Shikonin Inhibited Migration and Invasion of Human Lung Cancer Cells via Suppression of c-Met-Mediated Epithelial-to-Mesenchymal Transition
ABSTRACT
Epithelial-to-mesenchymal transition (EMT) is a major process to regulate cell migration and invasion. Inhibition of epidermal growth factor receptor (EGFR)-mediated EMT by tyrosine kinase inhibitors (TKIs) is a strategy to prevent lung cancer invasion. However, drug resistance is emerged and accelerated invasion through other signaling bypassing EGFR after TKIs therapy. c-Met signaling pathway is highly activated in EGFR-mutated lung cancer cells. Targeting c-Met signaling pathway may be a strategy to suppress EGFR-independent migration and invasion for lung cancer therapy. Therefore, we examined the anti-migration and anti-invasion abilities of shikonin, an active compound from Lithospermum erythrorhizon, in highly and ligand-induced c-Met activation lung cancer cells. Our results revealed that cell viability and cell cycle progression did not change under 1 μM of shikoinin treatment in highly c-Met expressive HCC827 lung cancer cells. Endogenous c-Met activation was dose-dependently inhibited and the migration and invasion activity of HCC827 cells were suppressed by shikonin treatment. Induction of E-cadherin expression and inhibition of vimentin, slug, and snail expression by shikonin was through c-Met-mediated PI3K/Akt and ERK signaling suppression. Furthermore, hepatocyte growth factor (HGF)-induced migration, invasion and EMT marker change were reversed by shikonin in low c-Met expressive A549 lung cancer cells. Inhibition of HGF-induced c-Met, PI3K/Akt and MEK/ERK activation were observed in shikonin-treated cells. Co-treatment of PI3K/Akt inhibitor or ERK inhibitor with shikonin enhanced shikonin-reversed HGF-regulated EMT marker expression. Taken together, the results suggested that the anti-migration and anti-invasion activities of shikonin was through c-Met inhibition and following by EMT suppression in lung cancer. J. Cell. Biochem. 118: 4639–4651, 2017. © 2017 Wiley Periodicals, Inc.
Lung cancers are the major health threat in the world, especially their high mortality rates [Jemal et al., 2011]. Human lung cancers are classified into two major categories, non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). NSCLC accounts for about 80% of entire lung cancers, especially in adenocarcinoma [Davidson et al., 2013]. Aberrant expression of EGFR in lung cancer has been demonstrated to correlate with a poor prognosis [Hirsch et al., 2003]. Dysregulation of EGFR tyrosine kinase activity is also high correlative with poor prognosis in lung cancers [Arteaga, 2003; Kosaka et al., 2004]. Treatment with EGFR tyrosine kinase inhibitors (TKIs) to inhibit EGFR kinase activity is current therapeutic strategy for lung cancer. However, drug resistance response is generated after TKIs treatment [Kobayashi et al., 2005]. Besides, recurrence by metastasis also cause poor survival rate in lung cancer patients after TKIs treatment. Therefore, searching another molecular target for metastasis and TKI-resistant lung cancer treatment is urgent. Recent studies disclose that amplification of MET gene prompts metastasis [Parikh et al., 2014] and it is a major mechanism to develop resistance of EGFR TKIs in lung cancer treatment [Engelman et al., 2007]. Thus, targeting c-Met signaling might provide another strategy for lung cancer treatment.
c-Met, a receptor tyrosine kinase receptor encoded by MET gene, also known as hepatocyte growth factor receptor (HGFR), regulates many physiological functions, such as proliferation, cellular motility, invasion, epithelial-to-mesenchymal transition (EMT), and angiogenesis [Jiang et al., 1999; Comoglio and Boccaccio, 2001]. During c-Met activation, hepatocyte growth factor (HGF) binds and induces c-Met dimerization, trans-phosphorylation and leads down-stream signaling activation, such as phosphoinositide 3-kinase (PI3K)/Akt, MEK/ERK, and signal transducer and activator of transcription (STAT) 3 [Birchmeier et al., 2003]. Dysregulation of c-Met signaling has been found in numerous cancers. For instance, MET gene activating mutation and/or amplification has been detected in lung cancer, especially in EGFR TKI-resistant lung cancer [Engelman et al., 2007; Lutterbach et al., 2007]. Amplification of MET gene increases phosphorylated c-Met expression and ErbB3 signaling and leads to resistant EGFR TKIs treatment [Engelman et al., 2007]. Furthermore, over-expression of c-Met has highly correlative with lymph node metastasis and decreases survival rate in patients with small oral tongue carcinoma [Lim et al., 2012].
Epithelial-to-mesenchymal transition (EMT) is a key process of tumor cells from epithelial phenotype to mesenchymal phenotype, which promotes cancer cells invasion and contributes to therapy resistance [De Craene and Berx, 2013]. The hallmark of EMT change in cancer cells is down-regulation of epithelial cell marker expression, E-cadherin, and meanwhile, up-regulation of mesenchymal cell marker expression, such as N-cadherin, vimentin, and snail transcriptional factor family [Thiery et al., 2009]. EMT can be induced by many growth factor, such as epidermal growth factor (EGF) and HGF [Thomson et al., 2005; Ogunwobi and Liu, 2011]. Increasing cell migration and invasion ability has been observed in c-Met-activated lung cancer cells and is through c-Met-regulated EMT change [Maulik et al., 2002; Canadas et al., 2014a]. EMT has also been indicated to correlative with drug resistance. Activation of EMT by TGF-β has been demonstrated to resist to crizotinib treatment in lung cancer cells and inhibition of EMT change by silencing vimentin expression restores the sensitive of crizotinib treatment [Kim et al., 2013]. Enhanced resistance to TKIs therapy by EMT activation is also observed in hepatocellular carcinoma and NSCLC [Thomson et al., 2005; Zhao et al., 2012].
Shikonin, an active naphthoquinone of Zi Cao, the root extract of Lithospermum erythrorhizon Sieb. et Zucc, has been used in traditional Chinese medicines for long time [Chen et al., 2002]. Shikonin possesses anti-inflammatory, anti-infection, and anti-tumor activities [Min et al., 2008; Lu et al., 2011]. Suppressing cancer cells proliferation by shikonin has been indicated through EGFR-dependent and -independent signaling pathway [Singh et al., 2003; Tian et al., 2015; Zhao et al., 2015]. Shikonin also inhibits cell invasion through down-regulation NF-κB-mediated matrix metalloproteinase-9 (MMP-9) expression in human adenoid cystic carcinoma cells [Min et al., 2011]. Furthermore, down-regulation Akt/mTOR- and ROS/ERK-mediated MMP-2/-9 expression by shikonin-suppressed cells metastasis is observed in prostate cancer cells [Chen et al., 2014]. Recent study demonstrates that inactivation of NF-κB-Snail signaling by shikonin suppresses lipopolysaccharide-induced EMT and followed by breast cancer cells migration and invasion inhibition [Hong et al., 2015]. However, the molecular mechanism of shikonin inhibits lung cancer cell migration and invasion is still unclear. In the present study, we demonstrated that shikonin-inhibited lung cancer cells migration and invasion was through c-Met-mediated EMT signaling pathway in highly c-Met expression HCC827 lung cancer cells. Furthermore, HGF-induced c-Met activation and c-Met-regulated EMT were also suppressed by shikonin in HGF-induced low c-Met expressive lung cancer cell model.
MATERIALS AND METHODS
CHEMICALS, REAGENTS, AND ANTIBODIES
Shikonin and anti-β-actin antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-p-c-Met, c-Met, p-EGFR, EGFR, p-ERK, ERK, p-Akt, slug, and snail antibodies were obtained from Cell Signaling Technology (Beverly, MA). Anti-Akt and anti-vimentin antibodies were acquired from GeneTex, Inc. (Irvine, CA). Anti-E-cadherin antibody was purchased from BD Biosciences, Inc. (San Jose, CA).
CELL CULTURE AND CELL VIABILITY ASSAYS
Non-small cell lung cancer cell lines H23, A549, and HCC827 were purchased from the American Type Culture Collection (Manassas, VA). All of cell lines were maintained in 5% fetal bovine serum-containing RPMI-1640 (Hyclone Laboratories, Logan, UT) and cultured at 37°C in 5% CO2 atmosphere. HCC827 cells (1 × 104/well) were seeded in 96-well plates for 24 h and then incubated with shikonin (0, 0.25, 0.50, 0.75, 1, and 2 μM) for 24 h. After treatment, cell viability was examined by MTT assay.
CELL CYCLE ANALYSES
Cell cycle analysis was performed as described previously [Yu et al., 2014]. Briefly, A549 and HCC827 cells were seeded and synchronized. After synchronization, shikonin-containing 5% fetal bovine serum media was incubated for 24 h. Cell lysate were then harvested, stained with 50 μg/mL of propidium iodide (Sigma Chemical, St. Louis, MO), and FACScan laser flow cytometer analysis system (Beckman Coulter, Fullerton, CA) was used to detect DNA contents.
IN VITRO WOUND CLOSURE
A549 and HCC827 cells (1 × 105 cells/well) were plated in 6-well plates for 24 h. Cells wounded by scratched with a pipette tip, incubated for 24 h with 0.5% FBS RPMI medium containing or not shikonin (0, 0.25, 0.5, 0.75, 1 µM). Cells were photographed using a phase-contrast microscope (×200), as the descriptions in Kao et al. [2012].
IN VITRO INVASION AND MIGRATION ASSAYS
In vitro invasion and migration assay were measured by modified from Chien et al. [2012]. Briefly, HCC827 and A549 cells were treated with serial concentrations of shikonin (0.25, 0.5, 0.75, 1 μM) for 24 h and cells were collected to plate on Boyden chamber (BD Bioscience, Bedford, MA) at cell density of 1 × 105 cells/well in serum free medium for 24 h incubation. For in vitro invasion assay, 8 μm pore polycarbonate filters were coated with 10 μL Matrigel (25 mg/mL; BD Bioscience, Bedford, MA) and the lower chamber was contained 5% FBS-containing RPMI-1640 medium. The invaded cells were fixed with methanol and stained with 0.1% crystal violet. Cell numbers were counted under a light microscope (×200). In vitro migration assay was carried out as described in vitro invasion assay without Matrigel coating. Triplicate samples were conducted, and data are expressed as the average cell number.
WESTERN BLOT ANALYSES
Western blot analysis was performed as described previously [Yu et al., 2014]. Briefly, cell lysates were prepared and then quantitated, electrophoresed via sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE), and transferred to Immobilon polyvinylidene difluoride membranes (Millipore Co., Billerica, MA). After transfer, the membranes were blocked and incubated with the indicated antibodies. The signals were detected by chemiluminescence (ECL kit, Amersham Pharmacia Biotech, IL, USA). The intensities of protein expression were then quantitated by a UVP BioSpectrum Imaging System ChemiDoc-It2 810 (UVP, LLC, CA, USA). The expression of β-actin was used as the internal control.
STATISTICAL ANALYSES
The results were expressed as the mean ± SD calculated from at least three independent determinations. One-way ANOVA coupled with student's t tests were used to compare individual experiments with the control value. A probability of P < 0.05 was considered to be a significant difference.
RESULTS
THE ANTI-PROLIFERATIVE EFFECTS OF SHIKONIN IN c-Met HIGHLY EXPRESSIVE HCC827 LUNG CANCER CELLS
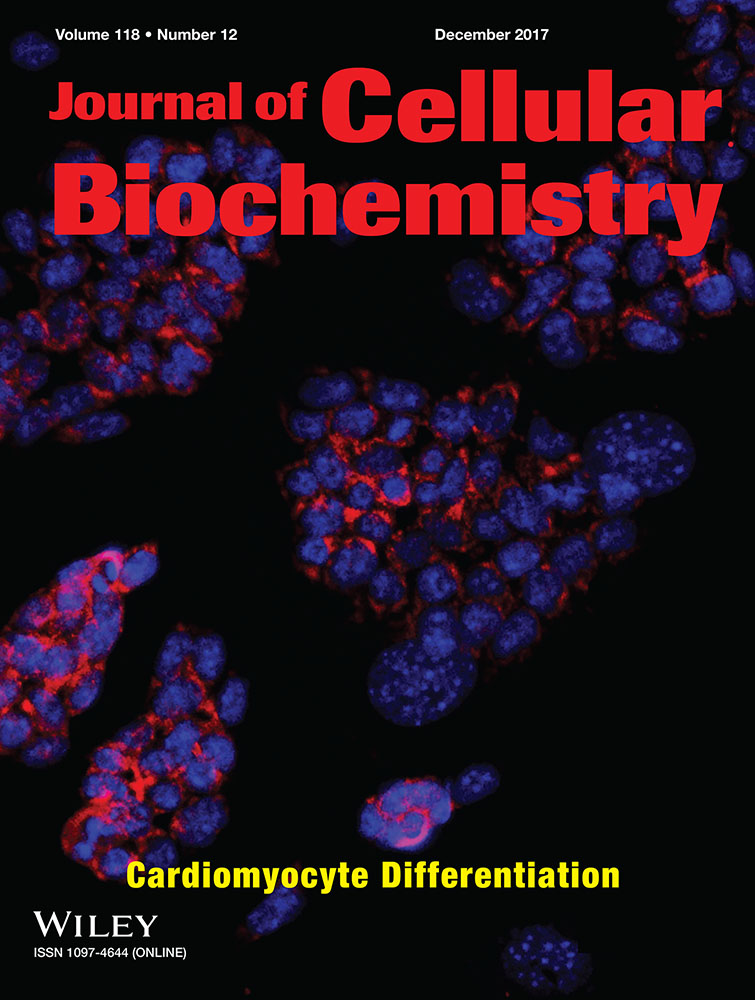
To understand the anti-proliferative effects of shikonin on c-Met highly expressive lung cancer cells, c-Met expression on different lung cancer cells was analyzed. As shown in Figure 1A, phosphorylated c-Met and c-Met were highly expressed in EGFR-mutated HCC827 lung cancer cells. Furthermore, phosphorylated c-Met and c-Met were also observed in EGFR wild-type lung cancer A549, rather than H23 cells (Fig. 1A). To further examine the inhibitory effects of shikonin on c-Met activation, HCC827 cells were treated with serial concentrations of shikonin (0, 0.25, 0.5, 0.75, 1 μM) for 24 h and phosphorylated c-Met was detected afterwards. The results revealed that shikonin inhibited c-Met phosphorylation in a dose-dependent manner in HCC827 lung cancer cells (Fig. 1B). Next, the anti-proliferative effects of shikonin on HCC827 were investigated by cell viability and cell cycle analysis. Treatment up to 1 μM of shikonin was not observed significant decrease in cell viability in HCC827 lung cancer cells. The cell viability was decreased about 27% in 2 μM of shikonin-treated cells (Fig. 1C). To further examine whether shikonin induced cell cycle distribution, HCC827 cells were treated with various concentrations of shikonin for 24 h and cell cycle analyses were performed. As shown in Figure 1D, shikonin did not influence cell cycle distribution up to 1 μM treatment. However, sub-G1 population cells accumulation and G1 population cells decrease was detected after 2 μM of shikonin treatment (Fig. 1D). According to the results, shikonin did not disturb cell viability and cell cycle distribution under 1 μM treatment in HCC827 lung cancer cells.
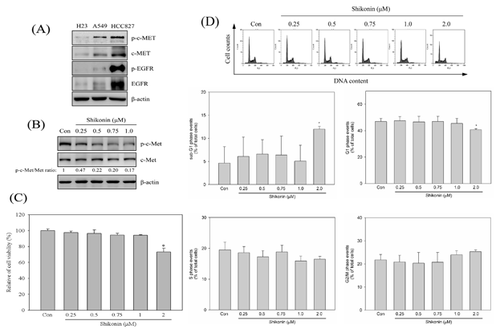
EFFECTS OF SHIKONIN ON MIGRATION AND INVASION IN HCC827 LUNG CANCER CELLS
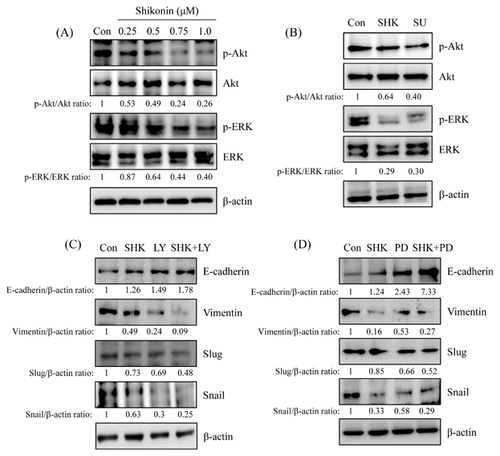
To examine the effects of shikonin in lung cancer cells migration and invasion, HCC827 cells were treated with various concentration of shikonin (0, 0.25, 0.5, 0.75, 1 μM) for 24 h. The wound healing, migration, and invasion assays were performed to evaluate the anti-migration and anti-invasive activity of shikonin. Our results revealed that shikonin inhibited HCC827 cell migration in a dose-dependent mode by wound healing and in vitro migration assay (Fig. 2A and B). In vitro invasion analysis also showed that shikonin inhibited HCC827 cell invasion in a dose-dependent mode (Fig. 2C).
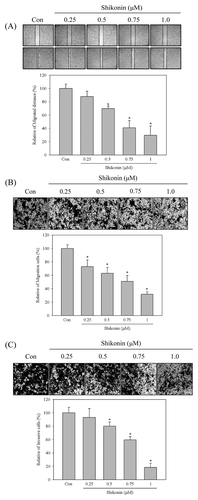
SHIKONIN-REGULATED EMT MOLECULE EXPRESSION WAS THROUGH c-Met SIGNALING
To further investigate whether the anti-migration and anti-invasion activities of shikonin were through EMT regulation, HCC827 cells were treated with various concentrations of shikonin for 24 h and the EMT marker expression was analyzed by Western blot. The results revealed that E-cadherin was increased by shikonin in a dose-dependent mode and was increased about 3.7-fold after 1 μM of shikonin treatment. Vimentin and snail were decreased in a dose-dependent mode (Fig. 3A) and were decreased to 0.21- and 0.37-fold in 1 μM of shikonin-treated cells, respectively. However, inhibition of slug expression was decreased after 1 μM of shikonin treatment and inhibited about 0.3-fold in compared with control (Fig. 3A). c-Met inhibitor, SU11274, was performed to examine the role of c-Met signaling in shikonin-regulated EMT. As shown in Figure 3B, induction of E-cadherin expression in shikonin-treated cells was similar in c-Met inhibitor treatment. Furthermore, the expression of vimentin and snail were also inhibited by c-Met inhibitor. However, c-Met inhibitor-repressed slug expression was more potent than shikonin-treated cells. (Fig. 3B).
SHIKONIN-REGULATED EMT MOLECULE EXPRESSION WAS THROUGH c-Met-MEDIATED Akt AND ERK SIGNALING
PI3K/Akt and MEK/ERK signalings are considered to involve in EMT regulation [Thiery et al., 2009]. To verify the role of PI3K/Akt and MEK/ERK signaling in shikonin-regulated EMT processes, the phosphorylation of Akt and ERK expression was first determined in shikonin-treated HCC827 cells. As shown in Figure 4A, phosphorylation of Akt and ERK were dose-dependently decreased after shikonin treatment. To understand whether shikonin-inhibited Akt and ERK phosphorylation resulted in c-Met inhibition, SU11274 was added to address this issue. The results showed that the expression of phospho-Akt and phospho-ERK was inhibited by c-Met inhibitor treatment (Fig. 4B). And the inhibitory effect of shikonin was similar to c-Met inhibitor. To further verify whether PI3K/Akt and MEK/ERK signalings were involved in shikonin-regulated EMT, pharmacological inhibitors of PI3K/Akt and MEK/ERK, LY294002 and PD98059, respectively, were examined. The outcomes revealed that the expression of E-cadherin in shikonin-treated cells was similar in PI3K/Akt or MEK/ERK inhibitor-treated cells (Fig. 4C and D). Interestingly, E-cadherin expression increased more dramatically in co-treatment with shikonin and PI3K/Akt or MEK/ERK inhibitor than pharmacological inhibitor treatment only. Treatment with PI3K/Akt or MEK/ERK inhibitor alone also inhibited vimentin, snail, and slug expressions. Co-treatment shikonin with PI3K/Akt or MEK/ERK inhibitor more significantly inhibited vimentin, snail, and slug expressions than pharmacological inhibitor treatment alone (Fig. 4B and C).
HGF-INDUCED EMT CHANGE WAS REVERSED BY SHIKONIN
The anti-migration and anti-invasion activities of shikonin might mediate c-Met signaling in HCC827 lung cancer cell model (Fig. 2 and 4). To explore the role of c-Met signaling in shikonin-regulated EMT, HGF, a well-known ligand of c-Met receptor, was added to address this issue. The normal c-Met expression of lung cancer cell line, A549 cells (Fig. 1A), was chosen as study model. The data displayed that the expression of E-cadherin was inhibited in a dose-dependent mode by HGF treatment. And the expression of vimentin, slug, and snail were dose-dependently increased in HGF-treated A549 lung cancer cells (Fig. 5A). Meanwhile, HGF-inhibited E-cadherin expression was reversed by shikonin treatment. Also, HGF-induced vimentin, slug, and snail expression was down-regulated by shikonin treatment (Fig. 5B).
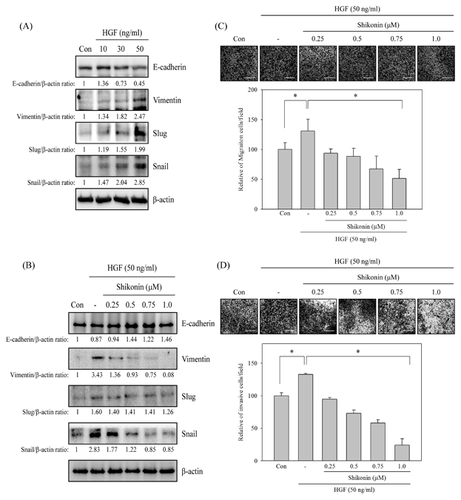
EFFECTS OF SHIKONIN ON HGF-INDUCED MIGRATION AND INVASION IN A549 LUNG CANCER CELLS
Subsequently, the effects of shikonin in HGF-induced lung cancer cell migration and invasion were further studied. A549 cells were pre-treated with or without various concentrations of shikonin (0, 0.25, 0.5, 0.75, 1 μM) for 30 min and then stimulated with HGF (50 ng/mL) for 24 h. The in vitro migration and invasion assays were performed to evaluate the anti-migration and anti-invasion activities of shikonin in HGF induction system. The results exhibited that treatment with HGF alone significantly induced A549 lung cancer cells migration and invasion. However, HGF-induced cell migration and invasion were inhibited by shikonin in a dose-dependent mode (Fig. 5C and D).
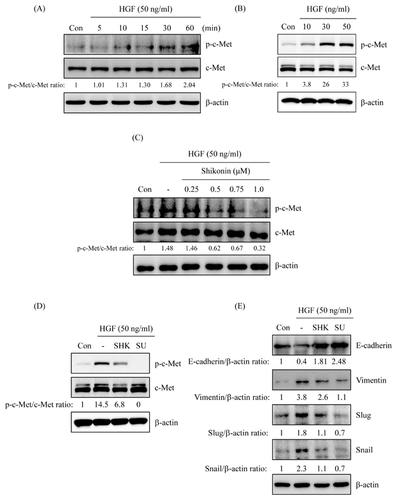
SHIKONIN INHIBITED HGF/c-Met-MEDIATED EMT CHANGE
To further verify shikonin-reversed HGF-induced EMT change was through c-Met signaling, HGF-induced c-Met activation was examined. The results showed that c-Met activation was observed after stimulation with 50 ng/mL of HGF for 10 min and sustained to 60 min (Fig. 6A). Furthermore, c-Met activation was dose-dependently induced by stimulation with HGF for 10 min (Fig. 6B). However, HGF-induced c-Met activation was dose-dependently inhibited by shikonin treatment (Fig. 3C). c-Met kinase inhibitor, SU11274, was examined to understand the mechanism of c-Met signaling involved in shikonin-reversed HGF-regulated EMT marker change. As shown in Figure 6D, HGF-induced c-Met activation was inhibited by shikonin and SU11274. In addition, shikonin-reversed HGF-down-regulated E-cadherin expression was similar to SU11274 treatment as shown in Figure 6E. Meanwhile, shikonin-inhibited HGF-up-regulated vimentin, slug, and snail expressions were comparable in SU11274-treated cells (Fig. 6E).
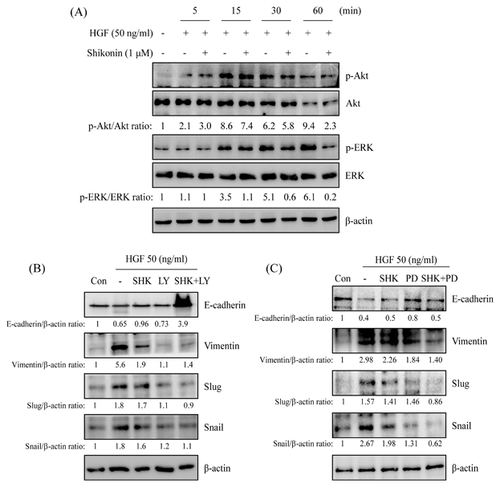
SHIKONIN-GOVERNED HGF-REGULATED EMT MOLECULE EXPRESSION WAS THROUGH Akt AND ERK SIGNALING
The studies discovered that shikonin-regulated EMT change might be through PI3K/Akt and MEK/ERK signaling pathway in c-Met highly expression HCC827 cells (Fig. 4). Phosphorylation of Akt and ERK expressions in HGF-stimulated A549 cells were determined to assess the roles of PI3K/Akt and MEK/ERK signaling in shikonin-regulated EMT change. As shown in Figure 7A, phosphorylation of Akt and ERK were dramatically increased at 15 min and sustained to 60 min after HGF stimulation and inhibited HGF-induced Akt and ERK phosphorylations in pre-treatment with shikonin (Fig. 7A). Pharmacological inhibitors of PI3K/Akt and MEK/ERK were examined to verify the role of PI3K/Akt and MEK/ERK signaling pathways in shikonin-reversed HGF-regulated EMT. The results discovered that shikonin-reversed HGF-inhibited E-cadherin expression was similar in PI3K/Akt and MEK/ERK inhibitor-treated cells (Fig. 7B and C). Interestingly, co-treatment with shikonin and PI3K/Akt or MEK/ERK inhibitor was more dramatically reversed HGF-inhibited E-cadherin expression than shikonin or pharmacological inhibitor treatment alone. Furthermore, PI3K/Akt and MEK/ERK inhibitors also inhibited HGF-induced vimentin, snail, and slug expressions. However, Co-treatment with shikonin and MEK/ERK inhibitor, rather than PI3K/Akt inhibitor, was synergistic inhibited HGF-induced vimentin, snail, and slug expressions than shikonin or pharmacological inhibitor treatment alone (Fig. 7B and C).
DISCUSSION
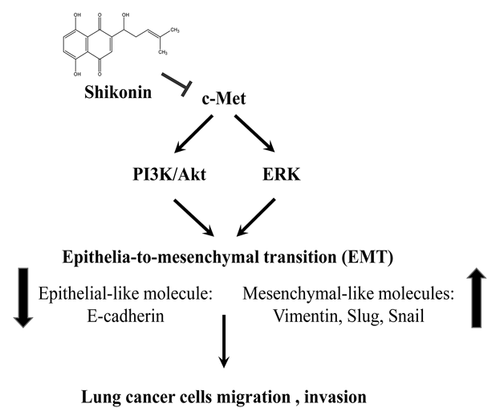
In this study, the anti-migration and anti-invasion activities of shikonin, an active compound from Lithospermum erythrorhizon, was examined in c-Met-regulated EMT. Inhibitory activity of shikonin on migration and invasion might be through c-Met suppression and resulted in PI3K/Akt- and ERK-mediated EMT inhibition in c-Met highly expression HCC827 lung cancer cells. Furthermore, HGF-induced migration, invasion, and EMT change were reversed by shikonin treatment in A549 lung cancer cells. HGF-induced c-Met activation and PI3K/Akt and MEK/ERK activations were also reversed by shikonin treatment (Fig. 8).
Epidermal growth factor receptor mutation in tyrosine kinase domain has been found in NSCLC. Most of these mutations are either in-frame deletions on exon 19 or point mutation at position 858 (L858R). These two type mutations of NSCLC are very sensitive to EGFR TKIs treatment, such as gefitinb (Iressa) or erlotinib (Traceva) [Arteaga, 2003; Kosaka et al., 2004]. Clinical studies have pointed out a 75% response rate for NSCLC patients who harbor these mutation [Riely et al., 2006]. However, acquired resistance on exon 20 of EGFR (T790M) emerges after long-term EGFR TKIs treatment [Kobayashi et al., 2005]. Irreversible EGFR TKIs are a potential therapeutic option for curing this resistance [Kwak et al., 2005]. Furthermore, amplification of the MET gene is another important acquired resistance by gefitinib treatment and is found more frequent in adenocarcinomas than in squamous cell carcinoma [Kobayashi et al., 2005; Engelman et al., 2007]. Overexpression c-Met has been demonstrated to associate with highly metastasis [Lim et al., 2012] and with poor prognosis in NSCLC [Kim et al., 2016]. Ectopic activation c-Met signaling pathway increasing lung cancer cell invasion and migration has been investigated [Maulik et al., 2002]. Suppression c-Met/PI3K/Akt signaling pathway inhibits EMT-induced migration and invasion in NSCLC and SCLC [Canadas et al., 2014a; Chen et al., 2016]. Accordingly, targeting MET signaling pathway may be a strategy for suppressing cancer cells migration and invasion in NSCLC. In the present study, the expression of c-Met and phospho-c-Met in EGFR-mutant HCC827 lung adenocarcinoma cells was higher than EGFR wild-type A549 and H23 lung adenocarcinoma cells (Fig. 1A). Additionally, the expression of phospho-c-Met was dose-dependently inhibited by shikonin in HCC827 cells (Fig. 1B). However, inhibition c-Met activation did not affect cell growth and cell cycle distribution under 1 μM of shikonin treatment (Fig. 1C and D). Cell growth inhibition and sub-G1 cells accumulation were only detected in 2 μM of shikonin-treated cells. Moreover, shikonin inhibited cell migration and invasion of HCC827 cell was found in a dose-dependent mode (Fig. 2).
Epithelial-to-mesenchymal transition is well known in involving cancer cell malignant and metastasis [De Craene and Berx, 2013]. EMT is controlled through down-regulation of epithelial-like molecules, such as E-cadherin, and up-regulation of mesenchymal-like molecules, such as vimentin. The up-regulation of transcription factors, such as snail family, which represses E-cadherin expression are also observed in EMT [Thiery et al., 2009]. Clinical studies indicate that mesenchymal-like features of cancer cells are associated with poor prognosis [Al-Saad et al., 2008]. Therefore, inhibiting cancer cells EMT may promote prognosis and improve the survival rate. Meanwhile, PI3K/Akt and MEK/ERK signaling pathways also involve in EGF/EGFR-induced EMT [Ahmed et al., 2006]. However, alternative routes activating PI3K/Akt and MEK/ERK through bypassing EGFR induction have been demonstrated the cause of acquired resistance. Acquired resistance by MET amplification in lung cancer through ErbB3 activation, a heterodimerized partner of EGFR, resulting the activation of PI3K/Akt signaling pathway is one of the conceivable mechanism bypassing EGFR induction [Engelman et al., 2007]. Furthermore, over-expression of c-Met promotes small oral tongue carcinoma cell invasion and metastasis [Lim et al., 2012]. Accordingly, we speculated that inhibition of c-Met signaling pathway may suppress EGFR-independent EMT in lung cancer cells. The results exposed that shikonin induced E-cadherin expression and inhibited vimentin and snail expression in a dose-dependent mode (Fig. 3A). Down-regulation of the protein levels of slung, another transcription factor of snail family, was only observed in 1 μM of shikonin-treated cells. Pharmacological inhibitor of c-Met (SU11274) also added to address the role of c-Met signaling pathway in shikonin-inhibited EMT. The data showed that shikonin-regulated EMT marker change was similar to SU11274 treatment (Fig. 3B). Moreover, shikonin also inhibited Akt and ERK phosphorylation (Fig. 4A). Co-treatment with shikonin and PI3K/Akt or MEK/ERK inhibitor dramatically induced E-cadherin expression. Meanwhile, the expression of vimentin and snail, but not slug, were significantly inhibited rather than inhibitor alone (Fig. 4C and D). Conclusively, the mechanism of shikonin inhibiting EMT-regulated migration and invasion through c-Met-mediated signaling pathways was suggested in HCC827 cells.
HGF is a blood-derived factor produced mainly from mesenchymal cells and acts on epidermal- or endothelial-derived cells [Bussolino et al., 1992]. It has been known that HGF involves in cancer growth and metastasis by enhancing cancer cell motility and angiogenesis [Trusolino and Comoglio, 2002]. Clinical studies indicated that serum concentration of HGF is highly correlative with lung cancer progression [Bharti et al., 2004; Ujiie et al., 2012]. High serum HGF concentration associates with a mesenchymal phenotype in SCLC patients [Canadas et al., 2014b]. Analyzing clinical lung cancer specimens from acquired resistance to EGFR TKIs shows high expression of HGF in Japanese patients. The expression of HGF is higher in acquired resistance tumor and associates with poor prognosis [Siegfried et al., 1997; Yano et al., 2011]. Furthermore, HGF also rescuing lapatinib-induces gastric cancer cell and cetuximab-treated colorectal cancer cell growth inhibition [Liska et al., 2011; Chen et al., 2012]. These discoveries indicate that down-regulating HGF expression or suppression of HGF/c-Met signaling pathway is novel strategy for inhibiting metastasis and drug resistance cancer. This studies disclosed that shikonin-inhibited migration and invasion were through c-Met-mediated EMT in HCC827 cells. The roles of HGF/c-Met-regulated EMT in shikonin-inhibited lung cancer cell migration and invasion were evaluated. Low c-Met expression A549 cells were chosen as a study model. The expression of E-cadherin was dose-dependently inhibited and the expression of vimentin, slug and snail were dose-dependently induced by HGF treatment (Fig. 5A). Pre-treatment with shikonin rescued HGF-inhibited E-cadherin expression. Meanwhile, HGF-induced vimentin and snail expressions were dose-dependently suppressed by shikonin treatment (Fig. 5B). Our results also exposed that HGF-induced migration and invasion was inhibited by shikonin (Fig. 5C and D). Suppression of HGF-induced slug expression was only detected in high dosage (1 μM) of shikonin-treated A549 cells. Inhibition of slug expression was only observed in high dosage of shikonin-treated HCC827 and HGF-induced A549 cells (Fig. 3 and 5). Shikonin-inhibited EMT through snail-dependent signaling pathway under low concentration treatment was suggested. And slug-dependent EMT signaling pathway may only involve in high dosage of shikonin-treated cells.
The expression of phospho-c-Met was inspected to further understand the repressive mechanism of shikonin in HGF-induced c-Met activation and c-Met-regulated EMT. HGF-induced c-Met activation in a time- and dose-dependent mode (Fig. 6A and B). Pre-treatment with shikonin inhibited HGF-induced c-Met activation (Fig. 6C). Furthermore, HGF-induced c-Met activation was also inhibited by SU11274 (Fig. 6D). In addition, HGF-down-regulated E-cadherin expression and -up-regulated vimentin, slug, and snail expressions were reversed by shikonin as well as c-Met inhibitor (SU11274) treatment (Fig. 6E). HGF-induced Akt and ERK activations were inhibited by shikonin treatment (Fig. 7A). Co-treatment with shikonin and PI3K/Akt or MEK/ERK inhibitor dramatically inverted HGF-down-regulated E-cadherin expression and repressed HGF-up-regulated vimentin, slug and snail expressions (Fig. 7B and C).
Epidemiological studies demonstrate that traditional Chinese herbal medicine possesses cancer prevention activities [Liu et al., 2015]. Lithospermum erythrorhizon Sieb. et Zucc, containing shikonin, has been used in traditional Chinese medicine and possesses anti-inflammatory [Chen et al., 2002; Lu et al., 2011] and anti-cancer [Singh et al., 2003; Tian et al., 2015] properties. However, the anti-migration and anti-invasion activities of shikonin are controversial. Shikonin has been indicated to prompt EMT through Zeb1 and Zeb2 up-regulation in skin wound healing [Yin et al., 2013]. The other study shows that shikonin-inhibited lipopolysaccharide-induced EMT is through inactivation of NF-κB/snail signaling pathway in breast cancer [Hong et al., 2015]. Therefore, the anti-migration and anti-invasion molecular mechanism of shikonin is needed to be clarified. Shikonin has been verified to inhibit EGFR activation resulting in the inactivation of EGFR-regulated signaling pathway [Singh et al., 2003; Tian et al., 2015; Zhao et al., 2015]. In the meantime, induction erlotinib-resistant glioblastoma cell death by combination with shikonin and erlotinib is also addressed [Zhao et al., 2015]. Together, the results uncover that suppressing drug resistance cancer cells growth by shikonin is through EGFR-independent mechanism. In the present study, shikonin inhibited endogenous c-Met activation and c-Met-mediated EMT in HCC827 cells (Figs. 1 and 3). Meanwhile, shikonin also inhibited HGF-induced c-Met activation in A549 cells (Fig. 6). Shikonin was ever demonstrated as an inhibitor of c-Met signaling pathway. However, it is still unclear how shikonin inhibits c-Met activation in a dose-dependent manner. Activation PI3K/Akt signaling bypassing EGFR is displayed through ErbB3-associated c-Met in gefitinb-resistance lung cancer cells [Engelman et al., 2007]. Shikonin disrupting c-Met/ErbB3 association and subsequently suppressing c-Met activation was therefore hypothesized. In addition, competition with HGF to bind c-Met by shikonin might be another mechanism. More experimental designs are needed to address our hypothesis. In conclusion, shikonin might be a potential anti-migration and anti-invasion candidate through c-Met inhibition in lung cancer cells (Fig. 8). It is worthy to further investigate the anti-invasive activity of shikonin in vivo model.
AUTHORS’ CONTRIBUTIONS
Yei-San Hsieh, Chiung-Ho Liao, Meng-Shih Weng conceived, designed the experiments. Wan-Shen Chen performed the experiments. Chiung-Ho Liao, Jih-Tung Pai, Meng-Shih Weng analyzed the data. Meng-Shih Weng wrote the manuscript.
ACKNOWLEDGMENT
This work was supported by grants from the Taoyuan General Hospital, Ministry of Health and Welfare, Taiwan, Republic of China, PTH10410.