berbamine + isoproterenol |
|---|---|---|---|---|
| GSH | 6.78 ± 0.17 | 3.862 ± 0.21* | 6.4 ± 0.16+ | 5.89 ± 0.15** |
| SOD | 114.54 ± 4.8 | 64.47 ± 6.1* | 112.17 ± 4.53+ | 94.19 ± 5.40** |
| CAT | 1.48 ± 0.04 | 0.71 ± 0.05* | 1.46 ± 0.09+ | 1.19 ± 0.06** |
| GPx | 17.51 ± 1.2 | 8.25 ± 1.5* | 16.95 ± 1.4+ | 14.30 ± 1.8** |
| GST | 557.7 ± 11.6 | 432.9 ± 12.5* | 552.4 ± 11.7+ | 527.1 ± 11.6** |
- Abbreviations: CAT, catalase; GPx, glutathione peroxidase; GSH, glutathione; GST, glutathione-S-transferase; SOD, superoxide dismutase.
- Results are shown as mean ± SEM (n = 6). One way analysis of variance followed by the Tukey multiple comparison test.
- Units are expressed as follows: GSH, nmoles of glutathione released/mg protein; SOD 1U, corresponds to the amount of enzyme required to give 50% inhibition of pyrogallol auto-oxidation; CAT, nm of H2O2 decomposed/min/mg protein; GPx, µg of GSH utilized/min/mg protein; GST, nm of CDNB conjugated/min/mg protein.
- * P < 0.001 compared with control.
- ** P < 0.001 compared with isoproterenol group.
- + Nonsignificant compared with control.
3.1.3 Effect of BBM on the activities of mitochondrial Kreb cycle and respiratory chain enzymes
In an attempt to identify the effect of BBM in the energy metabolism, we measured the changes in the activities of rat mitochondrial enzymes. The activities of ICDH, α-KGDH, SDH, and MDH were significantly (P < 0.001) lowered in the heart mitochondria of ISO-induced rats compared with the control. The results proved the adverse effect of ISO on Kreb cycle enzymes. Pretreatment with BBM followed by ISO administration significantly (P < 0.001) enhanced the activities of these enzymes compared with rats treated with ISO alone (Figure 2A).
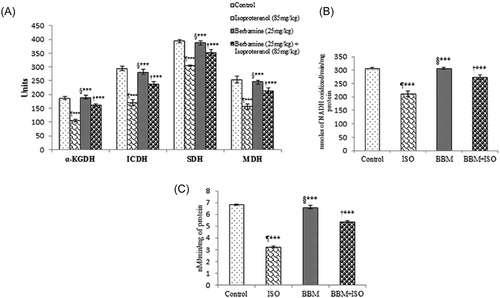
Effect of BBM pretreatment on the activities of mitochondrial enzyme in ISO-induced myocardial infarction in rats. A, Mitochondrial marker enzyme activities. α-KGDH, nmoles of ferrocyanide formed/h/mg protein; ICDH, nmoles of a-ketoglutarate formed/hour/mg protein; SDH, nmoles of succinate oxidized/min/mg protein; MDH, nmoles of NADH oxidized/min/mg protein. B, NADH dehydrogenase. C, Cytochrome c-oxidase activities. Values are expressed as mean ± SEM (n = 6). One way ANOVA followed by the Tukey multiple comparison tests. ¶***P < 0.001 compared with control group. †***P < 0.001 compared with isoproterenol group. §***Nonsignificant compared with control. BBM, berbamine; BBM + ISO, berbamine pretreated ISO-induced rats; ICDH, isocitrate dehydrogenase; ISO, isoproterenol; MDH, malate dehydrogenase; SDH, succinate dehydrogenase; α-KGDH, α-ketoglutarate dehydrogenase
Following decreased activities of Kreb cycle enzymes, we further examined the changes in the activities of respiratory chain enzymes. As shown in Figure 2B and 2C treatment of rats with ISO resulted in marked decrease in the activities of NADH dehydrogenase and cytochrome c-oxidase compared with control rats. The results showed that BBM treatment results in increased activity of these enzymes.
3.1.4 Effect of BBM on heart mitochondrial Ca2+ and ATP content
The effect of BBM on Ca2+ and ATP contents were evaluated in heart mitochondria. There was a considerable (P < 0.001) increase in the Ca2+ level in the heart mitochondrial of ISO group when compared with control group, whereas a significant (P < 0.001) decrease in mitochondrial ATP level was observed in the ISO-induced group. After pretreatment with BBM, the Ca2+ content returned to the normal level compared to the ISO group whereas, mitochondrial ATP level was increased significantly (P < 0.001) by BBM pretreatment compared with ISO-treated group (Figure 3).
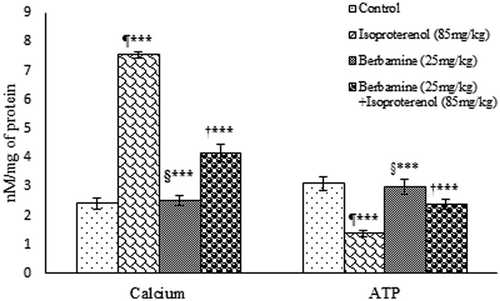
Effect of BBM on ISO-induced changes in heart mitochondrial calcium and ATP levels. Results are shown as mean ± SEM (n = 6). One way analysis of variance followed by the Tukey multiple comparison tests. ¶***P < 0.001 compared to control group. †***P < 0.001 compared to isoproterenol group. §***Nonsignificant compared with control. ATP, adenosine triphosphate; BBM, berbamine; ISO, isoproterenol
3.1.5 Effect of BBM on the structure of heart tissue mitochondria (TEM study)
The changes in the structure of heart mitochondria following incubation with ISO was studied through TEM. As shown in Figure 4, rats treated with ISO showed loss of myofibril alignment, reduction in the number of cristae, vacuolization, and swelling of mitochondria along with significant disruption of membrane integrity. On the other hand, BBM pretreatment restored morphology and reduced vacuolization and mitochondrial swelling, indicating a protective effect. Control and rats treated with BBM alone showed normal mitochondrial morphology.
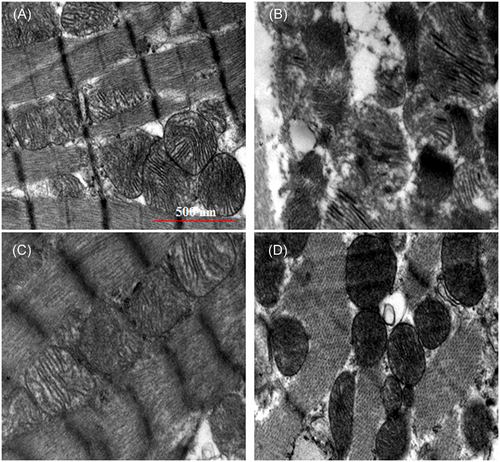
Transmission electron microscopic images of heart mitochondria. A, Control group showing the normal architecture of the heart mitochondria. B, ISO-induced group showing swelling of heart mitochondria with loss of cristae, irregular shape, and size and myofilaments with vacuolation. C, Normal + berbamine pretreated group showing normal heart mitochondria with normal myofibrils. D, Rats pretreated with berbamine followed by ISO-induced rats showing near normal architecture with myofibrils. Scale bar = 500 nm, ×20 000. ISO, isoproterenol
3.1.6 Effect of BBM on the surface topology of mitochondria (SEM study)
The examination of SEM represented in Figure 5 demonstrates that isolated mitochondria were affected by ISO treatment. It confirms that ISO administration damages the surface topology of mitochondria. The mitochondria isolated from ISO-treated rats showed a markedly contracted surface with membrane blebs. Whereas, BBM pretreatment significantly prevented the mitochondrial injury produced from ISO administration. However, BBM alone has no effect on isolated mitochondria as compared with control group.
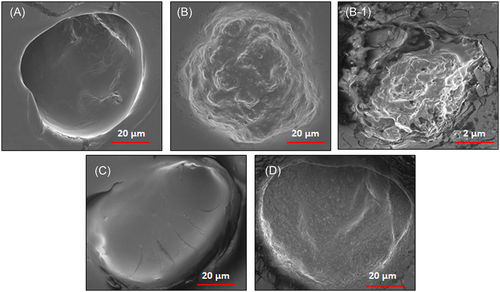
Scanning electron microscopic images of heart mitochondrial surface. A, Control group showing the normal architecture of mitochondrial surface. B and B-1, ISO-induced group shows convoluted membranes and contracted mitochondria along with membrane blebs covering its surface. C, Normal + berbamine-treated group shows normal heart mitochondria. D, Rats pretreated with berbamine significantly prevented the ISO-induced mitochondrial damage. Scale bar = 20 µm, ×6000. ISO, isoproterenol
3.2 Effect of BBM on ISO-induced apoptosis
3.2.1 BBM reduced TUNEL-positive cells in rat
To determine ISO-induced myocardial cell death TUNEL assay was carried out. TUNEL-positive cells are stained as brown nuclei. As shown in Figure 6A and 6B ISO administration resulted in myocardial apoptosis as evident from an increased number of TUNEL-positive cells compared with control rats. While BBM pretreatment significantly (P < 0.001) reduced the percentage of TUNEL-positive cells compared with control. A small number of TUNEL-positive cells were observed in both control and BBM alone treated group. These findings indicated that BBM effectively reduced the ISO-induced apoptosis in rats.
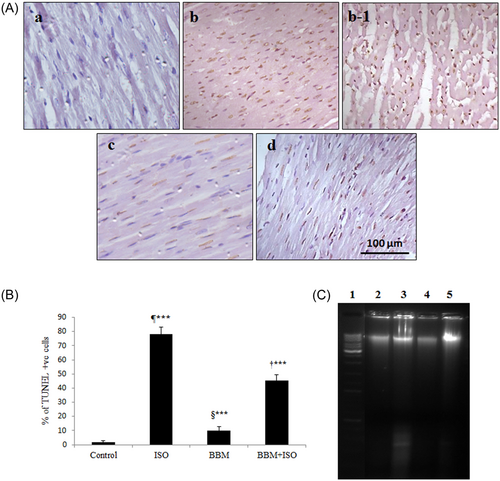
Effect of BBM on ISO-induced apoptosis was determined by TUNEL assay. A, TUNEL-positive cells are stained with brown nuclei. A, Control. b and b-1, Isoproterenol group. c, Berbamine group. d, Berbamine + isoproterenol group. Scale bar = 100 µm, ×400. B, Quantitative analysis of TUNEL staining in different treatment groups. C, Effect of BBM pretreatment on ISO-induced DNA fragmentation. Lane 1, marker DNA (10 kb); lane 2, control; lane 3, rats treated with isoproterenol; lane 4, rats pretreated with berbamine; lane 5, rats pretreated with berbamine followed by isoproterenol administration. Each bar represents mean ± SEM (n = 6). One-way analysis of variance followed by the Tukey multiple comparison tests. ¶***P < 0.001 compared with control group. †***P < 0.001 compared with isoproterenol group. §***Nonsignificant compared with control. BBM, berbamine; ISO, isoproterenol; BBM + ISO, berbamine pretreated ISO-induced rats; TUNEL, terminal deoxynucleotidyl transferase dUTP nickend labeling
3.2.2 BBM pretreatment protects heart tissue from ISO-induced DNA fragmentation
DNA fragmentation is a hallmark of apoptosis. Figure 6C shows the effect of BBM on ISO-induced apoptosis in rats. Rats treated with ISO resulted in increased DNA damage as evident from DNA laddering and smearing pattern in the agarose gel. On the other hand, ISO-induced DNA damage was substantially reduced in BBM pretreated rats. There was no DNA damage was observed in both control and BBM alone treated rats.
3.2.3 BBM modulates ISO-induced apoptotic pathway in myocardial tissue
Figure 7 shows the effect of BBM administration on ISO-induced changes in the protein expression of apoptosis signaling molecules such as Bax, Bcl-2, caspase-9, caspase-3, poly-ADP ribose polymerase (PARP), and cytochrome c in myocardial tissue of different experimental groups. In the present research, we observed decreased Bcl-2 expression and increased expression of Bax in ISO-administered rats compared with control rats. However, the altered protein expression of Bcl-2 and Bax was restored by BBM pretreatment compared with ISO alone induced rats. Furthermore, ISO administration also caused a significant upregulation of cytochrome c, cleaved caspase-9, caspase-3, and PARP expressions in myocardial tissue. While the expression of cytochrome c, cleaved caspase-9, caspase-3, and PARP were significantly downregulated in BBM pretreated group compared with ISO alone treated rats. These findings suggest that BBM pretreatment effectively inhibited ISO-induced apoptosis.
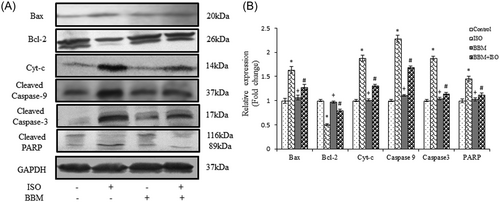
Effect of BBM on proapoptotic and antiapoptotic proteins. A, Immunoblot showing a significant increase in Bcl-2 and decrease in the levels of Bax, caspase-9, and PARP after BBM pretreatment. B, Densitometric analyses of Bax, Bcl-2, Cyt-c, caspase-9, caspase-3, and PARP protein expressions normalized with GAPDH. Each bar represents mean ± SEM (n = 6). One-way analysis of variance followed by the Tukey multiple comparison tests. *P < 0.001 compared with the control group. #P < 0.001 compared with the isoproterenol group. +Nonsignificant as compared with the control. BBM, berbamine; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; ISO, isoproterenol; PARP, poly-ADP ribose polymerase
4 DISCUSSION
Excess ROS damages the heart tissue and alters the membrane permeability leading to loss of membrane integrity which in turn leads to mitochondrial dysfunction.33 ISO treatment significantly increased the mitochondrial ROS indicating oxidative stress induction. Enhanced level of ROS causes mitochondrial membrane damage and leads to increased level of LPO. These findings are consistent with previous studies of ISO-induced mitochondrial oxidative stress.34 In the current study, the level of mitochondrial ROS and LPO reduced significantly in rats, which received BBM pretreatment. These results are in agreement with the earlier findings of Li et al (51), where paeonol and danshensu combination reduced the levels of oxidative markers through its antioxidant property and effectively mitigates the ISO-induced myocardial injury in rats. Results of our study indicate the free radical scavenging activity of BBM.
ROS generation and reduction in total cellular antioxidants were associated with stimulation of β-adrenergic receptor. ISO administration reduces the activity of mitochondrial antioxidant enzymes such as SOD, CAT, GST, and GPx and level of GSH, which leads to cardiac contractile dysfunction and cardiotoxicity finally results in myocardial necrosis.35 The use of antioxidants effectively eliminates the free radicals and prevents myocardial apoptosis.36 Administration with epicatechin and various plant-derived antioxidants were demonstrated to reduce the mitochondrial oxidative stress by enhancing the endogenous antioxidant pool.37 BBM pretreatment increased the activity of mitochondrial antioxidant enzymes and level of GSH in ISO-treated rats. Results of our study corroborate with the previous report of Punithavathi et al,38 where rutin effectively quenched the free radicals through its antioxidant property thereby protected mitochondria from ISO-induced damage.
The current study demonstrates the effect of BBM on ISO-induced mitochondrial dysfunction. In our experiment, the activities of Kreb cycle enzyme were protected from being altered when rats were pretreated with BBM and it protects the mitochondrial enzymes from ISO-mediated free radical attack and helps to maintain the mitochondrial energy level. According to Meeran et al,39 a decrease in the activities of mitochondrial enzymes results in ATP depletion in ISO-treated rats which contributes to mitochondrial dysfunction and development of myocardial ischemia. The results of our study are in accordance with the above report. Recently, a study published by Punithavathi and Prince.4 suggested that intake of quercetin and α-tocopherol and other dietary supplements offers protection against ISO-induced mitochondrial damage by increasing the activities of mitochondrial enzymes in rats. Our results are consistent with the observation of Khatua et al40 where garlic and diallyl disulfide functioned as a scavenger of toxic reactants and increased the activities of Kreb cycle enzymes.
The respiratory chain enzymes, NADH dehydrogenase, and cytochrome c oxidase are an integral part of the inner mitochondrial membrane and involved in ATP synthesis. It has been documented in the literature that unavailability of cardiolipin results in decreased activity of these enzymes in ISO-treated rats.41 In the current study, ISO significantly reduced the activities of mitochondrial respiratory chain enzymes, which may be due to increased phospholipid degradation. Padmanabhan and Prince.42 reported that ISO-induced effect on NADH dehydrogenase and cytochrome c oxidase enzyme activities. Our results showed activities of these enzymes significantly increased with BBM pretreatment in ISO-treated rats which might be due to its free radical scavenging nature which is in line with the previous report where N-acetylcysteine ameliorates ISO-induced mitochondrial dysfunction by increasing the activities of mitochondrial marker enzymes in rats.43
Intracellular (Ca2+)i overload, as a consequence of dysregulation of Ca2+ homeostasis, leads to an excess generation of ROS which in turn causes mitochondrial dysfunction and heart failure.44 ISO causes intracellular Ca2+ accumulation which disrupts the proton gradient within the mitochondrial membrane, leading to decreased ATP production.45 In the current study, ISO-treatment increased intracellular Ca2+ accumulation which affects the ionic gradients and alters the mitochondrial membrane structure and function, similar results were observed by Meeran et al.39 Our data demonstrate that BBM pretreatment significantly decreased intracellular Ca2+ level and ROS generation which could be due to its free radical scavenging nature. Decreased level of Ca2+ might have resulted in increased ATP production in rats pretreated with BBM followed by ISO administration. This corroborates with previous reports where caffeic acid supplementation prevented the ISO-induced intracellular Ca2+ accumulation and ATP depletion in heart mitochondria.46
Change in the mitochondrial structure is the main indicator of ISO-induced cardiac damage. ISO administration resulted in increased heart mitochondrial size and disruption of cristae. The increased size of mitochondria may be due to the growth of individual organelles or fusion of abutting mitochondria, or a combination of the two.47 Results from our study showed that ISO-induced degenerative changes are reversed in BBM pretreated rats. These results corroborate with the previous report where cardioprotection by phytic acid and maintenance of mitochondrial morphology has been observed in ISO-induced MI in rats.48 Consequently, BBM protects the heart mitochondria and sustained the function of heart mitochondria. Zheng et al15 reported that BBM possesses mitochondrial membrane stabilizing capacity. These properties may contribute to beneficial effects of BBM on ISO-induced mitochondrial damage in rats.
In addition, the SEM studies of mitochondrial surface revealed severe damage to the mitochondria following ISO administration, which is indicative of mitochondrial swelling which is in accordance with the previous study.49 According to Mukherjee et al3 melatonin offers mitochondrial protection against ISO-induced mitochondrial damage. Similar to the above findings, the current study shows a promising role of BBM on ISO-induced cardiac mitochondrial protection.
Apoptosis is a type of cell death regulated by a sequence of signal cascades under different situations. Bcl-2 family proteins such as Bcl-2, Bcl-W, and Bcl-Xl are a group of crucial factors which function as apoptotic inhibitors and Bax, Bak, Bad, Bcl-xS, and Bid are proapoptotic proteins, known to promote apoptosis.50 The changes in the expression of Bax/Bcl-2 family proteins usually decide the fate of the cell. Caspase family proteases play a vital role in the execution of cellular apoptosis. Excessive ROS generation induces Bax to permeabilize the external mitochondrial membrane resulting in the release of mitochondrial cytochrome c. Cytochrome c accelerates apoptotic protease-activating factor-1 (Apaf-1) and caspase-9 to form apoptosomes which trigger caspase-3 activation.51 PARP plays a vital role in DNA repair and programmed cell death, which is cleaved by activated caspase-3. Activation of caspase cascade results in cytoskeleton disassembly, chromatin fragmentation and finally cell death.52 In the current study, we observed decreased expression of antiapoptotic protein Bcl-2 and upregulated expression of Bax, cytochrome c, cleaved caspase-9, cleaved caspase-3, and PARP in ISO-treated rats indicating myocardial apoptosis.
To investigate the underlying molecular mechanism of BBM pretreatment on ISO-induced apoptosis, we examined the expression pattern of various apoptotic proteins. In the current study, BBM pretreatment prevented ISO-induced apoptosis by inhibiting cytochrome c release from mitochondria and subsequent downregulation of the expression of caspase-9, caspase-3, PARP cleavage, and inhibition of DNA fragmentation. In addition, ISO administration resulted in an enhanced number of TUNEL-positive cells which was reversed by BBM pretreatment. Our results corroborate with previous findings where epigallocatechin-3-gallate prevented ISO-induced apoptosis in rats.1 The results of this study suggest that BBM offers protection against ISO-induced mitochondria-dependent (intrinsic) pathway of apoptosis. The schematic representation of the proposed mechanism of action of BBM was depicted in Figure 8.
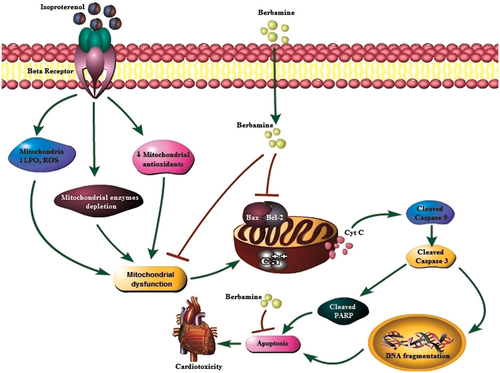
Schematic representation of mechanism of action of berbamine. LPO, lipid peroxidation; ROS, reactive oxygen spieces
In conclusion, the findings of the current study demonstrate the cardioprotective effect of BBM against ISO-induced apoptosis by preventing mitochondrial damage thereby maintaining the structure and function of mitochondria. This is the first study in elucidating the underlying molecular mechanism involved in the BBM-mediated cardioprotection on ISO-induced MI. Thus, the results of the current study confirm the cardioprotective efficacy of BBM.
ACKNOWLEDGMENTS
The author would like to acknowledge the UGC-BSR University Grant Commission (Basic Scientific Research) New Delhi, India for providing the financial support to carry out this study. All the authors acknowledge the financial support of UGC (SAP) and DST (FIST) to the Department of Biotechnology, Bharathiar University, Coimbatore.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.