BMP9/COX-2 axial mediates high phosphate-induced calcification in vascular smooth muscle cells via Wnt/β-catenin pathway
Abstract
Vascular calcification is a notable risk factor for cardiovascular system. High phosphate can induce calcification in vascular smooth muscle cells (VSMCs), but the detail mechanism underlying this process remains unclear. In the present study, we determined the relationship between high phosphate and bone morphogenetic protein 9 (BMP9) in VSMCs, the effect of BMP9 on calcification in VSMCs and the effect of COX-2 on BMP9 induced calcification in VSMCs, as well as the possible mechanism underlying this biological process. We found that high phosphate obviously up-regulates the expression of BMP9 in VSMCs. Over-expression of BMP9 decreases the level of alpha-smooth muscle cell actin (α-SMA) apparently, but increases the level of Runx-2, Dlx-5, and ALP in VSMCs. Meanwhile, BMP9 increases the level of OPN and OCN, promotes mineralization in VSMCs and induces calcification in thoracic aorta. High phosphate and over-expression of BMP9 increases the level of COX-2. Over-expression of COX-2 enhances the inhibitory effect of BMP9 on α-SAM and increases the level of OPN and OCN induced by BMP9. However, inhibition of COX-2 decreases the BMP9-induced calcification in VSMCs and thoracic aorta. For mechanism, we found that high phosphate or BMP9 increases the level of β-catenin and p-GSK3β in VSMCs, but no substantial effect on GSK3β. However, COX-2 inhibitor decreases the expression of β-catenin induced by BMP9. Our findings indicated that BMP9 is involved in the phosphate-induced calcification in VSMCs and COX-2 partly mediates the BMP9-induced calcification in VSMCs through activating Wnt/β-catenin pathway.
1 INTRODUCTION
Renal function failure is one of main cause for cardiovascular diseases related morbility and mortality, especially for patients with chronic kidney disease (CKD). Calcification of the VSMCs is one of the main complications for CKD, which may be initialized by the high level of calcium and/or phosphate.1-3 Therefore, high phosphate has been used as a model to recapture vascular calcification. It has been reported that the high phosphate-induced calcification may be resulted from inducing apoptosis and osteochondrocytic differentiation.4 Several important signal pathways have been activated in this process, such as BMPs/Smads and Wnt/β-catenin pathway.5, 6 However, the explicit mechanism of vascular calcification remains unclear yet.
In fact, the vascular calcification is very similar with the process of bone ossification. Bone morphogenetic proteins (BMPs) are a sub-group of TGF-β super-family and play an important role in the development and fracture healing process of skeletal system.7, 8 There are at least 15 members of BMPs, in which BMP2 and BMP7 have been validated with great potential for osteogenic induction.9, 10 BMP2 has been proved by FDA for the treatment for spinal or bone graft fusion for several years.10 Besides this, BMP2 also induces vascular calcification via Wnt/β-catenin pathway.6 BMP9, another member of BMPs, has been reported to be the most potent osteogenic BMPs for exhibiting markedly osteogenic potential either in vitro or in vivo experiments.11 Similarily, it has been reported that BMP9 can also induce osteogenic differentiation and calcification in smooth muscle cells via ALK1 dependent manner,12 which implied that BMP9 may also be involved in vascular calcification. But, the relationship between BMP9 and high phosphate in calcification remains unknown. Meanwhile, the explicit mechanism for BMP9 to induce vascular calcification keeps unclear yet.
Cyclooxygenase 2 (COX-2), as a pro-inflammation factor, is one of essential enzymes for the production of prostaglandins. Apart from inflammation, COX-2 is also associated with bone fracture healing process and ectopic calcification. COX-2 knockout delays the bone fracture healing and COX-2 inhibitor attenuates the ectopic ossification after trauma operation.13, 14 In addition, BMP9 up-regulates COX-2 when induce osteogenic differentiation in mesenchymal stem cells (MSCs) and inhibition or knockdown of COX-2 reduces the osteogenic potential of BMP9.15, 16 Hence, COX-2 may be potential to accelerate the calcification of VSMCs when presented inVSMCs. On the contrary, it has been reported that inhibition of COX-2 can increase the calcification in VSMCs,17 which implying that COX-2 may benefit to decrease the abnormal vascular calcification. Therefore, the role of COX-2 in vascular calcification need to be further investigated.
In this present study, we investigated the relationship between high phosphate and BMP9, the effect of BMP9 on the calcification, the role of COX-2 in the BMP9-induced calcification and the possible mechanism through which COX-2 affect the calcification induced by BMP9 in VSMCs.
2 MATERIALS AND METHODS
2.1 Cell culture and chemicals
Primary rat aortic smooth muscle cells (VSMCs) were used for this investigation. VSMCs were maintained in Dulbecco's Modified Eagle's Medium (DMEM), containing 10% fetal bovine serum (FBS), 100 U/mL of penicillin, and 100 µg/mL of streptomycin. The condition is kept at 37°C and 5% CO2. All primary antibodies were ordered from Santa Cruz Biotechnology (CA). COX-2 inhibitor NS-398 (#N194-25MG) was purchased from Sigma-Aldrich (St. Louis, MO). The phosphate (NaH2PO4, Cat# RDD007) was ordered from Sigma-Aldrich, and the final concentration of phosphate is 1.5, 2.5, or 3.5 mM.
2.2 Primary rat aortic smooth muscle cells (VSMCs) extraction
The primary rat aortic VSMCs were isolated and cultured as described.18 Briefly, Male Sprague-Dawley (SD) rats (150-180 g,10-12 w) were ordered from the Laboratory Animal Center of Chongqing Medical University. All animal experiments were approved by the institutional animal care and use committee (IACUC) of Chongqing Medical University (Chongqing, China). Removed the intima and adventitia carefully. Then, collected the arota and cut into small sections. Finally, the cells were grown in complete DMEM medium, treated with phosphate (1.5, 2.5, or 3.5 mM), AdGFP, AdBMP9, and/or NS-398 (30 µM). The expression of α-SMA was introduced as marker to identify the purity of VSMCs. The results showed that more than 90% of the cultured cells are VSMCs. The cells were used between the 3rd and 8th passage.
2.3 Construction of recombinant adenovirses for BMP9, COX-2, dnALK2, and GFP
The recombinant adenoviruses were constructed following the AdEasy system.19, 20 Briefly, the coding sequence (CDS) of human BMP9, COX-2, extraceullar part of ALK2 and green fluorescent protein (GFP) were amplified. These fragments were sub-cloned into shuttle vectors, respectively. Then, the shuttle vectors were linearized and transfected into HEK293 cells for packaging recombinant adenovirus, which were designated as AdBMP9, AdCOX-2, and AddnALK2. The recombinant adenovirus expressing GFP (AdGFP) only was used as vector control. All these recombinant adenoviruses were kindly provided by Dr T.C.-He (Medical Center of the University of Chicago).
2.4 RNA extraction, cDNA preparation, and polymerase chain reaction analysis
Total RNA were extracted with TRIzol reagents (Invitrogen, Carlsbad, CA) and then used to generate cDNA through reverse transcription (RT) reaction. The products were diluted 5-10 fold and used as templates for the following real-time PCR assay. All results were normalized with the level of glyceraldehyde phosphate dehydrogenase (GAPDH). The primers used in this investigation are presented in Table 1.
| Gene | Primer sequence (5′-3′) |
|---|---|
| SM22a | F: GCCTCAACATGGCCAACAAG R: CAGGCTGTTCACCAACTTGC |
| Runx-2 | F: AAGGAGCAGACCGTCAAAGG R: TAAGCTGGTCTGCAGAGTGC |
| Dlx-5 | F: AAAGCGCTCAACCCATACCA R: TTCTTTCTCTGGCTGGCTGG |
| ALP | F: CAACCTGACTGACCCTTCCC R: TTCTGGGAAGTCATGGTGCC |
| β-catenin | F: CAGCTCCCCTGACAGAGTTG R: CCAGTCCGAGATCAGCAGTC |
| GAPDH | F: AGACAGCCGCATCTTCTTGT R: CTTGCCGTGGGTAGAGTCAT |
- F, forward; R, reverse.
2.5 Western blot analysis
Cells were seeded in six-well plates and treated as the experimental design. At the indicated time points, cells were lyzed and the lysate were denatured by boiling for 10 min. The sample were separated by SDS-PAGE, transfered to polyvinylidene difluoride membrane, blocked with 10% skimmed milk and probed with primary anti-body for the target proteins. Finally, the target bands were developed with SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific, IL).
2.6 Matrix mineralization assay (Alizarin Red S staining)
Cells were seeded in 24-well plates and treated as the experimental design. After treating for 14 days, the mineralization nodules were determined with Alizarin Red S staining as described before.15, 16 Briefly, cells were fixed with 0.05% (v/v) glutaraldehyde at room temperature for 10 min and washed with distilled water. Then cells were incubated with 0.4% Alizarin Red S for 5 min, followed by gently washing with distilled water. Dried the plates in air at room temperature and then scanned. For quantification, the Alizarin Red S was dissolved with 10% acetic acid and the absorbance was determined at 405 nm with a micro-plate reader.21, 22 The results were performed in at least three independent experiments.
2.7 Aortic ring organ culture
The thoracic aorta were collected from Male Sprague-Dawley rats. Then the vessels were cut into segments as aortic rings with the width of 2-3 mm and cultured with complete DMEM medium for 24 h to reach steady state in six-well plates. Then, the aortic rings were treated with AdGFP, AdBMP9, NS-398 (30 µM), and/or AdBMP9 combined with NS-398 (30 µM) for 7 or 14 days. Finally, the aortic rings samples were collected and embedded in paraffin for histochemical evaluation. The results were performed in at least three independent experiments.
2.8 Von Kossa staining
The paraffin sections of thoracic arteries samples were deparaffined and incubated with 2% AgNO3 under ultraviolet light for 60 min. Then washed with distilled water for 5 min and treated with 5% sodium thiosuphate for 2 min. Followed by washing with distilled water for 5 min and counterstained with hematoxylin and eosin for 1-2 min. Finally, dehydrated with enthanol, cleared with xylene, and mounted with a resinous medium. Images were taken under microscope. The results were performed in at least three independent experiments.
2.9 Immunofluorescent staining
Cells were seeded in 48-well plate and treated as the experimental design. At the scheduled time point, cells were fixed with 4% formaldehyde shortly and washed carefully. Then permeablized with 0.3% Triton X-100 for 10 min and washed immediately. Followed by treating with H2O2 for 10 min and then incubated with primary anti-body for α-SMA, COX-2, or β-catenin overnight (4°C), the corresponding isotype IgG was used as negative control. Then cells were incubated with corresponding second anti-body conjugated with Dylight594. Finally, cells were stained with DAPI (1 µg/mL). The images were taken under microscope. The results were repeated at least in three independent experiments.
2.10 Statistical analysis
The data are presented as mean ± SD. For all quantitative analysis, each assay was performed in triplicate, and the results were repeated in at least three independent experiments. The statistical significance between groups were performed with GraphPad Prism software and t-test. The P value less than 0.05 was considered as statistically significant.
3 RESULTS
3.1 Effects of phosphate on inducing calcification in VSMCs
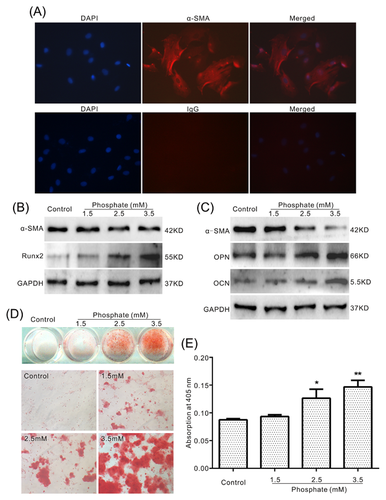
It is well known that high phosphate can induce calcification in VSMCs, but the explicit molecular mechanism underlying this process remains unclear. In this study, we aimed to unveil the possible mechanism for the phosphate-induced calcification in VSMCs. We extracted the primary VSMCs for the following study. The immunofluorescent staining results for a-SMA showed that over 90% cells are VSMCs (Figure 1A). The Western blot assay results showed that phosphate decreases the level of a-SMA, while increases the level of Runx2, OPN, and OCN simultaneously (Figures 1B and 1C). Alizarin Red S staining results showed that phosphate induces matrix mineralization in VSMCs apparently (Figures 1D and 1E). These results confirmed that phosphate is potent to induce calcification in VSMCs.
3.2 Effects of BMP9 on the expression of early osetogenic markers in VSMCs
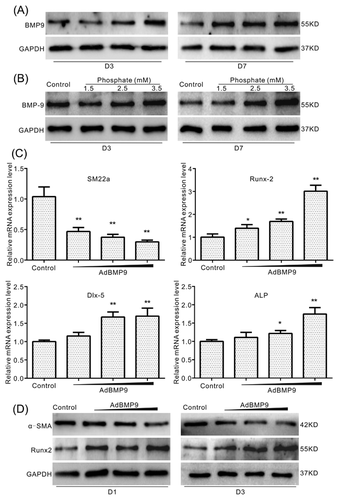
BMPs is one of the important factors to induce osteogenic differentiation, which also be involved in inducing calcification. To date, it remains unknown whether BMP9 is involved in calcification induced by high phosphate in VSMCs. The Western blot assay results showed that phosphate up-regulates the level of BMP9 concentration dependently in VSMCs (Figure 2A) and aorta ring (Figure 2B). Over-expression of BMP9 increases the mRNA level of Runx-2, Dlx-5, and ALP, while decreasing the mRNA level of SM22a (Figure 2C). Further assay results confirmed that over-expression of BMP9 decreases the protein level of a-SMA, but increases the protein level of Runx-2 substantially in VSMCs (Figure 2D). These results suggested that BMP9 may participate in high phosphate-induced calcification in VSMCs.
3.3 Effects of BMP9 on calcification in VSMCs and thoracic aorta
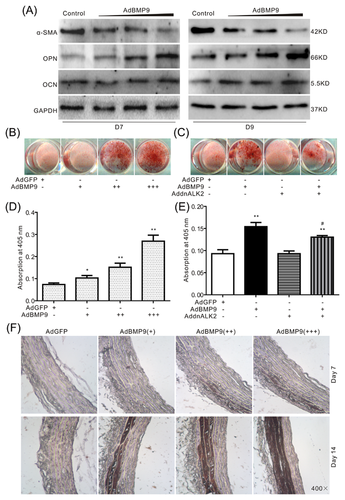
Next, we investigated whether BMP9 can induce calcification in VSMCs. Western blot assay results showed that over-expression of BMP9 increases the protein level of OPN and OCN, but decreases the level of α-SMA (Figure 3A). Meanwhile, over-expression of BMP9 also induces the matrix mineralization in VSMCs, but this effect is decreased greatly by the dominant negative mutation of ALK2, a plausible receptor for BMP9 (Figure 3B-E). The Von Kossa Staining results of aortic rings showed the over-expression of BMP9 markedly induces calcification in the vessels (Figure 3F). These data suggested that high phosphate-induced calcification of VSMCs may be mediated by BMP9. But how BMP9 promotes the calcification remains unclear.
3.4 Effects of high phosphate on the expression of COX-2 in VSMCs
COX-2 is one of the speed-limited enzyme for the production of prostglandins (PGs), which function as a pro-inflammatory factor. It has been reported that the inhibitor of COX-2 can reduce the ectopic ossification, which induced by trauma or surgical operation. Hence, we speculated that COX-2 may also be involved in high phosphate-induced calcification in VSMCs. The Western blot analysis results showed that high phosphate increases the level of COX-2 concentration dependently (Figure 4A). Further analysis results showed that BMP9 also up-regulates COX-2 apparently in VSMCs (Figures 4B and 4C). These data indicated that COX-2 may participate in the pathogenic process of calcification in vessels also.
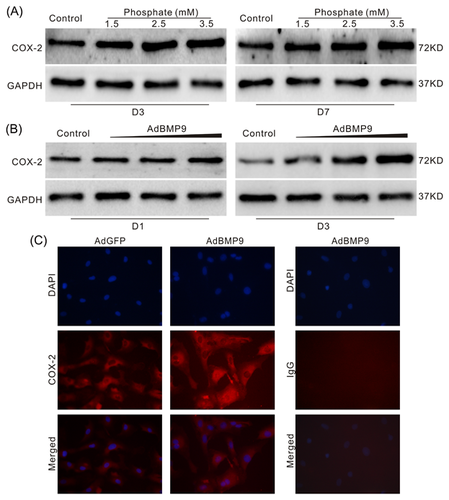
3.5 Effects of COX-2 on BMP9-induced calcification in VSMCs and thoracic aorta
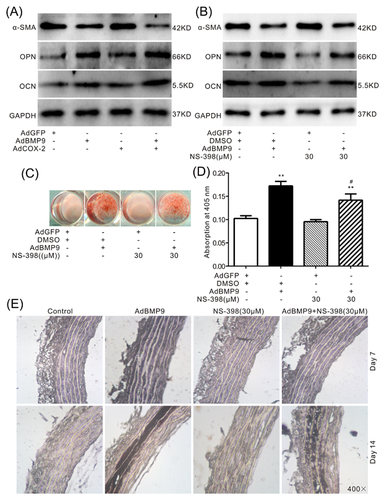
BMP9, as the most potent BMPs member to induce ossification, can up-regulate COX-2 in mesenchymal stem cells (MSCs). Therefore, BMP9-induced calcification in VSMCs may also be mediated by COX-2. Western blot assay results showed that over-expression of COX-2 enhances the inhibitory effect of BMP9 on α-SMA and promotes the BMP9-induced OPN and OCN in VSMCs (Figure 5A). On the contrary, specific inhibitor of COX-2 (NS-398) attenuates the inhibitory effect of BMP9 on α-SMA, as well as the BMP9-induced OPN and OCN in VSMCs (Figure 5B). Alizarin Red S staining results showed that BMP9 can obviously induce matrix mineralization in VSMCs, which can be greatly decreased by NS-398 (Figures 5C and 5D). The Von Kossa Staining results of aortic rings also showed that over-expression of BMP9 markedly induces calcification in the vessels, which can be substantially inhibited by NS-398 (Figure 5E). These results suggested that COX-2 may be a critical factors for BMP9 to induce calcification in VSMCs.
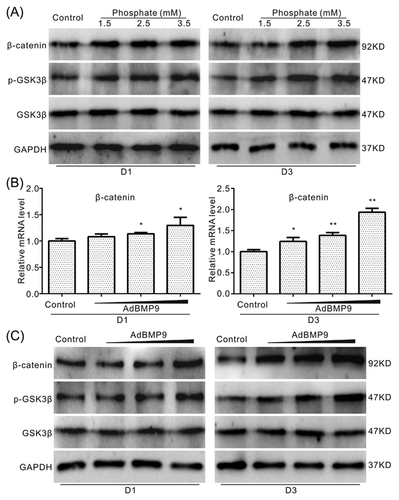
3.6 Effects of BMP9 on Wnt/β-catenin pathway in VSMCs
As Wnt/β-catenin signal has been reported playing an important role in high phosphate-induced calcification in VSMCs, we next investigated whether the effect of BMP9 on calcification in VSMCs is related with this signal. Western blot assay results showed that high phosphate increases the level of β-catenin and p-GSK3β apparently, but no substantial effect on GSK3β (Figure 6A). Real-time PCR assay results showed that BMP9 increases the mRNA expression level of β-catenin (Figure 6B). Further Western blot assay results showed that BMP9 exerts similar effect on the protein level of β-catenin, p-GSK3β and GSK3β as that of high phosphate in VSMCs (Figure 6C). These results suggested that BMP9 may mediate the high phosphate-induced calcification through activating Wnt/β-catenin signal in VSMCs.
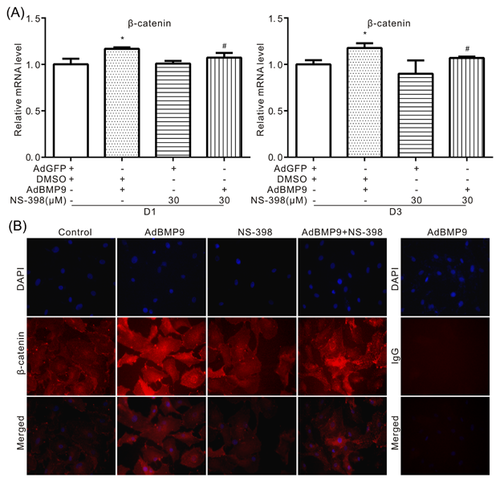
3.7 Effects of COX-2 on BMP9-induced activation of Wnt/β-catenin pathway in VSMCs
Because COX-2 can regulate the BMP9-induced calcification in VSMCs, so we next to determine whether COX-2 can affect the Wnt/β-catenin signal activated by BMP9. Real-time PCR analysis results showed that BMP9 can increase the mRNA level of β-catenin, which can be attenuated markedly by NS-398, specific inhibitor of COX-2 (Figure 7A). The immunofluorescent staining results also showed that NS-398 can reduce the level of β-catenin, which increased by BMP9 in VSMCs. These data suggested that COX-2 may promote the BMP9-induced calcification through enhancing the activation of Wnt/β-catenin signal in VSMCs.
4 DISCUSSION
Vascular calcification is one of the main complications of CKD, which may increase the cardiovascular diseases related morbility and mortality. To date, the molecular mechanism for calcification in VSMCs is not fully understood yet. In the present study, we demonstrated that BMP9 can be up-regulated by high phosphate and BMP9 can induce calcification in VSMCs directly. COX-2 partly mediates the effect of BMP9 on calcification by promoting the activation of Wnt/β-catenin signal in VSMCs.
Vascular calcification, as a risk factor for cardiovascular diseases, is associated with aging, diabetes mellitus, chronic renal diseases and high level of calcium and phosphate.23-25 It has been proved that VSMCs from vascular wall can differentiate to osteoblast-like cell and form matrix deposition.26 As the vascular calcification is very similar with bone formation, some essential transcriptional factors are also up-regulated in this process, such as Runx2, Osterix, and Msx2.26 Meanwhile, the pro-osteogenic factors or signals are also associate with the calcification of VSMCs, such as BMPs/Smads and Wnt/β-catenin signal.27, 28 Increasing evidences supported that high phosphate can induce calcification of VSMCs, the mechanism underlying this process may include up-regulating the osteogenic factors, inducing apoptosis and activating the endoplasmic reticulum stress.29-31 BMPs are very important to regulate the proliferation and differentiation and about 15 member have been identified. Some of them are potent to regulate bone development and fracture healing process, as well as the vascular calcification process, such as BMP2 and BMP7.32 BMP2 increases the up-take of phosphate and plays an important role in RANKL-promoted osteoblastic activity in VSMCs.33, 34 In postnatal life, BMP7 is mainly expressed in kidney and regulate skeletal remodeling and the phenotype of VSMCs.32 The polymorphisms of BMP7 are associated with inverse relationships between vascular calcification and bone mineralization.35 BMP9, the less studied member of BMPs, has been demonstrated to be the most potent factor to induce osteogenic differentiation in mesenchymal stem cells (MSCs). It has been reported that BMP9 exerts its physiological function through TGFβ/BMP type I receptor ALK1 and ALK2,36 then activates BMPs/Smads pathway or non-canonical BMPs/Smads pathway, such as p38 MAPK.37 Besides inducing osteogenic differentiation in MSCs, BMP9 also induces calcification in VSMCs with ALK1 dependent fashion.12 But how BMP9 was regulated in high phosphate-induced calcification keeps unknown. In this study, we found that high phosphate can markedly up-regulate BMP9 in VSMCs. Meanwhile, ALK2 mutation decreases the BMP9-induced calcification in VSMCs, as well as ALK1. Therefore, BMP9 may mediate the high phosphate-induced calcification in VSMCs through ALK1 and/or ALK2.
Inflammation has been associated with various diseases, so does the vascular calcification. Several pro-inflammatory factor has been involved in the calcification process in VSMCs, such as tumor necrosis factor-α (TNF-α).30, 38 High phosphate can induce not only vascular calcification but also systemic inflammation, which may contribute to the vascular calcification.39 COX-2 is one of the major pro-inflammatory factors and its inhibitors are widely used for anti-inflammation and analgesic clinically. But the relationship between phosphate-induced vascular calcification and COX-2 keeps unknown yet. Our previous studies indicated that BMP9 can obviously up-regulate COX-2 when commit MSCs toward osteoblastic lineage. Either knockdown or inhibition of COX-2 greatly decreases the BMP9-induced osteogenic differentiation in MSCs.15, 16 As high phosphate increases the expression of BMP9, so we speculated that the phosphate may up-regulate COX-2 in VSMCs too. Our data showed that high phosphate substantially increases the protein level of COX-2 in VSMCs, as well as BMP9. Hence, COX-2 may also participate in the calcification of VSMCs. Further study indicated that over-expression of COX-2 increases the BMP9-induced osteogenic markers in VSMCs. But, COX-2 inhibitor decreases the BMP9-induced calcification notably. These date suggested that COX-2 may participate in promoting the vascular calcification. On the contrary, reverse function of COX-2 on calcification has also been reported. It showed that inhibition of COX-2 can increase the calcification in VSMCs.17 The different phenotype of COX-2 on calcification may due to the difference of calcification inducing factors, the concentration of inhibitor and the microenvironment. Our data suggested that BMP9 partly mediates the high phosphate-induced calcification through up-regulating COX-2 at least.
Wnt/β-catenin signal is very important for the regulation of proliferation and differentiation. It has been proved that activation of Wnt/β-catenin signal is associated with vascular calcification.1, 40 High phosphate can induce the activation of Wnt/β-catenin pathway.41 Inhibition of this signal can reduce the transformation of VSMCs to osteoblastic lineage.42 But the detail of how Wnt/β-catenin pathway is activated during vascular calcification remainsunclear. BMP9 induces osteogenic differentiation in MSCs, which can be decreased greatly when Wnt/β-catenin signal was inhibited.43 BMP2 can induce vascular calcification via Wnt/β-catenin pathway.6 Thus, BMP9 may activate Wnt/β-catenin pathway when induce calcification in VSMCs also. Our data showed that high phosphate can activate Wnt/β-catenin pathway and BMP9 exhibits the same effect in VSMCs. Herein, Wnt/β-catenin pathway may partly mediates the BMP9-induced calcification in VSMCs. Our previous study demonstrated that COX-2 and BMP9 form a positive loop to regulate the osteogenic differentiation in MSCs. Inhibition of COX-2 attenuates the BMP9-induced osteogenic differentiation.15 Thus, COX-2 may regulate the BMP9-induced calcification in VSMCs via a Wnt/β-catenin pathway dependent manner also. The following data showed that BMP9 apparently increases the level of β-catenin in VSMCs, which can be decreased by COX-2 specific inhibitor. Thus, COX-2 may promote the BMP9-induced calcification in VSMCs through activating Wnt/β-catenin pathway partly.
Taken together, our study indicated that high phosphate up-regulates BMP9 when induce calcification in VSMCs and BMP9 can directly induce calcification in the same cells. COX-2 may partly mediate the BMP9-induced calcification through activating Wnt/β-catenin pathway in VSMCs, which might provide a novel target for the prevention and treatment of vascular calcification.
ACKNOWLEDGMENTS
This work was supported by the research grants from the Natural Science Foundation of China (NSFC, 81372120 and 81572226 to Bai-Cheng He), Chongqing Education Commission (KJ1500217 to Jin Cui), and Chongqing Science and Technology Commission (cstc2013jjB10021 to Zhong-Liang Deng).
DISCLOSURE
All authors state that they have no conflicts of interest.