S14G-humanin alleviates insulin resistance and increases autophagy in neurons of APP/PS1 transgenic mouse
Abstract
Alzheimer's disease (AD) is a progressive neurodegenerative disease characterized by Aβ plaque deposition in the brain, which is related to the disorder of autophagosome maturation, transport, and formation of autolysosome. Notably, abnormal insulin signaling is connected with cognitive dysfunction in AD. In this study, using APP/PS1 transgenic mice as AD model, we investigated the mechanism by which S14G-humanin (HNG) improved autophagy and insulin signaling in AD brain. Immunohistochemistry was used to determine the levels of mTOR and Aβ deposition, and Western blot analysis was used to determine IRS-1, IRS-1 pSEr636, ULK1, p62, LC3 I/LC3 II protein levels. Our results demonstrated that HNG could improve the learning ability and memory in APP/PS1 transgenic mice, possibly through decreasing IRS-1 Ser636 phosphorylation and mTOR protein expression in the hippocampus, thus improving insulin resistance in the brain. In addition, HNG increased ULK1 expression, decreased p62 and LC3 I/LC3 II protein levels, thus enhancing autophagy and decreasing Aβ deposition in the brain. Taken together, our results suggest that through the regulation of IRS-1/mTOR insulin signaling in the hippocampus, HNG increases the activity of autophagy and decreases Aβ deposition in the brain, and improves learning ability and memory of AD mice.
1 INTRODUCTION
Alzheimer's disease (AD) is a progressive neurodegenerative disease of nervous system that mainly affects middle aged and older people.1 The pathogenesis of AD is complicated and involves multiple factors. AD is characterized by the accumulation of Aβ peptides and hyperphosphorylated tau neurofibrillary tangles within the brain. Glycogen synthase kinase 3β is the major kinase responsible for tau phosphorylation and it is regulated by insulin signaling.2 Recent evidence suggests that abnormal insulin signaling is connected with cognitive dysfunction in AD development.3-5 It is known that malfunction of mTOR signaling pathway would lead to dysfunction or decreased activity of autophagy in the cells. The dysfunction of autophagosome maturation, transport, and autophagolysosome formation cause severe accumulation of autophagosome, which inhibits the clearance of β-Amloid (Aβ) and leads to plaque deposition and AD development.6 Moreover, the role of insulin resistance in the pathogenesis of AD has become to be appreciated.7 Therefore, how to alleviate insulin resistance, enhance autophagy function and decrease Aβ deposition in the brain will be important approaches for AD treatment.
Humanin is a small neuroprotective peptide identified from cDNA library of AD patient.8 Since its discovery, humanin has been shown to regulate many biological processes such as apoptosis, cell survival, metabolism, inflammation and oxidative stress. Humanin and its analogs have shown beneficial effects in age-related diseases including AD.9 S14G-humanin (HNG) is a derivative of humanin via a substitution of Gly for Ser14 of humanin and has shown neuroprotective effect in animal models of Aβ deposition, but it is unknown whether HNG protects neuron in these animals through the regulation of insulin resistance and autophagy. In this study, we used APP/SP1 transgenic mice as AD model and examined the effects of HNG on insulin resistance and autophagy in the brain.
2 MATERIALS AND METHODS
2.1 Reagents
HNG (purity >97%) was purchased from Sigma (St. Louis, MO). Rabbit anti IRS-1 antibody and rabbit anti pSer636 IRS-1 antibody were from Santa Cruz. Anti mTOR polyclonal antibody was from ZSGB-Bio (Beijing, China). Rabbit anti ULK1 antibody, anti ULK1 pSer757 antibody, anti Akt antibody, anti Akt pSer473 antibody, anti p62 antibody, and anti LC3 II/LC3 I antibody were purchased from Beijing Biosynthesis Biotechnology (Beijing, China). Mouse anti Aβ antibody was from Sigma.
2.2 Mice
This study was carried out in accordance with the recommendations of Animal Care Committee of China Medical University and the protocol was approved by Animal Care Committee of China Medical University. Nine months old APP/PS1 transgenic C57BL/6J mice and wild-type C57BL/6J mice (average body weight 25 ± 2 g) were provided from the Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences (Beijing, China). APP/PS1 double transgenic mice overexpress mutated human APP and PS1 (APPswe/PS1ΔE9) and effectively simulate the pathological features of AD patients.10 Twenty-four APP/PS1 transgenic mice were divided randomly into three groups, transgenic control group (Tg), HND low dose group (Tg/HNG-L), and HND high dose group (Tg/HND-H), each group had four male and four female mice. Four male and four female wild-type C57BL/6J mice were included as non-transgenic (NT) control group. NT and Tg groups were treated with 0.5 mL saline IP injection daily. Tg/HNG-L and Tg/HNG-H groups were injected with 50 μg/kg and 100 μg/kg HNG in 0.5 mL saline IP daily, respectively. All groups were treated for 4 weeks.
2.3 Morris water maze behavior test
Learning and memory ability of mice in each group was tested using Morris Water Maze (MWM) behavior test. The maze was set using an iron bucket with height of 40 cm and diameter of 125 cm. An 8 cm diameter cylinder platform was placed at quadrant I, and 30 cm deep water was added to let the platform submerged 0.5-1 cm under the water. The water temperature was maintained at 22 ± 1°C. On day 1 to day 5, the time for each mouse from getting into the water to find and climb on the submerged platform was recorded as escape latency. At the same time, the length of path by the mouse to find the platform was recorded as average path length. The longest searching time was set as 60 s. If a mouse could not find the underwater platform in 60 s, the mouse would be guided to the platform and rest on the platform for 10 s to enhance its memory. All mice were tested three times every day, with 30 min interval. On day 6, the underwater platform was removed and each mouse was tested.
2.4 Western blot analysis
Total protein lysates were extracted from mouse hippocampus, and protein concentration was determined using BCA method. A total of 40 μg total lysate was separated on SDS-PAGE gel and transferred to PVDF membranes. The membranes were blocked with 5% non-fat milk in 1 × PBS with 0.1% Tween 20 (PBST), then probed with antibody for IRS-1, pSer636 IRS-1, ULK1, pSer757 ULK1, Akt, pSer473 Akt, p62, LC3 II/LC3 I, or GAPDH in PBST overnight at 4°C. After washing with PBST three times, the membranes were incubated with HRP conjugated secondary antibody at room temperature for 1 h. The signal on the membranes was visualized with ECL reagent, captured using gel imaging system and analyzed with software Image J.
2.5 Immunohistochemistry
Mice were anaesthetized with sodium pentobarbital, the right half of the brain was collected and fixed in 4% paraformaldehyde, then embedded in paraffin for section. Brain tissue slides were dewaxed with xylene, then re-hydrolyzed with ethanol gradient solutions. Antigen was retrieved using heated 0.1 mmol/L sodium citrate, and the sections were blocked and incubated with anti-mTOR antibody (1:100 dilution) and anti-Aβ peptide 1-40 antibody (1:2000 dilution) at 4°C overnight. The sections were then incubated with biotin labeled secondary antibody at 37°C for 1 h. The signal was visualized using DAB staining kit. The intensity of staining was monitored under microscope. Three fields were randomly selected in hippocampus for each slide, the images were analyzed using Image-Pro Plus v6.0 to determine the integrated optical density of positive neuron in each slides.
2.6 Statistical analysis
All experimental results were represented as mean ± SD. The data were analyzed using SPSS13.0 statistical software. Comparison between groups was analyzed using one-way ANOVA method followed by Tukey's post hoc test. P < 0.05 indicated statistical significant difference.
3 RESULTS
3.1 HNG improved learning ability and memory of APP/PS1 transgenic mice
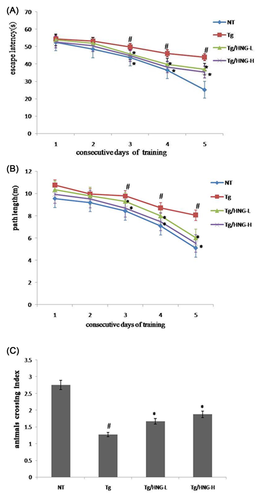
To investigate the effect of HNG on learning ability and memory in APP/PS1 transgenic mice, the Morris Water Maze experiments were performed and the escape latency and path length of all groups of mice were tracked for 5 days after 3 weeks of treatment with HNG (Figures 1A and 1B). All groups had decreased escape latency and path length with increased training. Compared to NT group, Tg group had significantly longer escape latency and path length (P < 0.05). These results indicated that APP/SPI transgenic mice had defects in spatial learning and memory. After HNG treatment, the escape latency and path length in both Tg/HNG-L and Tg/HNG-H groups were significantly decreased (P < 0.05) compared to Tg group. The results from spatial search test on day 6 showed that mice in Tg group had significantly less transpass of quadrant where the platform was located (Figure 1C). In contrast, mice in both Tg/HNG-L and Tg/HNG-H groups had significant increase of quadrant passage. Taken together, these data indicate that HNG could improve learning ability and memory in APP/PSI transgenic mice.
3.2 HNG regulated insulin signaling in the hippocampus of APP/PS1 transgenic mice
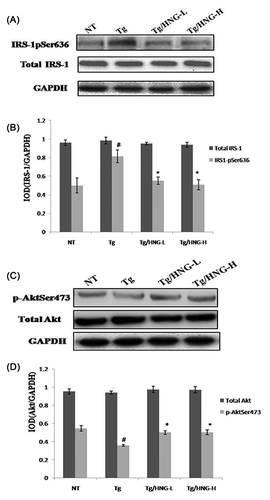
To understand how HNG improved brain function of APP/PS1 transgenic mice, we investigated the effect of HNG on insulin signaling pathway which played an important role in AD development. Insulin receptor substrate 1 (IRS-1) is a key factor in insulin signaling. Phosphorylation of IRS-1 at Ser636 could inhibit its activity and block insulin signaling. Therefore, we performed Western blot analysis to detect the phosphorylation of IRS-1 at Ser636. While total IRS-1 level among all four groups had no significant changes, the phosphorylation of IRS-1 at Ser636 was about twofold higher in Tg group than that in NT group (Figures 2A and 2B), suggesting that APP/PS1 transgenic mice had increased phosphorylation of IRS-1 that led to insulin resistance. After HNG treatment, Ser636 phosphorylation decreased in both Tg/HNG-L and Tg/HNG-H groups (Figures 2A and 2B). In addition, we detected the activity of Akt, an important downstream effector of IRS-1. While total Akt level among all four groups had no significant changes, the phosphorylation of Akt at Ser473 was significantly lower in Tg group than that in NT group. After HNG treatment, Ser473 phosphorylation significantly increased in both Tg/HNG-L and Tg/HNG-H groups (Figures 2C and 2D). These results suggest that HNG could inhibit Ser636 phosphorylation of IRS-1 and activate Akt to enhance insulin signaling and suppress insulin resistance.
3.3 HNG suppresses mTOR activity in the hippocampus of APP/PS1 transgenic mice
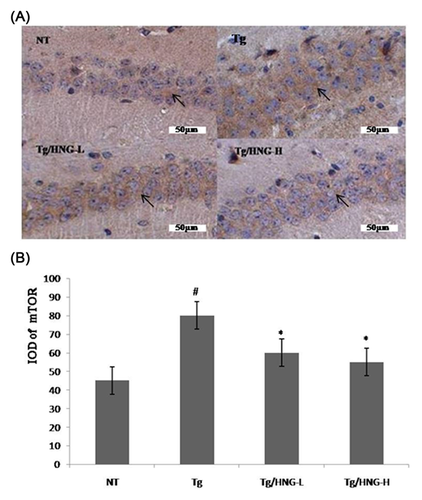
Serine/Threonine kinase mTOR is a key player of insulin signaling pathway. mTOR could negatively regulates insulin signaling through the phosphorylation of IRS-1 inhibitory serine sites Ser636, Ser639, and Ser312. To investigate the effects of HNG on mTOR activity, we performed immunohistochemistry staining of mouse hippocampus tissues. As shown in Figure 3, the number of mTOR positive neurons was higher in the hippocampus of Tg group than in that of NT group, indicating increased mTOR activity and suppressed insulin signaling pathway in the hippocampus of APP/PS1 mice. In contrast, the hippocampus of both Tg/HNG-L and Tg/HNG-H groups had decreased number of mTOR positive neurons (Figure 3), suggesting that HNG treatment reduced mTOR expression and improved insulin resistance in the hippocampus of AD mice.
3.4 HNG improves autophagy function in the hippocampus of APP/PS1 transgenic mice
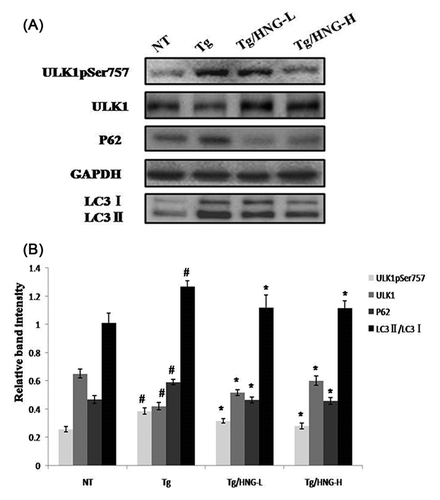
Autophagy is regulated by mTOR and dysfunction of autophagy is known to be involved in AD development. ULK1 is a conserved Ser/Thr kinase pivotal to autophagy induction, it regulates autophagy by phosphorylating downstream components of autophagic machinery.11 To investigate autophagy activity in the brain of AD mice, we compared the expression levels of key autophagy proteins ULK1, p62, LC3 I, and LC3 II in the hippocampus of NT, Tg, and HNG treated mice. As shown in Figure 4, ULK1 expression in the hippocampus of Tg group was lower than that of NT group, while p62, LC3 I, and LC3 II levels significantly increased (P < 0.05). In addition, we detected the activity of ULK1 using the phosphorylation of serine 757 of ULK1 as the marker because the phosphorylation of ULK1 at serine757 leads to the inhibition of ULK1 activity to promote autophagy. We found that ULK1 pSer757 level in the hippocampus of Tg group was higher than that of NT group. These data demonstrated that APP/PS1 transgenic mice had autophagy malfunction in the hippocampus, such as blockage of autophagy initiation, decrease of autophagy activity and accumulation of autophagic bodies. After 4 weeks of HNG treatment, both Tg/HNG-L and Tg/HNG-H groups had increased ULK1 expression, increased ULK1 activity (decreased level of ULK1 p Ser757), decreased p62 expression, and decreased LC3 II/LC3 I ratio in the hippocampus. These results suggested that HNG treatment could enhance autophagy flux in the hippocampus of APP/PS1 transgenic mice and decrease the accumulation of autophagic bodies.
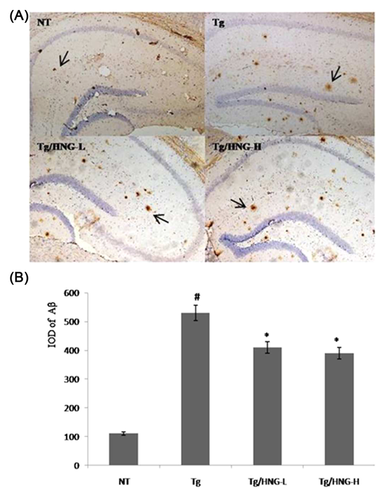
3.5 HNG alleviates the accumulation of Aβ deposition in the brain of APP/PS1 transgenic mice
Autophagy is a key cellular mechanism for the degradation of denatured or abnormal proteins in the cells. Since HNG treatment could enhance autophagy activity in the hippocampus, we wondered whether HNG help degrade Aβ and alleviate its deposition in the brain. Immunohistochemisty analysis of the hippocampus from all four groups showed that senile plaques from APP/PS1 transgenic mice group was about fivefold higher than that in NT group, demonstrating large accumulation of Aβ deposition in APP/PS1 transgenic mice (Figure 5). After 4 weeks of HNG treatment, Aβ deposition was significantly decreased in both Tg/HNG-L and Tg/HNG-H groups compared to Tg group (Figure 5). These results suggested that HNG treatment effectively decreased Aβ deposition in the hippocampus of APP/PS1 transgenic mice.
4 DISCUSSION
In this study, for the first time we demonstrated that HNG could improve brain insulin resistance in APP/PS1 transgenic mice. HNG treatment could enhance autophagy initiation and autophagy flux and decrease the accumulation of autophagosome to alleviate autophagy malfunction, leading to decreased accumulation of Aβ deposition in the hippocampus and improved spatial learning and memory.
Humanin (HN) was identified by the screening of cDNA libraryas a linear 24 amino acids peptide with inhibitory activity to neurotoxin. HN could cross blood brain barrier and offer neuroprotection in vivo.12 S14G is formed by the substitution of Ser14 with Gly, which results in a 1000-fold increase of neuroprotective activity.13 Both in vivo and in vitro studies have shown that HNG could protect neuron in AD through the inhibition of Aβ deposition, the improvement of synapse function, the activation of neurotropic factors, the alleviation of inflammatory response, and the inhibition of apoptosis.14, 15 However, the mechanisms by which HNG regulates brain insulin signaling and neuron autophagy remain unknown.
It is reported that abnormal insulin signaling is correlated with AD and increases risk of AD development.16 Abnormal insulin signaling was found at early stage of AD, and would worsen with the development of AD. IRS-1 is a key factor in insulin signaling pathway which has no kinase activity but its activity is regulated by phosphorylation/dephosphorylation. Serine phosphorylation, especially Ser 636, Ser 639 and Ser 312, of IRS-1 would inhibit IRS-1 activity and block the activation of downstream signaling factors, leading to insulin resistance.17 Our Western blot results showed that the phosphorylation of IRS-1 Ser636 was increased in APP/PS1 transgenic mice, indicative of insulin resistance in the brain of AD mice. After 4 weeks of HNG treatment, inhibitory phosphorylation of IRS-1 Ser 636 was decreased, suggesting that HNG could inhibit IRS-1 Ser636 phosphorylation in the hippocampus of APP/PS1 transgenic mice and alleviate insulin resistance to enhance insulin signaling.
Mammalian target of rapamycin (mTOR) is a Ser/Thr protein kinase that plays an essential role in insulin signaling pathway. Overexpression of mTOR could lead to over-phosphorylation of inhibitory serine sites (Ser 636, Ser 639, and Ser 312) in IRS-1. This will negatively regulate insulin signaling pathway and cause insulin resistance. Consistent with this, our results showed that the expression of mTOR was increased in APP/PS1 transgenic mice, which may cause the phosphorylation of IRS-1 Ser636 and lead to insulin resistance.18 Furthermore, HNG treatment downregulated mTOR expression, decreased the phosphorylation of IRS-1 Ser636, and alleviated insulin resistance.
Autophagy is a lysosome mediated cellular process to clear misfolded protein, old or damaged organelle in the eukaryotes. Autophagy is an important mechanism to maintain cellular homeostasis and prevent tissue necrosis. Recent studies showed the dysfunction of autophagy and the accumulation of autophagosome during AD progression.19, 20 Under physiological condition, autophagy activity remains at very low level. Upon abnormal protein accumulation, oxidative stress or lack of energy, autophagy will be induced. Ubiquitin like kinase 1 (ULK1) is a key regulator of autophagy initiation in mammalian cells and regulated by mTOR signaling. ULK1 can bind autophagy related gene 13 (ATG13) and FIP200 to form a complex, which then binds ATG17 and ATG9 to initiate autophagosome formation. The ratio of LC3 II/LC3 I represents the extent of autophagosome maturation and accumulation. Our results showed that in APP/PS1 transgenic mice, brain ULK1 protein level decreased, while ULK1 p Ser757, p62, and LC3 I/LC3 II levels increased. These results suggest that in APP/PS1 transgenic mice, autophagy is disrupted, such as blockage of autophagy initiation, low activity of autolysosome, and accumulation of autophagosome.21 HNG treatment could induce ULK1 expression and increase ULK1 activity in the brain to promote autophagy in APP/PS1 transgenic mice and enhance the clearance of abnormal proteins as shown by decreased p62 and LC3 I/LC3 II levels.
Aβ deposition and senile plaques are characteristic pathological changes in AD. Aβ can be cleared through enzymatic degradation, transport out and degradation in the cells. The accumulation of Aβ inhibits the activity of lysosomal protein degradation system, this would further worsen dysfunctional autophagy system in AD and lead to increased accumulation of autophagosome.22 Our results showed large number of Aβ plaques in the hippocampus of APP/PS1 transgenic mice. After HNG treatment, Aβ plaques in the hippocampus were significantly decreased. Our results suggest that HNG could increase Aβ degradation through enhancing autophagy.
mTOR is the crosstalk mediator between insulin signaling pathway and autophagy regulation. Insulin sensitivity is positively correlated with autophagy activity in pancreatic β cells, and insulin resistance is a common risk factor for both AD and type II diabetes.23, 24 These findings suggest that brain insulin signaling pathway, especially IRS-1/mTOR may be involved in autophagy regulation. Our results demonstrate that HNG could regulate insulin signaling through IRS-1/mTOR in the hippocampus of APP/SP1 transgenic mice, decrease insulin resistance, improve autophagy in the neuron, decrease Aβ deposition and plaque formation, and improve learning ability and memory. HNG may be a promising agent for AD treatment.
ACKNOWLEDGMENT
This study was supported by Natural Science Fund of Liaoning Province (No. 201601357).
CONFLICT OF INTEREST
No.
AUTHORS’ CONTRIBUTIONS
Kun Han and Ning Jia performed the experiments, Yi Zhong analyzed the data, Xiuli Shang designed the study.