CCL17-CCR4 axis promotes metastasis via ERK/MMP13 pathway in bladder cancer
Abstract
As an important chemokine receptor, the role of CCR4 in the progression of bladder cancer (BC) remains unknown. In this study, we have shown that CCR4 expression was upregulated in bladder carcinoma tissues compared with adjacent nontumor tissues. Kaplan-Meier survival analysis revealed that CCR4 expression was an independent prognostic risk factor in BC patients, and the addition of CCL17 induced CCR4 production and promoted migration and invasion of BC cells. In addition, CCR4 knockdown significantly attenuated the migratory and invasive capabilities of BC cells. Mechanistically, CCL17-CCR4 axis is involved in ERK1/2 signaling and could mediate the migration and invasion of BC cells by regulating MMP13 activation. This study suggests that CCR4 might represent a promising prognostic biomarker and a potential therapeutic option for BC.
1 INTRODUCTION
Bladder cancer (BC) is the ninth most common cancer and the thirteenth deadliest worldwide with 81 190 newly diagnosed cases and 17 240 deaths estimated in USA, 2018.1 Although the clinical treatment outcomes have improved in recent decades, there are many problems that requires attention, such as the short survival time of patients with invasive stage tumors or metastatic diseases. Many studies have shown that the progression of BC has a significant correlation with migration and invasion.2 Although tumor migration and invasion are some of the most serious aspects of BC and have a close relationship with the clinical survival of patients, the fundamental mechanisms of these processes remain unclear. Therefore, it is necessary to investigate the fundamental mechanisms of BC development to find effective therapeutic strategies to improve patients' survival.
Chemokines belong to a superfamily of small molecules whose effects are regulated by binding to G-protein-coupled receptors.3 They are involved with organ-specific metastasis of cancer by drawing tumor cells with matching chemokine receptors to specific sites. There are many studies proving the crucial function of chemokines and their receptors in cancer. For example, CXCR4 has been found to be involved with metastasis in more than 20 different cancers.4-6
As an important chemokine receptor, CCR4 is expressed on circulating and tissue-resident T cells, being prevailingly associated with regulatory T cells and Th2 cells.7-9 The function of CCR4 has been previously investigated in both hematologic malignancies and solid tumors, such as gastric cancer,10 breast cancer,11 and lung cancer.12 Various studies have suggested that CCR4 might be involved in CCL17/CCL22-induced migration of cancer cells. Nevertheless, there is little known about the function of CCR4 in the progression of BC. In this study, we have investigated CCR4 expression in BC tissues, its correlation with a variety of clinicopathological factors and the role of CCL17-CCR4 axis in cell invasion and metastasis. Underlying molecular mechanisms of CCR4 in BC metastasis were also revealed. Our results suggested that CCR4 plays an important role in BC progression.
2 MATERIALS AND METHODS
2.1 Cell culture and reagents
BC cell lines were purchased from the American Type Culture Collection (Manassas, VA). The cell lines, T24, 253J, were maintained in an RPMI 1640 medium, supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO). All of these cells were incubated at 37°C and 5% CO2. U0126 (MEK inhibitor) was purchased from MCE (Shanghai, China) and used in accordance with the manufacturer’s instructions. Recombinant human CCL17 was purchased from R&D Systems (Minneapolis, MN).
2.2 Generation of gene knockdown in stable cells
pGLV-GFP-CCR4 lentiviral vector and three small interfering RNA (shRNA) plasmids targeting different regions of CCR4 mRNA were purchased from Genechem (Shanghai, China).
Lentivirus particles were transfected into the BC cells in the presence of polybrene and selected for 2 weeks using 5 μg/mL puromycin. CCR4 sequences targeted were as follows: shRNA1: 5′-TTGAAAGATACTTGGACTA-3′; shRNA2: 5′-CTTAGGGATCATGCTGTTT-3′; and shRNA3: 5′-TTTCTGTTCAGCACTTGTT-3′.
2.3 Wound-healing assay and Matrigel invasion assay
The cells were cultured in serum-free medium for 24 hours and wounded with pipette tips. The fresh medium was then replaced. After 24 hours, the wound closing procedure was observed and photographed. The invasion assays were performed as previously reported. Briefly, 200 μL serum-free medium containing 2 × 105 cells was added into the upper chamber, and 600 μL medium with 10% serum and CCL17 was added into the lower chamber. The chamber coated with diluted Matrigel (BD Biosciences, Bedford, MA) was cultured in 37°C at 5% CO2 for 24 hours and fixed with methanol. The cells that invaded to the bottom of the membrane were stained with 1% crystal violet and photographed under microscope. Three independent experiments were conducted using the same conditions.
2.4 Real-time quantitative reverse-transcription PCR
Total RNA was isolated from cell lines and tissues using Trizol (Invitrogen, Camarillo, CA) according to the manufacturers’ instructions. Complementary DNA was synthesized using a reverse-transcription kit (Invitrogen, Camarillo, CA). Quantitative polymerase chain reaction (PCR) was performed using SYBR Green PCR Master Mix (Applied Biosystems, Austin, TX).
The primers for CCR4 were as followed: forward 5′-CCCACGGATATAGCAGACACC-3′ and reverse 5′-GTGCAAGGCTTGGGGATACT-3′. Relative mRNA expression was calculated by comparative Ct method, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the control. All the experiments were carried out in triplicates.
2.5 Patients and immunohistochemical analysis
The collection of specimens from 122 patients was authorized by Shandong University Qilu Hospital. These patients were all received radical cystectomy with BC, diagnosed by imageological examinations, and pathological results, at our institution from 2009 to 2014. Patients were excluded from this study if their information missed. Patients with autoimmune disease, cancer in other systems, received neoadjuvant chemotherapy and radiotherapy were also excluded from study. The specimens were used after obtaining informed consent from the patients. The staining of these specimens was conducted according to the manufacturer’s protocol (Immunostain SP kit, DakoCytomation). The antibodies used for immunohistochemical (IHC) analysis included antibody against CCR4 (ab1669, Abcam, Cambridge, MA). For IHC staining, briefly, the paraffin-embedded sections of BC tissues were deparaffinized and then heated in a pressure pot for 3 minutes to retrieve the antigens. Then, the sections were incubated with primary antibodies overnight at 4°C. Antibody binding was detected using a peroxidase-conjugated secondary antibody at 37°C for 30 minutes. A DAB substrate kit was used to perform the chromogenic reaction. The intensity of the staining was evaluated using the following criteria: 0, negative; 1, low; 2, medium; and 3, high. The extent of staining was scored as 0, 0% stained; 1, 1% to 25% stained; 2, 26% to 50% stained; and 3, 51% to 100% stained. The final scores were calculated by multiplying the scores of the intensity with those of the extent and dividing the samples into four grades: 0, negative; 1 to 2, low staining; 3 to 4, medium staining; and 4 to 6, high staining. The following criteria were used to quantify the expression levels of CCR4 in BC tissues: high expression, the final scores were 4 to 6; low expression, the final scores were 0 to 3.
2.6 Immunofluorescence
The BC cells were seeded on coverslips and cultured until they reached 60% to 70% confluence. The cells were washed with PBS and fixed in 4% paraformaldehyde for 15 minutes at room temperature (RT). The cells were then washed three times with cold PBS before permeabilization with 0.25% Triton X-100 in PBS for 15 minutes at RT. The cells were washed three times with PBS and blocked with 1% bovine serum albumin in polybutylene succinate-co-butylene terephthalate at RT for 30 minutes and were incubated with primary antibody overnight at 4°C, as described by the manufacturer. CCR4 expression was detected by incubating the cells for 1 hour with a secondary antibody conjugated with IF488 (1:1000). The cells were counterstained with 4′, 6-diamidino-2-phenylindole (Sigma-Aldrich, St. Louis, MO), and the images were captured using a fluorescence microscope.
2.7 Immunoblotting
Western blot analysis was performed as previously described [1.35]. Briefly, 100 µg of protein was separated by 10% sodium dodecyl sulfate polyacrylamide-based discontinuous gel and transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% nonfat milk for 2 hours and were incubated at 4°C overnight with primary antibodies. The primary antibodies for CCR4 were purchased from Abcam, and the antibodies against ERK (AF0155), p-ERK (AF1015), MMP3 (AF5355), and GAPDH (AF0911) were purchased from Affinity Biosciences (Affinity, Cincinnati, OH). Horseradish peroxidase–conjugated secondary antibodies were used, and the protein bands were visualized using an Odyssey scanner (LI-COR Biosciences, Lincoln, NE).
2.8 Statistical analysis
Statistical analyses were performed using the IBM SPSS Statistics Program. Each experiment was performed in triplicates, and the values are presented as the mean ±standard deviation, unless otherwise stated. The variance between the groups was statistically compared. Student t-test was used to compare the mean values. Kaplan-Meier curves were analyzed for relevant variables. The log-rank test was used to analyze the differences in survival times among the patient subgroups. The risk factors associated with the prognoses of these patients were evaluated using Cox’s proportional hazard regression model. All the probability values had a statistical power level of 90%, and a two-sided level of 5%. P < 0.05 was considered to be significant.
3 RESULTS
3.1 Correlation of CCR4 expression with clinicopathological characteristics and prognosis of bladder cancer patients
As shown in Figure 1A, the expression of CCR4 at the protein level was significantly increased in BC samples compared with paired normal tissues from seven patients. In addition, we examined the expression of the CCR4 protein by IHC staining in a retrospective cohort of 122 BC specimens from patients (Figure 1B).
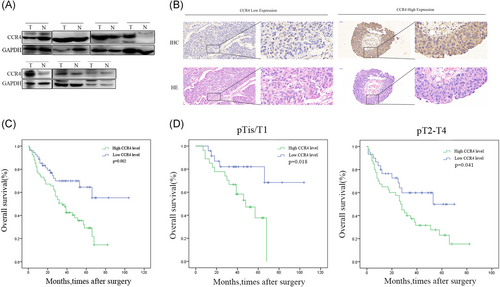
Correlation of CCR4 expression with clinicopathological characteristics and prognosis of bladder cancer patients. A, Representative Western blot images of CCR4 expression in seven paired bladder cancer samples. B, IHC staining of CCR4 proteins in bladder cancer samples and hematoxylin-eosin (HE) staining in the same place. C, Bladder cancer patients with high expression of CCR4 presented have worse overall survival, and low expression was opposite (P = 0.002). D, There was no clear difference between different pathological T stage. Adjustments of brightness, contrast, and size are applied to the whole images of Western blot–based analyses without elimination of any information present in the original, including backgrounds. Data are presented as mean ± SD. HE, hematoxylin-eosin; IHC, immunohistochemical; SD, standard deviation
To explore the clinical significance of CCR4 in BC, we analyzed the CCR4 expression pattern in 122 BC specimens by IHC. The clinicopathologic characteristics of 122 patients are shown in Table 1. In detail, higher level CCR4 expression was detected in 68 (55.7%) tumor tissues, whereas only 54 (44.3%) specimens exhibited low/absent level (Table 1). Subsequently, the correlation between CCR4 expression level and clinicopathological parameter in patients, and patients with higher expression of CCR4 (with a cutoff at median) presented advanced grade, tumor stage, and metastasis status. However, no significant difference was observed between the two groups on age, sex, tumor grade, or metastasis status. Kaplan-Meier survival analysis revealed that patients with low CCR4 expression had more favorable overall survival (P = 0.002, respectively) compared with high CCR4 expression (Figure 1C). In addition, we analyzed whether the different pathological T stages were associated with the OS (Figure 1D). No difference was observed in patients with pTis/T1 for OS (P = 0.018), and in patients with pT2-4 (P = 0.041). The results of univariate analysis and multivariate analysis by Cox proportional hazards models suggested that CCR4 expression was an independent prognostic risk factor (P = 0.003, P = 0.007, Table 2).
| All patients (n = 122) | CCR4 expression | P value | ||
|---|---|---|---|---|
| Low/absent (n = 54) | High(n = 68) | |||
| Sex | 0.211 | |||
| Male | 108 (88.5%) | 47 | 61 | |
| Female | 14 (11.5%) | 7 | 7 | |
| Age, y | 0.201 | |||
| <65 | 47 (38.5%) | 22 | 25 | |
| ≥65 | 75 (61.5%) | 32 | 43 | |
| Tumor size | 0.873 | |||
| <4 cm | 35 (28.7%) | 12 | 23 | |
| ≥4 cm | 59 (48.4%) | 26 | 33 | |
| unknown | 28 (22.9%) | 15 | 13 | |
| Tumor grade | 0.644 | |||
| Low grade | 12 (9.8%) | 4 | 8 | |
| High grade | 110 (90.2%) | 50 | 60 | |
| Pathological T Stage | 0.278 | |||
| pTis/T1 | 51 (41.8%) | 24 | 27 | |
| pT2-4 | 71 (58.2%) | 30 | 41 | |
| Lymph node metastasis | 0.076 | |||
| pNx/pN0 | 105 (86.1%) | 47 | 58 | |
| pN+ | 17 (13.9%) | 7 | 10 | |
| Metastasis | 0.741 | |||
| M0 | 115 (94.3%) | 52 | 63 | |
| M1 | 7 (5.7%) | 2 | 5 | |
| TNM stage | 0.104 | |||
| Ois-I | 50 (41%) | 23 | 27 | |
| II-IV | 72(59%) | 31 | 41 | |
- pN+ = pN1, pN2, or pN3.
| Univariate analysis | ||
|---|---|---|
| OS | ||
| Variable | HR (95%CI) | P value |
| Sex | 0.55 | |
| Male | 1.000 (reference) | |
| Female | 0.513 (0.260-1.016) | |
| Age, y | 0.742 | |
| <65 | 1.000 (reference) | |
| ≥65 | 1.09 (0.653-1.819) | |
| Tumor size | 0.181 | |
| <4 cm | 1.000 (reference) | |
| ≥4 cm | 0.672 (0.376-1.203) | |
| Tumor grade | 0.968 | |
| G1-2 | 1.000 (reference) | |
| G3 | 1.016 (0.462-2.236) | |
| Pathological T stage | 0.008 | |
| pTis/T1 | 1.000 (reference) | |
| pT2-4 | 2.053 (1.203-3.503) | |
| Lymph node metastasis | 0.236 | |
| pNx/pN0 | 1.000 (reference) | |
| pN+ | 0.663 (0.337-1.307) | |
| Metastasis | 0.008 | |
| M0 | 1.000 (reference) | |
| M1 | 0.339 (0.153-0.751) | |
| TNM stage | 0.009 | |
| Ois-I | 1.000 (reference) | |
| II-IV | 2.067 (1.203-3.552) | |
| CCR4 | , | 0.003 |
| Low/absent | 1.000 (reference) | |
| High | 0.428 (0.244-0.749) | |
| Multivariate analysis | ||
|---|---|---|
| Variable | OS | |
| HR (95%CI) | P value | |
| Pathological T stage | 0.814 | |
| pTis/T1 | 1.000 (reference) | |
| pT2-4 | 0.783 (0.101-6.067) | |
| Metastasis | 0.176 | |
| M0 | 1.000 (reference) | |
| M1 | 0.564 (0.246-1.294) | |
| TNM stage | 0.404 | |
| Ois-I | 1.000 (reference) | |
| II-IV | 2.416 (0.305-19.157) | |
| CCR4 | 0.007 | |
| Low/absent | 1.000 (reference) | |
| High | 0.451 (0.253-0.806) | |
- pN + = pN1, pN2, or pN3.Abbreviations: CI, confidence interval; HR, hazard ratio; OS, overall survival.
3.2 Expression of CCR4 in bladder cancer cell lines
To investigate whether CCR4 expression is correlated with BC development and progression, we first analyzed CCR4 expression in BC cell lines. According to the results of Western blot, real-time PCR, and immunofluorescence, CCR4 express in BC cell lines T24 and 253J (Figure 2A-C).
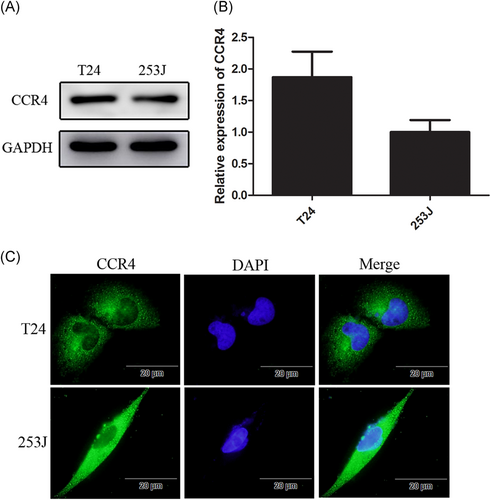
Expression of CCR4 in bladder cancer cell lines. A and B, CCR4 expression in T24 and 253J detected by Western blot and qRT-PCR. C, Bladder cancer cells (1.0 × 105 cells/well) are seeded into six-well plates and cultured until they reach 60% to 70% confluence. Cells are incubated with anti-CCR4 antibody and detected using a second antibody conjugated with FITC (green). Cells are counterstained with 4′,6-diamidino- 2-phenylindole (blue). Data are presented as mean ± SD. FITC, fluorescein isothiocyanate; SD, standard deviation
3.3 Induction of the migration and invasion in CCL17-treated bladder cancer cells
To elucidate the role of CCL17, which can specifically bind to CCR4, in the invasiveness and motility of BC cells, wound-healing migration and Matrigel invasion assays were performed. According to the results, treatment of T24 and 253J cells with CCL17 (100 ng/mL) for 24 hours significantly increased invaded cells compared with the control cells (Figure 3A). Besides, both 253J and T24 cells treated with CCL17 for 24 hours exhibited relative distance compared with control (Figure 3B). These results indicated that CCL17/CCR4 can enhance the motility of the T24 and 253J cells. Western blot assay results confirmed the promotion of CCR4 expression in CCL17-treated cells (Figure 3C).
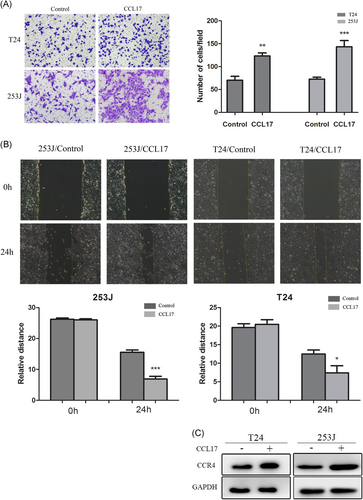
CCL17 promotes bladder cancers invasion and migration, and induces CCR4 expression. A, T24 and 253J are placed in Transwell inserts and treated with CCL17 (100 ng/mL). After 24 hours incubation, T24 and 253J cells that had migrated through the membrane are stained (magnification 200×). B, Migration of bladder cancer cells is assessed with a wound-healing assay. C, T24 and 253J cells are treated with CCL17(100 ng/mL) for 24 hours, and Western blot for CCR4 is performed. Adjustments of brightness, contrast, and size are applied to the whole images of Western blot–based analyses without elimination of any information present in the original, including backgrounds. Data are presented as mean ± SD. All experiments are performed in triplicate, and mean values are shown. *P < 0.05, **P < 0.01, and ***P < 0.001. SD, standard deviation
3.4 CCR4 promotes cell invasion and migration in bladder cancer
To investigate the role of CCR4 in BC cells, we employed lentivirus-mediated shRNA to knock down CCR4 in T24 and 253J cells, respectively, with the treatment of CCL17 (100 ng/mL). The effect of knockdown was confirmed by Western blot analysis, and the cells infected with sh-CCR4 exhibited a lower expression (Figure 4A). Transwell assay results showed that the number of invaded cells were significantly decreased in CCR4-knockdown cells(Figure 4B) compared with cells infected with control. In addition, wound-healing assay results showed that the relative distance of wound edges of CCR4-knockdown cells was significantly wider than those of control cells (Figure 4C). To elucidate the signaling pathways involved in the cellular responses, we investigated the MAPK signaling. The results of Western blot showed that the expression of p-ERK and MMP13 was reduced in the CCR4-knockdown cells (Figure 4D).
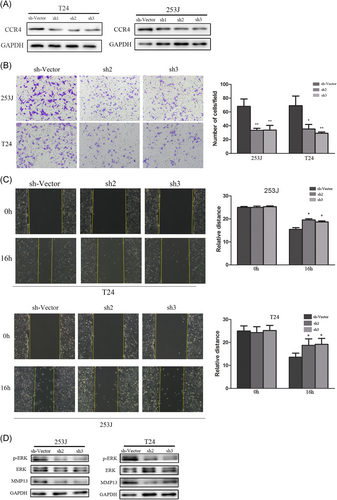
CCR4 facilitates bladder cancer cells metastasis in vitro and regulated the expression of MMP13 (A) T24 and 253 J cells transfected with sh-CCR4, respectively, were subject to Western blot. B, Invasive behavior was evaluated using Matrigel invasion assays after knockdown of CCR4 in bladder cancer cells, after 16 hours, with the treatment of CCL17(100 ng/ml) (magnification 200×). C, The migratory capacity of CCR4-knockdown cells was analyzed by wound-healing assay. D, p-ERK, ERK, and MMP13 expressions were determined by Western blot analysis. Adjustments of brightness, contrast, and size are applied to the whole images of Western blot–based analyses without elimination of any information present in the original, including backgrounds. Data are presented as mean ± SD. All experiments are performed in triplicate, and mean values are shown. *P < 0.05, **P < 0.01. SD, standard deviation
3.5 Association between ERK1/2 signaling and MMP13 effect on the expression of CCL17-CCR4 axis induced bladder cancer cells metastasis
To further investigate the exact roles of ERK1/2 signaling in the CCL17-CCR4 axis–induced molecular events of T24 and 253J cells, ERK1/2 specific inhibitor U0126 was used. As shown in Figure 5A and 5B, migratory and invasive effects by CCL17 were enhanced by CCL17 induction. However, this effect of CCL17 was suppressed by a U0126 pretreatment, characterized by an attenuated mobility in both 253J and T24 cell lines subsequently. Besides, CCL17 could improve the expression of p-ERK and MMP13 in bladder cell lines (Figure 5C). However, ERK1/2 and MMP13 expression induced by CCL17 were then reversed by pretreating U0126 in T24 and 253J cells (Figure 5C). These results suggested that ERK1/2 signaling was involved in the migration, invasion, and MMP13 expression through the activation of the CCL17-CCR4 axis.
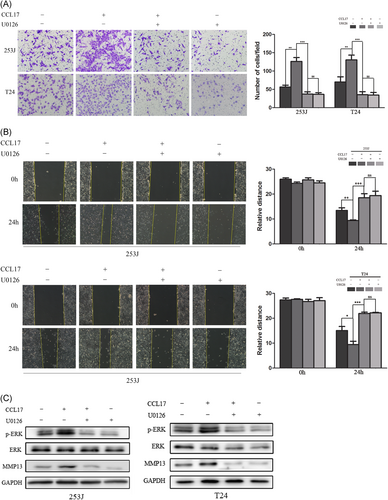
ERK1/2 signaling is associated with MMP13 expression through activation of the CCL17-CCR4 axis. A,B T24 and 253J cells were treated with CCL17 (100 ng/mL) alone or in combination with the ERK1/2 inhibitor U0126 (10 μM) for 24 hours. U0126 is pretreated for 40 minutes. C, Western blot show the expression of p-ERK and MMP13 with the treatment of U0126 and CCL17. Adjustments of brightness, contrast, and size are applied to the whole images of Western blot–based analyses without elimination of any information present in the original, including backgrounds. Data are presented as mean ± SD. All experiments are performed in triplicate, and mean values are shown. *P < 0.05, **P < 0.01, ***P < 0.001. SD, standard deviation
4 DISCUSSION
In the past decade, great efforts have been made to explore the molecular mechanism of invasion and metastasis for cancer cells. Nevertheless, the crucial mechanisms about metastasis of BC remain incompletely understood. Many studies have approved that chemokines and their receptors are involved in the complex processes of cancer metastasis.4 In this study, we have showed that chemokine receptor CCR4 might play a crucial role in the metastasis of BC through a clinical investigation and in vivo exploration.
CCR4 has been demonstrated to be a risk factor in various types of cancers, such as breast cancer, lung cancer, and gastric carcinoma, and higher expression of CCR4 in patients potentially contribute to metastasis.10, 13-16 Olkhanud et al14 showed that the process of breast cancer lung metastasis was correlated with CCR4 expression and regulatory T cells. Moreover, CCL17/CCL22-CCR4 axis played an important role in migration and invasion in prostate cancer.17 Gastric carcinoma patients with higher CCR4 expression have a significantly poorer prognosis than patients with lower CCR4 expression.10 Ishida et al13 suggested that CCR4 also plays an important role in immunity related to occurrence and development of cancer, because tumor cells in patients with CCR4 + T-cell leukemia or lymphoma can arm themselves as regulatory T cells, leading to an immune escape and tumor survival facing immune response from the host.13 In the current study, a number of patients with higher expression of CCR4 were found to be more susceptible to BC metastasis, though no significant clinicopathological difference was observed. Cox proportional hazard model analysis showed that significant poor prognosis was found to be associated with a higher level of CCR4.This contradiction might contribute to the fact that most of the patients included in this study had muscle-invasive BC, because BC patients always suffer a poor survival even though receiving the standard therapy according to reports.18, 19 Indeed, more BC patients should be included to validate this conclusion clinically. Still, our above findings suggest that chemokine receptor CCR4 may play an important role in BC cells metastasis.
Also, CCR4 has been demonstrated to be an oncogene in in vitro research. The migratory response of gastric cancer cell line was attenuated using CCR4 antibodies.10 Alhaidari et al20 suggested that CCR4 presents a higher expression in colon cancer cells, and its expression can support migration of cancer cells. RhoA/Rho-Kinase signaling has been found to contribute to the migratory promotion of these cells. In this study, CCL17 treatment resulted in a significant increase in CCR4 protein levels in BC cells and improved the metastasis ability of bladder cells. However, silencing of CCR4 reduced the migratory and invasive capacity of BC cells in vitro.
Many studies have reported that MMPs participate in the process of tumor progression and are responsible for the degradation of the extracellular matrix, which results in the migration and invasion of tumor cells.2, 21-23 The expression of MMP13 is known to be a critical factor in the progression and development of BC, as reported in previous studies. It has been reported that MMP13 is involved in cancer metastasis in hepatocellular carcinoma.24 Stromal MMP13 is involved in tumor microenvironment and promotes lung metastasis of breast cancer.25 In our study, expression level of p-ERK and MMP13 was found to be decreased under conditions of CCR4 knockdown in BC cells. These results provided new clues on the underlying mechanism that CCL17-CCR4 axis could promote BC cells invasion by the upregulation of MMP13.
In order to identify the potential signaling pathways involved in the regulation of MMP13 expression, the small chemical inhibitor, U0126, was used against the ERK1/2 pathway. It was observed that MMP13 expression was decreased by the blocking of ERK signaling in U0126 pretreated BC cells. Meanwhile, the ability of invasion and migration was decreased with the pretreatment with U0126 of BC cells. According to previous studies, the ERK/MMP pathway is involved in the migration and invasion of BC cells.26 Liang et al27showed that that interleukin-1-induced MMP9 expression is required for the activation of ERK signaling. Collectively, our results showed that ERK might play an important role in the regulation of MMP13 expression and metastasis of BC.
5 CONCLUSION
The current study indicated a correlation between CCR4 expression and the clinical prognosis of BC patients. Downregulation of CCR4 influenced BC metastasis in vitro, which was assumed to activate the ERK/MMP13 pathway. Therefore, CCR4 might be considered as a potential target for anticancer treatment of BC.
6 LIMITATION
However, no significant difference was observed in the clinical feature between patients with higher and lower expression of CCR4. More patients should be included in future investigation.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (Grant 81470987 and 81170702 to B Shi), the Tai Shan Scholar Foundation to B. Shi, the Science and Technology Development Project of Shandong Province (Grant 2014GSF118054 to B. Shi), Science Foundation of Qilu Hospital of Shandong University (Grant 2015QLMS28 to B. Shi).
CONFLICTS OF INTEREST
None of the contributing authors have any conflicts of interest, including specific financial interests or relationships and affiliations relevant to the subject matter or materials discussed in the manuscript.