Short-Term Hypoxia Reverses Ox-LDL-Induced CD36 and GLUT4 Switching Metabolic Pathways in H9c2 Cardiomyoblast Cells
ABSTRACT
High levels of circulating low-density lipoproteins (LDL, plasma proteins that carry cholesterol and triglycerides) are associated with type 2 diabetes, arteriosclerosis, obesity, and hyperlipidemia. In the heart, the accumulation of oxidized low-density lipoprotein (Ox-LDL) has been proposed to play a role in the development of cardiovascular disease. We obtain cholesterol from animals and animal-derived foods such as milk, eggs, and cheese. In previous studies, the ratio of low-density lipoprotein (LDL) and high-density lipoprotein (HDL) was shown to be important for our health. High levels of LDL cholesterol lead to atherosclerosis, increasing the risk of heart attack and ischemic stroke. In this study, we utilized Ox-LDL-treated H9c2 cardiomyoblast cells as a simulated hyperlipidemia model. CD36 metabolism pathway proteins (phospho-Akt, SIRT1, PGC1α, PPARα, CPT1β, and CD36) increased at low doses of Ox-LDL. However, high doses (150 and 200 mg/dL) of Ox-LDL reduced the levels of these proteins. Interestingly, expression of GLUT4 metabolism pathway proteins (phospho-PKCζ) were reduced at low doses, while the expression of phospho-AMPK, phospho-PI3K, phospho-PKCζ, GLUT4, and PDH proteins increased at high doses. Ox-LDL acute treatment induces apoptosis in cardiomyocytes as evidenced by apoptotic nuclei apparition, caspase-3 activation, and cytochrome c release from mitochondria. In our results, Ox-LDL induced lipotoxicity in cardiomyocytes, and subsequent exposure to short-term hypoxia or reversed the Ox-LDL-induced metabolic imbalance. The same result was obtained with the pharmacological activation of SIRT1 by resveratrol and si-PKCζ. The mechanism of metabolic switching during Ox-LDL lipotoxicity seems to be mediated by SIRT1 and PKC ζ. J. Cell. Biochem. 118: 3785–3795, 2017. © 2017 Wiley Periodicals, Inc.
The energy source of the heart is fatty acid and glucose, which are carried into the cardiac cells by cluster of differentiation (CD36) and glucose transporter type 4 (GLUT4), respectively [Luiken et al., 2009]. A healthy heart metabolic regulation is linked with cardiac function. Cardiac hypertrophy and heart failure are closely related to metabolism dysfunction [Lopaschuk et al., 2010]. Cardiomyocytes are able to switch fatty acids (50–70%) and glucose (30–50%) depending on the different environment, for example, ischemia or anoxia [Stanley et al., 1992, 1997]. In our previous studies, hypoxia and intermittent hypoxia had both damaging and protective effects in different physiological conditions. The short-term intermittent hypobaric hypoxia in myocardial reperfusion provides benefits and prevents adverse cardiac remodeling [Lin et al., 2008]. In a rat model, short-term hypoxia and long-term hypoxia have different effects on the survival and apoptosis pathways in rat hearts [Lee et al., 2006]. In cardiopulmonary training, short-term hypoxia challenges may be beneficial for cardiac function, contributing to the improvement of training [Hoppeler and Vogt, 2001]. Our study concluded that short-term intermittent hypoxia for 1 and 4 days appeared to exert protective effects on hearts, whereas long-term intermittent hypoxia for 1 and 2 weeks appeared to exert deleterious effects in the rat hearts and was regulated by the B-cell lymphoma 2 (Bcl2) family and cytochrome c oxidase [Lee et al., 2006]. Resveratrol is a type of natural phenol, and there is some evidence of the biochemical and physiological action of resveratrol in heart disease, for example, estrogenic [Chottanapund et al., 2014], antiplatelet [Vilahur and Badimon, 2013], and anti-inflammatory properties [Latruffe et al., 2015]. Recent studies determined the cardioprotective efficacy of resveratrol, both in acute and chronic experiments models. Resveratrol attenuates myocardial ischemic reperfusion injury [Zheng et al., 2015], atherosclerosis [Buttari and Profumo, 2014], hypercholesterolemia [Deng et al., 2015] and reduces ventricular arrhythmias [Chen et al., 2008]. Resveratrol supplementation at high doses has an effect on blood pressure [Liu et al., 2015]. Resveratrol treatment, as an adjunct to pharmacological management in type 2 diabetes mellitus, significantly improved multiple cardiometabolic biomarkers [Hausenblas et al., 2015]. Moderate red wine drinking is known to reduce the risk of heart disease [Ferrieres, 2004; Szmitko and Verma, 2005], stimulate endothelial nitric oxide synthase (eNOS) activity [Duffy and Vita, 2003] and, as an antioxidant, contribute to the inhibition of platelet aggregation [Olas and Wachowicz, 2005]. Therefore, the cardioprotective effects of resveratrol are considered to be the best method of cardioprotection rather than a direct therapy [Das and Maulik, 2006]. Low-density lipoprotein (LDL) is often related to the human risk of cardiovascular disease [Steinberg, 1997], which are oxidized by the endothelium, which can be easily retained by the proteoglycans [Steinberg and Witztum, 2002]. When the LDL particles increase in the blood vessels they contribute to the development of atherosclerosis [Itabe, 2009; Dashti et al., 2011], stroke, and vascular disease, resulting in plaque and clot formation [Parthasarathy et al., 2010]. The aim of our present study was to check whether short-term hypoxia protects cardiomyocyte injuries against Ox-LDL-induced toxicity. We further explored the switch of the fatty acid and glucose metabolism pathways during Ox-LDL treatment. Further we analyze whether 5′ adenosine monophosphate-activated protein kinase (AMPK), phosphatidylinositide 3-kinases (PI3K) and the survival pathway are regulated after Ox-LDL treatment and short-term hypoxia for 0.5 or 2 h. Additionally, we determined whether Ox-LDL and hypoxia treatment induced the translocation of CD36 and GLUT4 receptors to the sarcolemma.
MATERIALS AND METHODS
CELL CULTURE
Rat embryonic cardiac myoblasts (ATCC, Manassas, VA) H9c2 cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM, Sigma–Aldrich, MO) supplemented with 10% cosmic calf serum (CCS) (HyClone), 1 mM pyruvate, 2 mM glutamine, 100 U/mL penicillin and 100 mg/mL streptomycin. For each experiment, the H9c2 cardiomyoblast cells were used between passages 20 and 45 and then were placed in a hypoxia chamber (95% N2, 1% O2) (NexBiOxy, Hsinchu, Taiwan) inside a humidified incubator (Thermo, NY). This chamber was filled with a gas mixture (21% O2 and 5% CO2) at 37 °C for the indicated time and calibrated in 1% oxygen concentration by an oxygen analyzer (NexBiOxy). In this study, the H9c2 cells were pretreated with resveratrol and PKC ζ siRNA using the indicating amounts (20 nM) for 24 h before being exposed to hypoxia for 0.5 h. In addition, the H9c2 cells were subsequently pretreated with 150 mg/dL Ox-LDL for 24 h and exposed to short-term and long-term hypoxia. For short time hypoxia, cells were exposed to 1% oxygen for 5 min, 30 min and 2 h. For long time hypoxia, cells were exposed to 1% oxygen for 6, 12, and 24 h.
WHOLE CELL EXTRACTION
Cultured H9c2 cells were scraped and washed twice with PBS. Then, the cell suspension was spun down, and the cell pellets were lysed for 20–30 min in lysis buffer (50 mM Tris (pH 7.5), 0.5 M NaCl, 1.0 mM EDTA (pH 7.5), 10% glycerol, 1 mM BME, 1% IGEPAL-630 and proteinase inhibitor) and was spun down at 14,010 g for 30 min. The supernatant was collected in a new eppendorf tube and stored at −80°C.
LOWRY PROTEIN ASSAY
Bovine Serum Albumin (BSA) was diluted to 0, 0.1, 0.25, 0.5, 0.75, 1 mg/mL and used as the standards in the Lowry method for determining protein concentration using Folin–Ciocalteu reagent for enhanced color development. The protein was first reacted with alkaline cupric sulfate in the presence of tartrate (2% Na–K tartrate:1% CuSO4 · 5H2O:2% Na2CO3 in 0.1 N NaOH = 1:1:98) during a 15–20 min incubation at room temperature. During this incubation, a tetradentate copper complex formed from four peptide bonds and one atom of copper. Following the incubation, Folin phenol reagent was added. It is believed that the color enhancement occurs when the tetradentate copper complex transfers electrons to the phosphomolybdic/phosphotungstic acid complex. Finally, the OD values were read at 750 nm after a 20–30 min room temperature incubation.
WESTERN BLOT ANALYSIS
Proteins were separated by an 8–13% SDS–PAGE and transferred onto a PVDF membrane (Millipore, Belford, MA). The membranes were blocked with 5% non-fat dry milk blocking buffer in 20 mM Tris-HCl, pH 7.6, 150 mM NaCl, and 0.1% Tween 20 for at least 1 h at room temperature or overnight at 4°C. The membranes were incubated with the following primary antibodies: (1) β-actin (Santa Cruz); (2) phospho-Akt (Santa Cruz); (3) phospho-PI3K (Cell Signaling); (4) phospho-PKCζ (Cell Signaling); (5) SIRT1 (Santa Cruz); (6) PGC1α (Santa Cruz); (7) PPARα (Santa Cruz); (8) CD36 (Santa Cruz); (9) phospho-AMPK (Cell Signaling); (10) GLUT4 (Santa Cruz); (11) CPT1β (Genetex); (12) pyruvate dehydrogenase (Cell Signaling); (13) caspase 3 (Cell Signaling); and (14) cleaved caspase 3 (Cell Signaling) in the above solution on an orbital shaker at 4°C overnight. Following the primary antibody incubation, the membranes were incubated with horseradish peroxidase-linked secondary antibodies (anti-rabbit, anti-mouse, or anti-goat IgG) for 1–1.5 h. Densitometric analyses of the immunoblots was performed using the Fuji LAS 3000 imaging system. For repeated blotting, the nitrocellulose membranes were stripped with restore Western blot stripping buffer at room temperature for 20 min.
GENE SILENCING THROUGH siRNA
The specific double-stranded small interfering RNA (siRNA) sequences were obtained from Sigma–Aldrich. The cells, at 50% confluence, were replaced into fresh culture medium containing serum 2 h before the transient transfection, and then an siRNA targeting PKC ζ and the non-specific siRNA (scrambled) were introduced in the cells using the PureFection™ Nanotechnology-based Transfection Reagent (System Biosciences) following the manufacturer's protocol. The effective gene silencing was measured by Western blot.
ELISA ASSAY OF MEMBRANE PROTEIN
The H9c2 cells were seeded at a density of 1 × 105 cells/well into 24-well plates, and the cells were exposed to Ox-LDL for 24 h. The cells were washed for 5 min with 1 × PBS three times and blocked with 5% goat serum at 37°C for 1 h. The cells were washed for 5 min with 1 × PBS three times and were incubated with 1:1000 diluted primary antibodies against CD36 (Santa Cruz) and GLUT4 (Santa Cruz) at 4°C overnight and were then incubated with secondary antibodies at 37°C for 1.5 h. The antibody interactions were visualized with the 3, 3′, 5, 5′-Tetramethylbenzidine (TMB) substrate (Thermo Scientific) at 37°C for 30 min before adding H2SO4 at room temperature, and the OD values were read at 450 nm.
LIPOPROTEIN SEPARATION
The lipoprotein separation of human plasma was obtained from the Taichung Blood Bank (Taichung, Taiwan), and LDL was isolated using sequential ultracentrifugation (1.3344 g/mL) in a KBr solution containing 30 mM EDTA, stored at 4°C in a sterile and dark environment, and used within 3 days as previously described [Havel et al., 1955]. Immediately before the oxidation tests, LDL was separated from the EDTA and from the diffusible low-molecular-mass compounds by gel filtration on a PD-10 Sephadex G–25 M gel (Pharmacia) in 0.01 M phosphate-buffered saline (PBS: 136.9 mmol/NaCl, 2.68 mmol/KCl, 4 mmol/Na2HPO4, and 1.76 mmol/ KH2PO4) at pH 7.4. Cu2+ modified LDL (1 mg protein/mL) was treated with LDL exposing it to 10 µM CuSO4 for 24 h at 37°C.
NEONATAL CARDIOMYOCYTE PRIMARY CULTURE
Animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85–23, revised 1996) under a protocol approved by the Animal Research Committee of China Medical University, Taichung, Taiwan. Neonatal rat ventricular cardiomyocytes (NRVMs) were isolated and cultured using the commercially available Neonatal Rat Cardiomyocyte Isolation Kit (Cellutron Life Technology, MD) according to the manufacturer's guidelines. Hearts from one-day-old newborn Sprague–Dawley rats (BioLASCO, Taipei, Taiwan) were separated, and the ventricles were pooled. The ventricular cells were released with digestion buffer at 37°C. Ventricular cardiomyocytes were grown in NS medium with 10% fetal bovine serum (FBS), 2 mM glutamine, 100 g/mL streptomycin, and 100 g/mL penicillin. After 2–3 days, the cells were seeded in DMED medium and were treated with different drugs.
ANNEXIN V-FITC/PI STAINING
The H9c2 cells seeded at a density of 5–5.5 × 105 cells/well into 6 cm dishes, and after 24 h the H9c2 cells were treated with Ox-LDL and resveratrol or si-PKCζ. Apoptotic cells were monitored by FACSC and flow cytometery (BD, CA) using the Annexin V-FITC Apoptosis Detection Kit (BioVision). The total cells and supernatants were collected, washed, and incubated for 10 min with 1× binding buffer containing annexin V-conjugated FITC and propidium iodide (PI). Annexin V-FITC staining was used to detect the cell membrane exposure of phophatidylserine (PS), and the cells that have lost membrane integrity were stained by PI. Cells that were Annexin V positive were considered early apoptotic cells. Cells positive for both annexin V and PI were considered late apoptotic cells, whereas, the viable cells were unstained.
THIOBARBITURIC ACID-REACTIVE SUBSTANCES (TBARS) ASSAY
The H9c2 cells were seeded at a density of 8 × 105 cells/well in 10 cm dishes. After 24 h, the cells treated with Ox-LDL and resveratrol or si-PKCζ. The H9c2 cells were monitored using the method described by Khuwaja et al. [2011], which was modified for the estimation of lipid peroxidation in the H9c2 cells [Islam et al., 2002]. The 500 µL homogenate was pipetted into a glass tube and 5% trichloroacetic acid (TCA) and 0.67 thiobarbituricacid (TBA) was added. Then, each sample was centrifuged at 1000g for 15 min. The supernatant was transferred to another tube and placed at 100°C for 10 min. After 10 min, each sample was cooled. Then, the absorbance of the color was read at 535 nm. The TBARS (nmol/mL/mg total protein protein) was calculated using the rate of lipid peroxidation.
STATISTICAL ANALYSIS
The statistical results are presented as the mean ± S.D. All of statistical analyses were performed with GraphPad Prism software 5, consisting of an ANOVA test followed by Tukey's post-hoc test when group differences were detected at the 5% significance level. Differences were considered significant when P < 0.05.
RESULTS
OX-LDL AND LDL-INDUCED CELL DEATH IN H9c2 CARDIOMYOBLAST CELLS
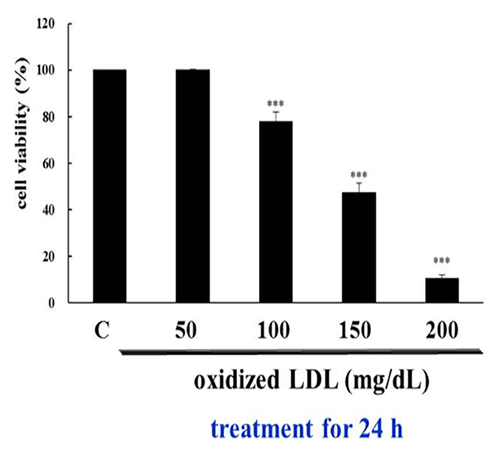
H9c2 cells were treated with various concentrations of ox-LDL and LDL for 24 h, and their viability was measured with the 3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Treatment with 50–200 mg/dL LDL had no effect on the H9c2 cells, but in 100 mg/dL Ox-LDL, the cells showed a significant decrease in viability. The data are expressed as the percentage of control cells and presented as the mean ± S.D of the three different experiments. The asterisks indicate the significant differences (*P < 0.05, **P < 0.01, and ***P < 0.001) between the LDL and Ox-LDL-treated and media control groups (Fig. 1).
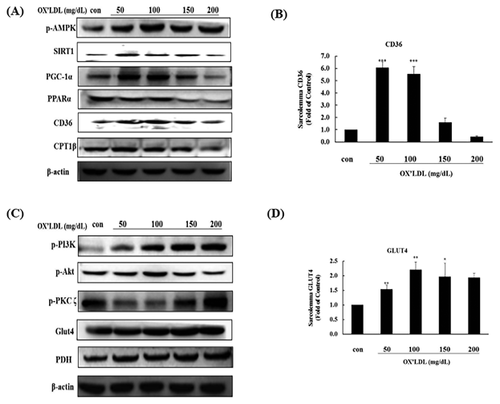
DIFFERENT DOSES OF OX-LDL AFFECT CHANGES IN THE CD36 AND GLUT4 METABOLISM PATHWAYS IN H9c2 CELLS
We investigated the effects of a range of concentrations (50–200 mg/dL) of Ox-LDL on the metabolic pathways of H9c2 cardiomyoblast cells to mimic a lipotoxic environment. The expression of CD36 metabolism pathway proteins (phospho-AMPK, SIRT1, PGC1α, PPARα, CPT1β, and CD36) increased at low doses of Ox-LDL. However, high doses of Ox-LDL reduced the levels of these proteins (Fig. 2A), and significant reductions in CD36 membrane protein levels were observed at the 150 and 200 mg/dL doses (Fig. 2B). Conversely, the expression of GLUT4 metabolism pathway proteins (phospho-PKCζ) was reduced at low doses, while the expression of phospho-Akt, phospho-PI3 K, phospho-PKCζ, GLUT4, and PDH proteins increased at high doses (Fig. 2C). GLUT4 protein expression was significantly increased at the 50 mg/dL and 100 mg/dL doses (Fig. 2D).
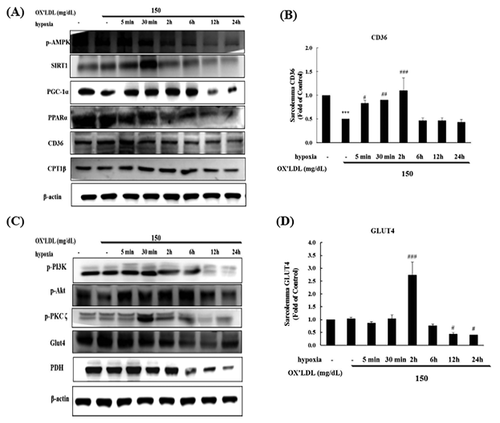
TIME DEPEDENT EFFECTS OF HYPOXIC PRECONDITIONING ON CD36 AND GLUT4 METABOLIC PATHWAYS IN H9c2 CELLS
To look for time-dependent effects, H9c2 cells were treated with 150 mg/dL Ox-LDL and a preconditioning hypoxic treatment for 5 min–24 h, and we investigated the effects on the metabolic pathways in H9c2 cardiomyoblast cells. The expression of CD36 metabolism pathway proteins (phospho-PI3K, phospho-Akt, SIRT1, PGC1α, PPARα, CD36, and CPT1β) increased after short-term exposure to Ox-LDL, while long-term exposure resulted in reduced levels of the CD36 metabolism pathway protein expression (Fig. 3A). CD36 membrane protein levels were significantly reduced at 6–24 h (Fig. 3B). The expression of GLUT4 metabolism pathway proteins (phospho-PI3K, phospho-PKCζ, phospho-AMPK, GLUT4, and PDH) was not significantly changed after short-term exposure, while phospho-PI3K, phospho-PKCζ, phospho-AMPK, GLUT4, and PDH proteins levels were significantly decreased after long-term exposure (Fig. 3C). The expression of the GLUT4 protein was significantly reduced at 12 and 24 h and significantly increased at 2 h. Phospho-PKCζ protein levels were significantly reduced at 12 and 24 h, and significant correlations were found with GLUT4 (Fig. 3D).
EFFECT OF RESVERATROL AND SHORT-TERM HYPOXIA ON THE CD36 METABOLISM PATHWAY IN H9c2 CELLS TREATED WITH OX-LDL
H9c2 cells were pretreated with 25 µM of the SIRT1 activator resveratrol following co-treatment with 150 mg/dL Ox-LDL for 24 h. SIRT1, PGC1α, PPARα, CD36, and CPT1β protein expression levels were reduced by treatment with 150 mg/dL Ox-LDL for 24 h in the H9c2 cells. However, changes in SIRT1, PGC1α, PPARα, CD36, and CPT1β protein expression were reversed by treatment with 0.5 h short-term hypoxia and resveratrol (Fig. 4A). CD36 membrane protein expression was significantly reduced by treatment with 150 mg/dL Ox-LDL for 24 h in the H9c2 cells. However, CD36 membrane protein levels significantly increased when the H9c2 cells were pretreated with 0.5 h short-term hypoxia and resveratrol; short-term hypoxia and resveratrol even showed a synergistic effect (Fig. 4B).
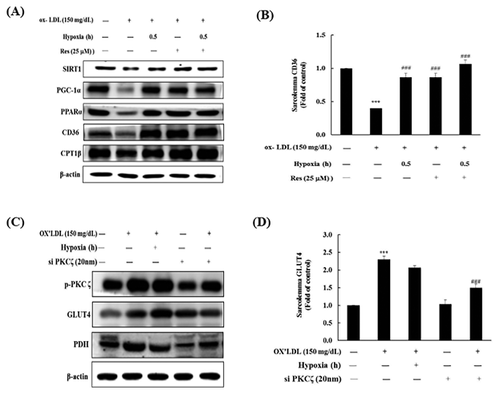
ROLE OF PKCζ ON GLUT4 METABOLIC PATHWAY PROTEINS DURING OX-LDL TREATMENT IN H9c2 CELLS
Phospho-PKCζ, GLUT4, and PDH protein expression levels increased following treatment with 150 mg/dL Ox-LDL for 24 h in the H9c2 cells. Additionally, phospho-PKCζz, GLUT4, and PDH protein expression was enhanced by treatment with 0.5 h short-term hypoxia and si-PKCζ (Fig. 4C). GLUT4 membrane protein levels significantly increased when the H9c2 cells were pretreated with 150 mg/dL Ox-LDL for 24 h. GLUT4 membrane protein levels also significantly increased after treatment with 0.5 h short-term hypoxia and si-PKCζ (Fig. 4D).
RESVERATROL AND 0.5 h SHORT-TERM HYPOXIA RESCUED OX-LDL-INDUCED APOPTOSIS IN H9c2 CELLS
To explore whether the SIRT1 activator resveratrol and 0.5 h short-term hypoxia had an effect on apoptosis induced by Ox-LDL, we treated H9c2 cells that had been pretreated with 150 mg/dL Ox-LDL with these compounds. Apoptosis in the Ox-LDL group was 4.3 times greater compared with the control. However, treatment with resveratrol and 0.5 h short-term hypoxia significantly decreased the amount of apoptosis (Fig. 5A).
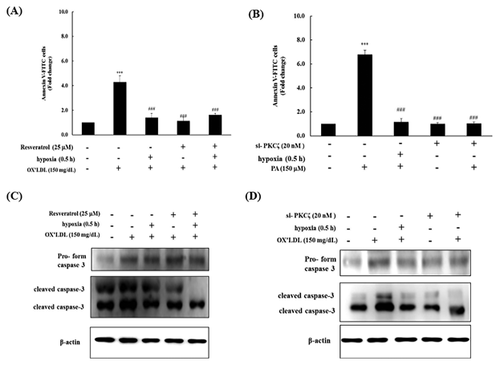
si-PKCζ AND SHORT-TERM HYPOXIA PREVENTS OX-LDL-INDUCED H9c2 CELL APOPTOSIS
H9c2 cells were transfected with 20 nm si-PKCζ and treated with 150 mg/dL Ox-LDL for 24 h following co-treatment with 0.5 h short-term hypoxia. The percentage of cells undergoing apoptosis significantly increased following Ox-LDL treatment; however, pretreatment with si-PKCζ significantly reduced the apoptosis levels (Fig. 5B).
EFFECTS OF RESVERATROL AND 0.5 h SHORT-TERM HYPOXIA ON THE APOPTOSIS MARKER CASPASE-3 IN OX-LDL-TREATED H9c2 CELLS
To further examine the effects of resveratrol and short-term hypoxia on the apoptosis marker caspase-3, H9c2 cells were pretreated with 25 µM of the SIRT1 activator resveratrol following co-treatment with 150 mg/dL Ox-LDL for 24 h. Western blots indicated that the protein levels of the caspase-3 pro-form in the Ox-LDL-treated group were significantly increased. However, the caspase-3 pro-form levels were significantly increased in the cells pretreated with short-term hypoxia, resveratrol, or short-term hypoxia and resveratrol. Cleaved caspase-3 levels were significantly increased in the Ox-LDL-treated group but were significantly decreased in the cells treated with resveratrol, short-term hypoxia or resveratrol and short-term hypoxia (Fig. 5C).
EFFECTS OF si-PKCζ AND SHORT-TERM HYPOXIA ON THE APOPTOSIS MARKER CASPASE 3 IN OX-LDL-TREATED H9c2 CELLS
To further examine the effects of si-PKCζ and short-term hypoxia on the apoptosis marker caspase-3, H9c2 cells were transfected with 20 nm si-PKCζ following co-treatment with 150 mg/dL Ox-LDL for 24 h. Western blots indicated that the protein levels of the caspase-3 pro-form were significantly increased in the Ox-LDL-treated group but were significantly decreased in the group pretreated si-PKCζ or short-term hypoxia. Cleaved caspase-3 levels were significantly increased in the Ox-LDL-treated group but were significantly decreased when the cells were treated with si-PKCζ and short-term hypoxia (Fig. 5D).
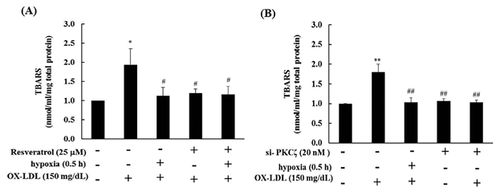
EFFECT OF RESVERATROL AND SHORT-TERM HYPOXIA ON THE LEVEL OF TBARS IN OX-LDL-TREATED H9c2 CELLS
To further examine the effects of resveratrol, si-PKCζ and short-term hypoxia on the lipid peroxidation marker TBARS, H9c2 cells were pretreated with 25 µM of the SIRT1 activator resveratrol or transfected with si-PKCζ following co-treatment with 150 mg/dL Ox-LDL for 24 h. TBARS were significantly increased in the Ox-LDL group compared with the control group. Pretreated with resveratrol, si-PKCζ and short-term hypoxia for 0.5 h reduced the TBARS level significantly (Fig. 6A and B).
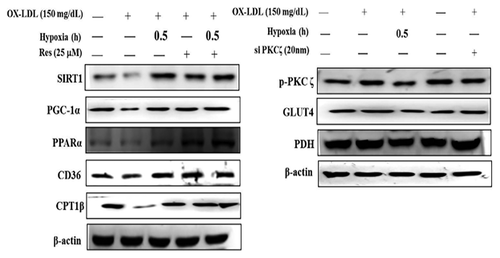
SHORT-TERM HYPOXIA REGULATES OX-LDL-INDUCED ALTERED CD36 AND GLUT4 METABOLIC PATHWAYS IN NEONATAL RAT CARDIOMYOCYTES
Neonatal rat cardiomyocytes were pretreated with 25 µM of the SIRT1 activator resveratrol for 24 h. After 24 h, the cells were co-treated with 150 mg/dL Ox-LDL for 24 h followed by treatment with short-term hypoxia for 0.5 h. The protein expression of SIRT1, PGC1α, PPARα, CD36, and CPT1β was reduced in neonatal rat cardiomyocytes following 24 h treatment with 150 mg/dL Ox-LDL. However, the protein expression of SIRT1, PGC1α, PPARα, CD36, and CPT1β was reversed by short-term hypoxia and resveratrol. The protein expression of phospho-PKCζ, GLUT4, and PDH increased with 150 mg/dL Ox-LDLL treatment for 24 h in neonatal rat cardiomyocytes. Additionally, phospho-PKCζ, GLUT4, and PDH protein expression was enhanced by short-term hypoxia and si-PKCζ (Fig. 7).
DISCUSSION
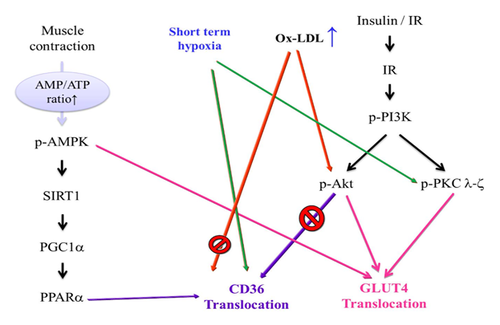
Many atherosclerosis cases have been attributed to Ox-LDL, and Ox-LDL contributes to the plaque formation in arteries, which causes lipid accumulation in the arteries [Ross, 1999]. Endothelial cell dysfunction by Ox-LDL is a key step for atherosclerosis progression [Menon and Kurup, 1976]. The endothelial cells reduce the release of nitric oxide (NO), increase adhesion molecules, and secrete chemoattractants and growth factors. Ox-LDL is taken up by macrophages, resulting in foam cell formation. High LDL-cholesterol levels are a risk for heart disease [Sato et al., 1976]. Guidelines from the American Heart Association, the National Institutes of Health (NIH), and the National Centers for Environmental Prediction (NCEP) for optimal LDL cholesterol levels and low to high risk heart disease levels served as our reference standard dose for treatment. CD36, also known as fatty acid translocase, uptakes many ligands, such as Ox-LDL [Endemann et al., 1993; Nicholson et al., 1995]. In current studies, CD36-targeted nanovesiciles can detect atherosclerotic lesions and macrophage accumulation by the high affinity of the CD36 for Ox-LDL. CD36 plays a key role in mediating recognition and uptake [Nie et al., 2015], and CD36 is a biomarker of atherosclerosis [Yazgan et al., 2014]. In our results, the fatty acid-CD36 metabolic pathway significantly increased in low dose Ox-LDL but decreased in high dose Ox-LDL. In other words, a high dose of Ox-LDL leads the fatty acid-CD36 metabolic pathway to switch to the glucose-GLUT4 metabolic pathway (Fig. 2). Hypoxia is usually related to atherogenesis [Soler-Argilaga et al., 1974; Marsch et al., 2014], and here we test the use of short-term hypoxia and resveratrol on Ox-LDL-induced lipotoxicity in H9c2 efficacy, which will reverse the metabolic imbalance (Fig. 4). Recent studies indicate that hypoxic exercise training has a cardioprotective effect in healthy humans [Bailey et al., 2000]. Furthermore, in a cultured human coronary arterial endothelial cell (HCAEC) model, Ox-LDL increased lipid peroxidation and decreased superoxide dismutase activity and hypoxia-reoxygenation mediated apoptosis via the downregulation of bcl-2, the upregulation of Fas protein, and the activation of the protein kinase C (PKC) and protein tyrosine kinase (PTK) pathways [Li et al., 1998]. Resveratrol, as a SIRT1 activator, has many health effects, such as heart protection [Tome-Carneiro et al., 2013], anti-cancer benefits [Carter et al., 2014], and metabolism regulation [Poulsen et al., 2013], and it has been reported to improve mitochondrial functional and combat against metabolism disease [Lagouge et al., 2006]. In the murine macrophage cell line RAW264.7, resveratrol suppressed Ox-LDL-induced apoptosis and reduced ROS production via the inhibition of the LOX-1, p38, and MAPK signaling pathways [Guo et al., 2014]. In our results, resveratrol and short-term hypoxia reversed Ox-LDL-induced apoptosis and reduced the protein expression of cleaved caspase 3 (Fig. 5). In recent exercise reports, Ox-LDL increased oxidative stress, ROS production and was associated with atherogenesis progress. Mild, moderate, and heavy exercise enhanced superoxide dismutase activity, and glutathione reductase content protected individuals against Ox-LDL-induced oxidative stress in a human model [Wang et al., 2006].
Some phytochemicals have benefits for vascular function and cardiometabolic health. The flavonoids epicatechin and quercetin improved fasting plasma insulin and insulin resistance [Dower et al., 2015] in a glabridin study by inhibiting the early inflammatory processes in atherosclerosis [Kang et al., 2015] and anthocyanins, which are mediated via interleukin 6 (IL-6) and vascular cell adhesion molecule 1 (VCAM-1) inflammatory mediators to regulate oxidized LDL-induced cardiovascular disease [Amin et al., 2015]. In a human model, high n6 fatty acid ratios (polyunsaturated fatty acids) are associated with lower LDL oxidation and inflammation, which decrease cardiovascular risk [Kaikkonen et al., 2014]. In a Cryptotanshinone treatment study, the oxidized LDL-induced ROS is reduced by regulating the NF-κB pathways [Zhao et al., 2015]. Furthermore, we attempted to utilize short-term hypoxia to rescue the oxidized LDL-induced cardiometabolic imbalance and determine if it has clinical efficacy. In summary, we have demonstrated that short-term hypoxia affects the Ox-LDL-induced metabolic imbalance, and the efficacy is similarly to resveratrol. Ox-LDL decreased the fatty acid-CD36 metabolic pathway and increased the glucose-GLUT4 metabolic pathway at a high dose (150 mg/dL) in H9c2 cells. The mechanism of switching seems to be mediated by SIRT1and PKC ζ, and we determined that short-term hypoxia reduced cell apoptosis as well as resveratrol and si-PKC ζ. Furthermore, we consider that short-term hypoxia has benefits for heart disease (Fig. 8).
ACKNOWLEDGMENTS
This study is supported by DMR-104-007 and in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence MOHW105-TDU-B-212-133019.