TRPV5/V6 Channels Mediate Ca2+ Influx in Jurkat T Cells Under the Control of Extracellular pH
ABSTRACT
Regulation of cytoplasmic free calcium concentration [Ca2+]i is a key factor for the maintenance of cellular homeostasis in different cell types, including lymphocytes. During T lymphocyte activation as well as production of cytokines, sustained Ca2+ influx is essential, however, it remains unclear how this influx is regulated. Previously, we reported the expression and functional activity of calcium channels TRPV5 and TRPV6 (transient receptor potential vanilloid type 5 and 6) in human leukemia Jurkat T cells. In this study, using single channel recordings, we found that activity of calcium channels TRPV5/V6 in Jurkat T cells is subject to strong control of external stimuli such as a low- or high-pH stressor. We showed that extracellular acidic pH reduces the activity of TRPV5/V6 channels, whereas alkaline pH increases the activity of TRPV5/V6 channels in Jurkat T cells. Using calcium imaging, we found that Ca2+ influx in Jurkat T cells displayed sensitivity to extracellular pH, similar to that shown for the calcium channels TRPV5/V6. Double immunostaining of Jurkat T cells revealed that TRPV5 and TRPV6 channels colocalize with clathrin and the early endocytosis marker, EEA1. Moreover, we demonstrated that a specific inhibitor of clathrin-dependent endocytosis, dynasore, blocked TRPV5/V6 activity, and Ca2+ influx into Jurkat T cells. Overall, our findings indicate that strong environmental cues may affect the intracellular calcium level in Jurkat T cells by influencing the traffic of TRPV5/V6 channels in lymphocytes. J. Cell. Biochem. 117: 197–206, 2016. © 2015 Wiley Periodicals, Inc.
Changes in intracellular Ca2+ are of pivotal importance for many processes such as cell growth, motility, secretion/exocytosis, etc. Sustained Ca2+ entry is critical for all physiological functions in T cells, including proliferation, cytokine production, as well as differentiation of naive T cells into Th1, Th2, Th17, and other T cell subtypes. The molecular mechanisms of Ca2+ signaling as well as the consequences of this signaling are well-characterized in cells of the immune system. Ca2+ signaling pathways in lymphocytes have a number of specific features compared with other non-excitable cells. For instance, lymphocytes have an extremely low amount of Ca2+ in intracellular stores, as estimated by the tiny amplitude of Ca2+ response that is triggered by ionomycin in a Ca2+-free solution. In addition, T cell Ca2+ stores are known to be very leaky [Randriamampita and Trautmann, 2004]. The small amplitude of Ca2+ release in T cells indicates that the Ca2+ increase is mainly shaped by Ca2+ influx.
Despite the tremendous efforts of multiple laboratories, the identification of the molecules involved in shaping Ca2+ influx in T cells remains undetermined. Several ion channels have been identified in lymphocytes that mediate Ca2+ influx, including Ca2+ release-activated Ca2+ (CRAC) channels and transient receptor potential (TRP) channels. Bulk of the available evidence suggests that CRAC channels form the primary route of Ca2+ entry into T cells and mast cells. Two key molecules STIM1 [Roos et al., 2005; Liou et al., 2007] and ORAI1 [Feske et al., 2006; Vig et al., 2006; Zhang et al., 2006] make up the store-operated CRAC channels. After the depletion of Ca2+ from the endoplasmic reticulum, STIM1 transitions to endoplasmic reticulum–plasma membrane junctions, thus activating ORAI1, a pore-forming subunit of the CRAC channel [Stathopulos et al., 2006; Liou et al., 2007; Luik et al., 2006]. While the role of STIM and ORAI proteins in Ca2+ influx is undisputed [Liou et al., 2005; Feske et al., 2006; Stathopulos et al., 2006], the role of members of TRP family in Ca2+ signaling pathways remains obscure. Nevertheless, several studies have proposed that some entry pathways involved in Ca2+ signaling in lymphocytes are mediated by TRP [Wenning et al., 2011].
In humans, the TRP channel family consists of six subfamilies: TRPC, TRPV, TRPM, TRPA, TRPP, and TRPML. According to recent data, T cells predominantly express channels belonging to the TRPC and TRPM subfamilies including TRPC1, TRPC3, TRPC5, TRPM2, TRPM4, and TRPM7 [Wenning et al., 2011]. Most TRP channels are non-selective and permeable to several cations including Ca2+ and Na+ [Owsianik et al., 2006; Ramsey et al., 2006]. TRP channels contribute to changes in the cytosolic free Ca2+ concentration either by acting as Ca2+ entry pathways in the plasma membrane or via changes in membrane potential, which modulates the driving force for Ca2+ release from several cell organelles.
Most Ca2+-permeable TRP channels are only poorly selective for Ca2+ with a permeability ratio relative to Na+ (PCa/PNa) in the range of 0.3 to 10. TRPV5 and TRPV6 channels are an exception, they are the two most highly Ca2+-selective TRP channels with PCa/PNa > 100 [Nilius et al., 2001; Den Dekker et al., 2003]. TRPV5/V6 is primarily expressed in the apical membrane of distal convoluted tubules (DCT2) and connecting tubules (CNT) cells in the kidney, and in the apical membrane of CNT cells in the intestine [Peng and Hediger, 2002; Hoenderop et al., 2005]. The TRPV5/V6 channels in the kidney and intestine cells are one of the primary targets for regulation of calcium homeostasis by hormones. They also seem to be involved in acid/base disorders [Hoenderop et al., 2003; Hoenderop and Bindels, 2005; Hoenderop et al., 2005].
Several lines of evidence indicate that TRPV5 activity is sensitive to pH. In vitro studies indicate that intra- and extracellular pH directly regulates the activity of TRPV5. Acidification inhibits, whereas alkalization stimulates TRPV5 activity, this is most likely mediated by conformational changes of the channel pore helix [Yeh et al., 2003; Yeh et al., 2005]. In addition, recent evidence revealed that plasma membrane expression of TRPV5 and TRPV6 is under the control of extracellular pH. It was demonstrated that extracellular alkalization results in a rapid elevation of the TRPV5 delivery rate to the plasma membrane, whereas acidification resulted in retrieval of TRPV5 from the plasma membrane to the cytosolic compartment [Lambers et al., 2007]. Thus, the existing data suggests that extracellular pH may have a major role in the physiological regulation of the levels of intracellular Ca2+ by controlling the number of TRPV5 channels at the cell surface.
Previously, we have reported the expression and functional activity of TRPV5/V6 in Jurkat T cells [Vassilieva et al., 2013]. The objective of this study was to investigate the role and regulatory mechanisms of TRPV5/6 channels in Jurkat T cells. Using patch-clamp, calcium imaging, and immunofluorescent protein labeling, we established that calcium channels TRPV5/V6 mediate the Ca2+ influx in Jurkat T cells. It was found, that extracellular pH is involved in regulation of this Ca2+ influx in Jurkat T cells and most likely affects the traffic of TRPV5/V6 channels.
MATERIALS AND METHODS
CELL ISOLATION
Human T cells of leukemia line Jurkat T were obtained from the Cell Culture Collection (Institute of Cytology, St. Petersburg, Russia) and were maintained in RPMI medium with 10% FBS, supplemented with 0.2 M l-glutamine, 25 U/ml gentamicin at 37°C in a humidified 5% CO2 atmosphere. The cells were passaged every 2–3 days to maintain a cell concentration of 1 × 106/ml.
ELECTROPHYSIOLOGY AND DATA ANALYSIS
At the beginning of the experiment, Jurkat T cells were placed on poly-l-lysine-coated glass coverslips. The recording chamber (0.1 ml in volume) was filled with divalent free (DVF) external solution in which a giga-seal was formed. Single channel currents were recorded using standard outside–out and cell-attached mode of the patch–clamp technique. We used a micropipette puller P-97 (Sutter Instrument, Novato, CA) to manufacture patch pipettes with a resistance of 8–15 MΩ when filled with solution. Membrane currents were measured using an Axopatch 200B patch–clamp amplifier (Axon Instruments/Molecular Devices, Eugene, OR) with low-pass filtered at 1 kHz and digitized at 5 kHz with an A/D converter. Data was collected and analyzed with pClamp software. Membrane voltage was calculated as the potential of the intracellular membrane side minus the potential of the extracellular one. The recordings were performed at room temperature (22–23°C) on the stage of an inverted Zeiss Axiovert Z1 microscope. Data analysis was performed using Microcal Origin 6.2 software (OriginLab). Data is presented as means ± SE; n = number of the experiments. The patch pipette solution in outside–out mode contained (in mM): 140 K-aspartate or Cs-aspartate, 5 NaCl, 10 HEPES/TrisOH, 10 BAPTA, 10 d-glucose (pH 7.3). The bath solution (and patch pipette solution for cell-attached mode) contained (in mM): 140 Na-methanesulfonate, 5 KCl, 10 HEPES/TrisOH, 10 HEDTA, and 10 d-glucose (pH 7.3). Bath solutions for outside–out experiments with potassium currents contained (in mM): 140 NaCl, 6 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES/TRIS, and 10 d-glucose (pH 7.3). The patch pipette solution contained (in mM): 140 K-aspartate, 2 MgCl2, 1 CaCl2, 10 EGTA, 10 HEPES/TrisOH, (pH 7.3). All chemicals were from Sigma–Aldrich (St. Louis, MO).
MEASUREMENTS OF [Ca2+]i
Jurkat T cells at a density of 106/ml were washed with serum-free medium and loaded with 5 µM Fura-2AM (Molecular Probes, Eugene, OR) for 40 min at room T. After loading, cells were washed and kept in the dark at room T until use. At the beginning of the experiment, cells were transferred on a perfusion chamber and imaged using an AxioObserver.Z1 inverted microscope (Carl Zeiss MicroImaging GmbH, Germany) with a Plan-Apo-chromate × 40/0.95 objective for taking measurements. Fura-2AM fluorescence was excited alternately by light of 340 and 380 nm from an illuminator with a Lambda DG-4 high-speed wavelength switcher (Sutter Instrument Co.). Registration process control and analysis were performed using AxioVision 4.8.2 software (Carl Zeiss MicroImaging GmbH). Post-processing of the images included the subtraction of background fluorescence using the built-in functions of the software. At the end of each experiment, the solution in the perfusion chamber was replaced with a calcium-containing solution with Ionomycin, calcium ionophore (10 µM), then the solution was replaced by a calcium-free solution containing 5 mM MnCl2 for fluorescence quenching. The changes in fluorescence were converted into [Ca2+]i using the formula: [Ca2+]i = [kD × (R –R min)/(Rmax – R)] × Sf2/Sb2 where the dissociation constant (kD) of Fura-2 AM was assumed to be 225 nM and Sf2 and Sb2 are the fluorescent values for Ca2+ free (f) and bound (b) of the indicator.
IMMUNOFLUORESCENCE STAINING
For the detection of TRPV5, TRPV6, EEA1 and clathrin heavy chains, immunofluorescent single and double labeling was performed on Jurkat T cells. Cells were plated on a poly-l-lysine-coated coverslip, fixed with 4% paraformaldehyde in PBS, permeabilized with 0.25% Tween 20 in PBS and then blocked with 10% donkey serum (1 h at 24°C). After which the cells were incubated with primary antibodies diluted in PBS (1:100) and incubated at 4°C overnight. Then the cells were reacted with secondary antibodies, also diluted in PBS (1:200) for 40 min at 37°C. Finally, nuclei were stained by DAPI (0.05 µg/ml). Cell membranes were stained by CTX (cholera toxin B subunit). After staining, cover slips were mounted with Vectashield Mounting Medium (Vector Laboratories) and imaged with a confocal microscopy (Leica TCS SP5; Leica Microsystems).
ImageJ software was used to provide a quantitative measurement of the co-localization of TRPV5 and TRPV6 with clathrin and EEA1. Pearson's correlation coefficient was used for quantitative measurement of the degree of overlap between fluorescence signals obtained in two channels. Differences in the intensities of the signals were ignored, but similarity between shapes was considered. A Pearson's correlation coefficient was calculated for each antibody pairing from confocal images acquired using sequential scanning. Background correction values were identical for all images. A small box was drawn around the cell of interest using the square tool. The Pearson's correlation coefficients were calculated from multiple images, averaged, and a standard error of the mean was calculated.
All antibodies used in the present study are commercially available. Anti-TRPV5 antibodies were H-99 (Santa Cruz Biotechnology, Santa Cruz, CA), EB08515 (Everest Biotech, Upper Heyford, Oxfordshire, UK). Anti-TRPV6 antibodies were H-90 (Santa Cruz Biotechnology). Anti-clathrin heavy chain antibodies and anti-EEA1 antibodies were 610499 and 610456, respectively (BD Transduction laboratories). Secondary antibodies were FITC-conjugated goat anti-mouse antibodies and Cy3-conjugated goat anti-rabbit antibodies (Jackson ImmunoResearch Labs, UK)
RESULTS
EXTRACELLULAR pH AFFECTS THE ACTIVITY OF TRPV5/V6 CHANNELS IN JURKAT T CELLS
Using the patch–clamp method, we have previously detected the functional activity of TRPV5/V6 channels in Jurkat T cells [Vassilieva et al., 2013]. The single channel's activity has been observed at negative potentials in outside–out patches in absence of extracellular Ca2+ and Mg2+, with Na+ as a charge carrier. Voltage-current relations obtained from unitary currents recorded at different voltages displayed typical for calcium channels TRPV5/V6 inward rectification and a single channel conductance of 38 pS. The endogenous expression of TRPV5/V6 were confirmed by real-time PCR and Western blot analysis [Vassilieva et al., 2013]. Here, we investigated the mechanisms of regulation of TRPV5/V6 channels in Jurkat T cells.
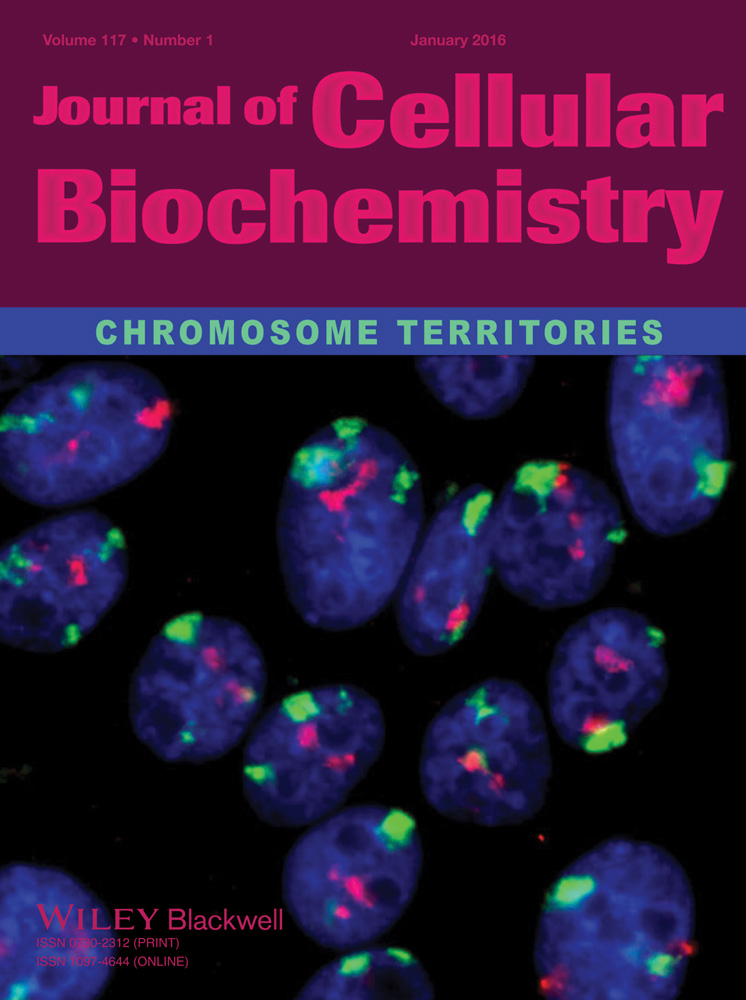
Several lines of evidence indicated that TRPV5/V6 channels’ activity is sensitive to pH. Regulation of ion channels by pH may occur via direct proton titration of the channel or indirectly and/or by the control of cell surface delivery of the channels [Yeh et al., 2003; Yeh et al., 2005; Lambers et al., 2007]. To assess the influence of extracellular pH on TRPV5/V6 activity in Jurkat T cells, electrophysiological recordings were performed in solution with acidic, basic, and close to neutral pH. Continuous single-channel activity was obtained in outside–out patches at membrane potential –70 mV and could be observed for 10–30 min in absence of any stimulus in solution with pH 7.3. In some cases, the activity of up to four channels was observed in the plasma membrane of isolated patches. After the development of channel activity an extracellular solution with pH 7.3 was replaced by a solution with pH 6. Figure 1A, shows that extracellular acidification results in a rapid decrease of TRPV5/V6 currents. It should be noted that washout did not restore the channel activity (n = 5). To check, whether the acidic pH acts directly on the channel, or its effect is mediated by processes occurring inside the cell, we conducted patch–clamp experiments using cell-attached configuration. In these experiments, changes in extracellular pH could not directly act on a channel pore, as the pore of the channel was exposed in the pipette solution with constant pH 7.3. Nevertheless, results showed that acidification of the external solution to pH 6.0 led to the suppression of channel activity in cell-attached experiments (n = 3) (Fig. 1B).

Then, to check the specificity of pH effect on TRPV channels, we investigated the action of acidic pH on potassium currents recorded in Jurkat T cells in outside–out patches at membrane potential +50 mV. As demonstrated on Figure 1C, outward potassium channels were not affected by the acidification (pH 6.0) of the bath solution. Washout of the acidic solution also does not alter the activity of the potassium channels (Fig. 1C). Next, we investigated the effect of increasing pH on the activity of TRPV5/V6 channels in Jurkat T cells. Figure 2A demonstrates that alkalinity of the solution in a bath did not affect the channels activity in isolated outside–out patches. The channel activity, expressed as NPO in the neutral and alkaline medium had similar values (0.74 ± 0.28, n = 4) and (0.80 ± 0.2, n = 4), respectively (Fig. 2B). Besides, we performed a series of experiments in which the Jurkat T cells were initially placed into a basic (pH 8.2), acidic (pH 6.0) or close to neutral (7.2) solution. Then, the plasma membrane fragments were isolated, in the same solutions, and channel activity was examined in outside–out patches. Under these conditions single TRPV5/V6 channel activity was observed in 71% of the patches performed in pH 8.2. (n = 14) and in 55% of the patches performed in pH 7.3 (n = 9). No channel's activity has been registered in acidic (pH 6.0) solutions (n = 5). Altogether, these results allow us to conclude that extracellular pH regulates the activity of TRPV5/6 channels in Jurkat T cells, but, apparently, does not act directly on the channel pore.
It is well-known, that an intrinsic physiological effect of extracellular pH change on the cells is the regulation of trafficking processes like endo- and exocytosis [Keyes and Rudnick, 1982; Glunde et al., 2003]. Recently, it has been demonstrated that the number of TRP channels at the cell surface is regulated by endo/exocytosis. Moreover, recent study revealed that intra- and extracellular pH dynamically controls the cell surface delivery rate and subsequent overall activity of functional TRPV5 channels [Lambers et al., 2007; van de Graaf et al., 2008]. In accordance with this data, we further tested whether inhibition of intracellular traffic influences TRPV5/V6 currents in Jurkat T cells. To test these pathways directly, we used dynasore, a cell-permeable inhibitor of dynamin GTPase activity and blocker of dynamin-dependent endocytosis [Kirchhausen et al., 2008]. Figure 2C shows typical outside–out TRPV5/6 currents before and after treatment with dynasore in Jurkat T cells (n = 5). As is apparent from this representative trace, a 100 μM dynasore application decreases the activity of TRPV5/V6 channels.
SENSITIVITY OF INTRACELLULAR Ca2+ TO RUTHENIUM RED IN JURKAT T CELLS
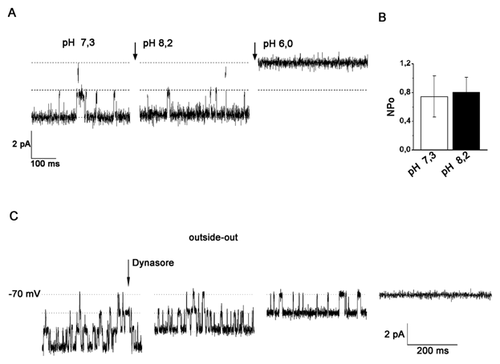
The Ca2+-selective properties of TRPV5/V6 allow them to play a crucial role in regulation of Ca2+ transport in epithelial cells [Den Dekker et al., 2003; Nijenhuis et al., 2003]. We hypothesized that TRPV5/V6 channels detected in Jurkat T cells may participate in the regulation of intracellular calcium in these cells. To verify this, cultured Jurkat T cells were loaded with the calcium-sensitive probe Fura-2AM, and Ca2+ fluxes were measured with the imaging system (see Material and Methods) from the changes in intracellular Ca2+ concentration ([Ca2+]i) elicited by concentration jumps in extracellular Ca2+ ([Ca2+]i). Cells were initially placed in a Ca2+ free (0 mmol/l) medium and then calcium free medium was replaced with a high Ca2+ (2 mmol/l) medium. Afterward, changes in free [Ca2+]i were examined in individual Jurkat T cells using fluorescence-ratio imaging of cells loaded with Fura-2AM. The results showed that Jurkat T cells react rapidly to changes in extracellular Ca2+. Short-term changes in the concentration of extracellular calcium elicited a rise in [Ca2+]i, from the level of 93 ± 11 nM to a peak of 526 ± 44 nM (n = 17) without any delay (Fig. 3). In the majority of cases, the initial rising phase was quite abrupt, reaching a peak within several seconds. Then, within a few minutes, the original (resting) level of intracellular calcium was restored, as result of the balancing work of intracellular buffering systems, pumps, ion channels, etc.
Earlier, in patch–clamp experiments on human blood lymphocytes (HBLs) and Jurkat T cell [Vassilieva et al., 2013], we showed the sensitivity of TRPV5/V6 channels in these cells to ruthenium red, a widely used, although not completely selective, inhibitor of TRPV5 and TRPV6 channels [Hoenderop et al., 2001; Schoeber et al., 2007]. In order to test if TRPV5/V6 channels mediate the Ca2+ influx in these cells, ruthenium red was used in Ca2+ measurements. The relative changes in intracellular free calcium were calculated in the presence of 2 mM extracellular calcium before and after the addition of 50 µM ruthenium red in a bath. Figure 3A and B, represents the averaged data for several cells and demonstrates that calcium influx was significantly reduced (from 494 ± 62 to 199 ± 32 nM, n = 9) in the presence of ruthenium red.
REGULATION OF INTRACELLULAR Ca2+ BY EXTRACELLULAR pH
As already described above, extracellular pH affected the activity of calcium channels TRPV5/V6 in patch–clamp experiments in Jurkat T cells. Therefore, we assumed that pH might likewise affect the level of intracellular Ca2+ in these cells. To test this hypothesis, the influx of 2 mM Ca2+ in cells was estimated in solutions with pH 7.3, pH 6.0, and pH 8.2. As demonstrated on Figure 3C and D, the acidification of extracellular solution almost completely abolished the rise in [Ca2+]i elicited by concentration jumps in extracellular Ca2+. Under these conditions the [Ca2+]i reached a peak of 225 ± 26 nM (n = 9) in pH 6.0, (compared with 566 ± 70.5 nM in pH 7.3). In contrast, alkalization of the external solution in Jurkat T cells resulted in a significant increase in [Ca2+]i reaching a peak of 888 ± 56 nM (Fig. 3C and D). Thus, lowering of pH effectively and reversibly suppresses the Ca2+ influx, whereas increasing pH substantially increases Ca2+ influx in Jurkat T cells. It is essential to note that Ca2+ influx in Jurkat T cells displayed sensitivity to extracellular pH at levels close to that shown for activity of calcium channels TRPV5/V6.
Our findings suggest that [Ca2+]i influx in Jurkat T cells depends on the activity of TRPV5/V6, and consequently, on the number of functional TRPV5/V6 channels presented at the cell surface of the plasma membrane. Consequently, we supposed that processes associated with endo/exocytosis may also affect the Ca2+ influx through the TRPV5/V6 channels into T cells. To test this hypothesis the Ca2+ influx in Jurkat T cells was investigated in the presence of dynasore. As illustrated in Figure 3E and F, the blocking of dynamin-dependent endocytosis results in decrease of Ca2+ influx in Jurkat T cells within several minutes.
CO-LOCALIZATION OF TRPV6 AND TRPV5 CHANNELS WITH CLATHRIN AND EEA1
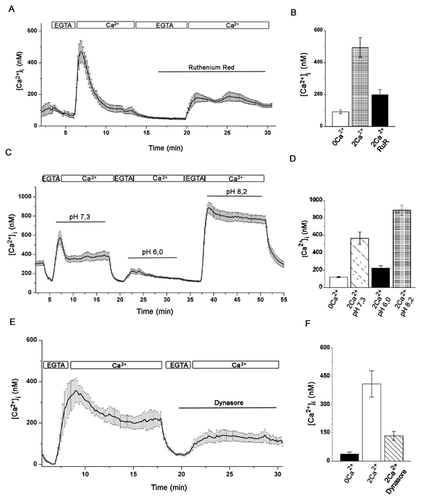
To further assess the information about surface expression and subcellular localization of TRPV5 and TRPV6 channels in Jurkat T cells, we employed immunofluorescence staining with specific anti-TRPV5 and anti-TRPV6 antibodies. Figure 4A and C shows the staining with anti-TRPV5 in Jurkat T cells. Figure 4B demonstrates a similar pattern for anti-TRPV6 immunoreactivity. The pattern of staining was consistent for the anti-TRPV5 antibodies H99 and EB08515 as well as for the anti-TRPV6 antibodies H90 and E16. A distinguishing feature of lymphocytes is their small amount of cytoplasm, with most of the cell being occupied by the nucleus (Fig. 4). Although antibodies are localized to the plasma membrane, staining is also present in the cytoplasmic compartment. In multiple independent experiments, this overall pattern held constant, although, the relative extent of cytoplasmic versus membrane stain varied. A lack of clear membrane staining for TRPV5 channels due to a predominant cytoplasmic localization has been described previously. Thus, it has been shown that a significant population of TRPV channels is held dormant in cytoplasmic vesicles in many cell systems, with channel activation depending upon transient vesicle insertion into the plasma membrane [Cayouette and Boulay, 2007; Lambers et al., 2007; Cha et al., 2008].
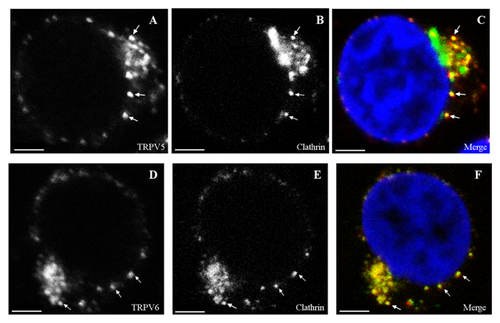
A number of mechanisms including both clathrin-dependent endocytosis as well as a number of clathrin-independent pathways can mediate endocytosis of proteins. Studies into the mechanisms underlying the regulated endocytosis of TRPV5 revealed that TRPV5 is predominantly internalized via a dynamin- and clathrin-dependent pathway [van de Graaf et al., 2008]. To test these pathways directly in Jurkat T cells, we used double staining with specific anti-TRPV5 or TRPV6 antibodies and specific anti-clathrin antibodies. Analysis of the confocal images (Fig. 5C and F) revealed co-localization of TRPV5 (Pearson coefficient range 0.51–0.7) and TRPV6 (Pearson coefficient range 0.47–0.58) with clathrin, implicating clathrin in TRPV5/V6 endocytosis in Jurkat T cells.
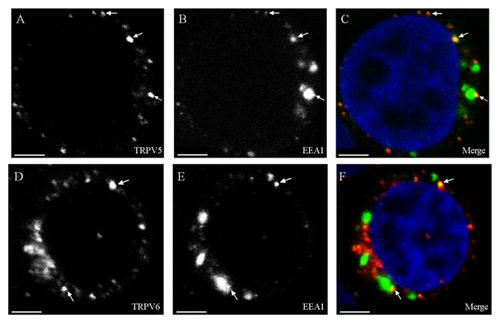
In addition, we used antibodies against EEA1, a 162-kDa autoantigen, that have been shown to be specifically associated with the cytoplasmic face of the early endosome membrane. Unlike many other endosomal proteins, EEA1 has not been detected on other membrane compartments and appears to be one of the most specific early endosomal markers known to date. Localization of EEA1 showed a slight overlap with that of TRPV5 (Pearson coefficient range 0.25–0.3) and of TRPV6 (Pearson coefficient range 0.21–0.28) (Fig. 6C and F).
DISCUSSION
In this paper, we have analyzed mechanisms involved in the regulation of intracellular Ca2+ in Jurkat T cells. To explore the Ca2+ influx in these cells, we mimicked conditions in the natural environment, so no artificial blockers of Ca2+ ATPase were used. Calcium influx in non-stimulated Jurkat T cells was estimated from the variations in intracellular Ca2+ concentration [Ca2+]i elicited by concentration jumps in extracellular Ca2+ [Ca2+]i. Using Ca2+-sensitive fluorescent dye we found that small changes in extracellular pH (within physiological limits) could significantly affect Ca2+ entry into Jurkat T cells, elicited by the lowering of intracellular Ca2+. Acidification of extracellular solutions (pH 6.0) significantly decreased, whereas alkalization of solution (pH 8.2) increased the Ca2+ entry. As already noted above, the rise of intracellular Ca2+ in T cells is mostly provided by calcium ions coming from the external environment through the plasma membrane ion channels. We explored ways that could potentially regulate this Ca2+ influx. Our previous studies have demonstrated the expression of functional TRPV5/V6 channels in Jurkat T cells [Vassilieva et al., 2013]. The activity of TRPV5/V6 channels was recorded in isolated outside–out patches at negative potentials close to resting potential. This constitutive activity of TRPV5/V6 calcium channels allowed us to anticipate the involvement of these channels in regulation of intracellular Ca2+ in Jurkat T cells. In our experiments calcium influx was susceptible to ruthenium red, inhibitor of TRPV5 and TRPV6 channels [Hoenderop et al., 2001; Den Dekker et al., 2003], which confirmed this hypothesis.
Extracellular pH has long been known to affect the rate and magnitude of ion transport processes via regulation of ion channel activity. Several lines of evidence indicate that activity of recombinant TRPV5/V6 is sensitive to pH [Yeh et al., 2003; Yeh et al., 2005; Lambers et al., 2007]. Here, we found that single channel activity of TRPV5/V6 channels in Jurkat T cells is affected by pH. The constant activity of TRPV5/V6 channels at resting potential is not diminished during the long time of registration; however, a weak acidification of the external solution in a short time resulted in the inhibition of active channels. Conversely, the rise of extracellular pH increased the activity in some rare cases, but in most experiments had no effect on channel activity in outside–out patches. Regulation of ion channels by pH may occur via direct proton titration of the channel or may be mediated by some other mechanism. There is some data indicating that the intra- and extracellular pH can directly adjust the activity of TRPV5 through conformational changes of the channel pore helix [Yeh et al., 2003; Yeh et al., 2005]. However, our electrophysiological data does not support this hypothesis. First, suppression of channel activity, as a result of acidification, was obtained both in outside–out and cell-attached mode. Moreover, this effect was irreversible in Jurkat T cells. Secondly, alkalinization of the solution did not affect the TRPV5/V6 channel activity in isolated patches, though it did increase the channel activity in intact cells. Several lines of evidence indicate the involvement of extracellular pH in the regulation of trafficking processes like endo- and exocytosis and lysosomal trafficking [Keyes and Rudnick, 1982; Glunde et al., 2003].
TRPV5/V6 is predominantly active at physiological membrane potentials. Therefore, TRPV5/V6 -mediated Ca2+ influx is largely dependent on the amount of TRPV5/V6 channels at the cell surface. Our data supports the hypothesis that extracellular pH may affect the intracellular transport of TRPV5/V6 channels, their surface expression, and therefore, overall channel activity [Lambers et al., 2007]. The insensitivity of TRPV5/V6 channels to alkaline pH, in isolated patches, can be explained by the loss of the elements necessary for intracellular transport of vesicles containing channels. Conversely, if the cell remained intact with intact intracellular machinery, vesicles fuse with the membrane at an alkaline pH, and we could see an increase in channel activity in outside–out patches.
Endocytosis of proteins can be mediated by a number of mechanisms, including both clathrin-dependent endocytosis and a number of clathrin-independent pathways. In our patch–clamp experiments, a potent inhibitor of dynamin – dynasore, suppressed the activity of TRPV5 and TRPV6 channels, suggesting the participation of dynamin and clathrin-dependent endocytic pathways in the channel activity. Dynasore also inhibited calcium entry in Ca2+ measurements in Jurkat T cells. Participation of clathrin-mediated endocytosis (CME) in TRPV5/V6 traffic was confirmed by our immunofluorescent data that show co-localization of TRPV5 and TRPV6 with clathrin. We also showed a slight overlap of EEA1 with TRPV5 and TRPV6, which pointed on possible association of TRPV5/V6 with early endosomes.
Thus, as a result of the present study, we were able to show that activity of TRPV5/V6 channels in human lymphocytes is sensitive to extracellular pH. At the same time, it was shown that the Ca2+ influx in Jurkat T cells, induced by the decrease in intracellular Ca2+, is affected by extracellular pH. Furthermore, it was shown that both Ca2+ influx and TRPV5/V6 channels in Jurkat T cells are inhibited in the presence of the endocytosis blocker, dynasore. Finally, this calcium influx was inhibited by ruthenium red. Altogether, our data suggests that the TRPV5/V6 channels, at least in part, provide Ca2+ entry, induced by calcium deficiency in Jurkat T cells. Extracellular pH through intracellular traffic can affect the activity of TRPV5/V6, and therefore, Ca2+ influx in these cells.
TRPV5/V6 channels are primarily expressed in the epithelium of the kidney and intestine [Peng et al., 2001; Song et al., 2003], where pH plays an important role in calcium flow regulation. Apropos of the immune system, alterations in the microenvironment at the sites of infection and inflammation have long been known (for examples, see [Rotstein et al., 1987; Rotstein et al., 1988; Grinstein et al., 1991]). For instance, a characteristic feature of inflammatory locus is local acidosis, which is attributed to the local increase of lactic-acid production by the anaerobic, glycolytic activity of infiltrated neutrophils, and to the presence of short chain, fatty acid by-products of bacterial metabolism [Grinstein et al., 1991]. The interstitial fluid of tumors and abscesses also has shown pH values of less than 6.0, averaging 0.2–0.6 units lower than mean extracellular pH of normal tissues (for review, see [Kraus and Wolf, 1996]). Acidic microenvironments may play a role in inhibiting immune function in certain respiratory conditions such as cystic fibrosis and during neoplastic growth and invasion [Kraus and Wolf, 1996; Helmlinger et al., 1997]. Experimental evidence is emerging gradually for an inhibition of lymphocyte activity when the pH levels surrounding tumors are reduced. Evidently, the inter-relationship between extracellular pH and the activities of ion channels cannot be ignored, especially in light of a myriad of findings implicating a role for TRP channels in human pathophysiology and disease.
Acknowledgements
This work was supported by grants from the Russian Foundation for Basic Research [15-04-00938] and the Molecular and Cell Biology Program of the Russian Academy of Sciences.