PRDM1, a Tumor-Suppressor Gene, is Induced by Genkwadaphnin in Human Colon Cancer SW620 Cells
ABSTRACT
Genkwadaphnin (GD-1) is isolated from the flower buds of Daphne genkwa Siebold et Zuccarini (Thymelaeaceae), and it has been used as a traditional Korean and Chinese medicine. In this study, the authors observe that GD-1 inhibits the growth of the colon cancer cell line, SW620, through the up-regulation of p21 expression in a PRDM1-dependent manner. After treatment with GD-1, the transcriptional repressor PRDM1 is prominently induced in SW620 cells. Furthermore, GD-1 induce the phosphorylation of PKD1 and MEK and subsequently provide PRDM1 enhancement, resulting in the suppression of c-Myc expression and the up-regulation of p21. PKD1 knockdown using siRNA abrogates PRDM1 expression by GD-1 and subsequently disrupts the regulation of c-Myc and p21 expression. Treating SW620 cells with GD-1 inhibits cell-cycle progression and is characterized by the down-regulation of c-Myc followed by the up-regulation of p21 expression. The up-regulation of p21 by GD-1 induces the growth arrest of the SW620 colon cancer cell line. Based on these data, the authors propose that GD-1 has tumor-suppressor activity that may contribute to the anti-tumor effects of PRDM1 in colon cancer. J. Cell. Biochem. 117: 172–179, 2016. © 2015 Wiley Periodicals, Inc.
Abbreviations
-
- GD-1
-
- Genkwadaphnin
-
- PRDM1
-
- positive regulatory domain I-binding factor 1
-
- PKD1
-
- protein kinase D1
-
- MEK
-
- mitogen-activated protein kinase kinase
INTRODUCTION
Genkwadaphnin (GD-1) is isolated from natural products extracted from the dried flower buds of Daphne genkwa Sieb. et Zucc [Park et al., 2007]. It is commonly used as a traditional Korean and Chinese medicine for trauma as well as for diuretic, antitussive, expectorant, and edema applications, but its specific biological activity has not been well defined [Hong et al., 2010]. The structural characterization of GD-1 is reported to be a series of daphnane-type diterpenes, 12-O-benzoyl-5-hydroxy-6,7-epoxy-resiniferonol-9,13,14-orthobenzoate, by a comparison of its physicochemical and spectral data with those in the literature [Hall et al., 1982, 1986; Liou et al., 1982; Yesilada et al., 2001; Zhang et al., 2006; Park et al., 2007; Hong et al., 2010]. GD-1-induced apoptosis in human myelocytic HL-60 cells and suppressed tumor growth in a Lewis lung carcinoma (LLC)-inoculated mouse model [Park et al., 2007]. GD-1 was evaluated for its inhibitory effects on melanogenesis against α-melanocyte-stimulating hormone (α-MSH)-activated B16 melanoma cells [Bang et al., 2013]. GD-1 also induced reactive oxygen species (ROS)-mediated apoptosis of squamous cell carcinoma (SCC) cells [Li et al., 2014]. Our previous study showed that GD-1 is involved in the activation of the PKD1 and/or ERK/MEK pathways, which activate NF-kB, triggering IFN-γ production in NK-92 cells [Kang et al., 2014]. In this study, the anti-proliferative activity of GD-1 was investigated in PR domain containing 1 (PRDM1), c-Myc, and p21 production in SW620, a colon cancer cell line.
PRDM1 is a transcriptional repressor of the IFN-β promoter, and it plays an essential role in many cell lineages and in early development [Keller and Maniatis, 1991]. Most studies have focused on the functional role of PRDM1 in lymphocytes, and it is known for its ability to induce B cell differentiation into plasma cells [Shaffer et al., 2002]. Several factors have been implicated in the activation of PRDM1 transcription during the differentiation of B cells, including IL-21, IL-10, and IL-6 and the phosphorylation of STAT3 and STAT5. In addition, NF-kB, AP-1, and IRF4 activate PRDM1, although their precise mechanisms of action are not fully understood [Calame, 2008].
PRDM1 is a master regulator of plasma cell differentiation, and it functions as a tumor-suppressor gene in the pathogenesis of B cell lymphoma [Pasqualucci et al., 2006; Mandelbaum et al., 2010]. Several reports state that PRDM1 has been detected in non-hematopoietic cancer cells [Yan et al., 2007; Romagnoli et al., 2012; Yu et al., 2012]. It has been reported that PRDM1 is a mediator of Ras/Raf/AP-1 signaling, which promotes cell migration in lung cancer cells, and is a downstream effector of the NF-κB, RelB/Bcl-2/Ras-driven pathway that promotes breast cancer cell migration. Colorectal cancer cells have also been shown to express PRDM1, which represses the TP53 gene and thus maintains cell growth. However, the signal mechanism of activation and the function of PRDM1 in non-hematopoietic cancer cells are yet to be identified.
The proto-oncogene c-Myc has been implicated in the pathogenesis of most types of human tumors [Gabay et al., 2014]. Many studies have shown that ectopic c-Myc could block the differentiation of B cells into immunoglobulin-secreting plasma cells [Lin et al., 1997]. Recent studies have demonstrated that PRDM1 may bind to the c-Myc gene promoter and directly regulate the expression of the c-Myc gene [Lin et al., 1997]. Down-regulation of c-Myc levels also seems to have a direct effect on p21 transcript levels. The inverse correlation between c-Myc and p21 expression was apparent when suberoylanilide hydroxamic acid was shown to be able to down-regulate c-Myc levels and simultaneously increase p21 levels in multiple myeloma cells [Gui et al., 2004]. Treatment with yuanhuacine, another component of daphnane diterpene ester isolated from the flower buds of Daphne genkwa, resulted in the up-regulation of p21 expression and a significant G2/M phase arrest in T24T, HCT116, and A549 cells [Hong et al., 2011; Zhang et al., 2014].
The authors investigated whether the anti-tumor effects of GD-1 in cancer cells may involve the modulation of PRDM1 expression and whether PKD1 activation by GD-1 affects PRDM1 expression in the human colorectal cancer cell line SW620. In this study, the authors analyzed PRDM1 expression by GD-1 and the upstream-signaling events that lead to c-Myc repression involving the PKD1 and MEK pathways in response to GD-1. GD-1 is characterized by the down-regulation of c-Myc followed by the up-regulation of p21. The authors determined that GD-1 has anti-cancer effects through the interworking of PKD1/PRDM1/c-Myc/p21 in SW620 human colon cancer cells.
MATERIALS AND METHODS
ISOLATION OF GD-1
GD-1 was isolated from the dried flower buds of Daphne genkwa as previously reported [Park et al., 2007]. Briefly, the dried flower buds of Daphne genkwa were extracted with MeOH and the methanolic extract was resuspended in water.
CELL CULTURE AND PREPARATION
All human myeloid cells (U937, Jurkat, K562, and NK-92) and human colon cancer cells (HCT116, KM12c, SW620, and Colo205) were obtained from the American Type Culture Collection (ATCC) and cultured in a humidified incubator with 5% CO2 at 37 °C. The human IL-2-dependent NK lymphoma, NK-92, was maintained in α-MEM (Life Technologies, Karlsruhe, Germany) containing 20% FCS (HyClone, Logan, UT), 2 mM l-glutamate, 100 μg/ml penicillin, and 100 μg/ml streptomycin (Life Technologies) and supplemented with 100 U/ml IL-2 (Chiron, Emeryville, CA). U937, Jurkat, and K562 cells were maintained in RPMI-1640 (Life Technologies) containing 10% FCS. HCT116, KM12c, SW620, and Colo205 cells were maintained in DMEM (Life Technologies) containing 10% FCS.
INHIBITOR TREATMENT AND siRNA TRANSFECTION
CID 755673, an inhibitor of PKD1 (Tocris Bioscience, MO), and PD98059, an inhibitor of MEK (Sigma–Aldrich, St. Louis, MO), were added to the medium at various concentrations between 0 and 50 μM in DMSO (Sigma–Aldrich). DMSO was used as a vehicle control. The siRNA directed against human PKD1 (sc-36245), PRDM1 (sc-37714), and control siRNA (sc-37007) were from Santa Cruz Biotechnology (Santa Cruz, CA). For the transient transfection of single siRNAs, cells were incubated in the presence of siRNA (10 nM final) and Attractene Transfection Reagent (QIAGEN, Valencia, CA) according to the manufacturer's instructions.
REVERSE TRANSCRIPTASE (RT)-PCR ANALYSIS
A two-step RT-PCR reaction was performed using reverse transcriptase with oligo-dT primers and M-MLV reverse transcriptase (Promega, Madison, WI) with specific primer pairs. Total RNA was isolated using a standard protocol, and cDNA was synthesized using M-MLV reverse transcriptase following the manufacturer's instructions. One microliter of the synthesized cDNA was used per 20 μl PCR reaction—which was comprised of 0.5 U Taq DNA polymerase, 1× buffer, and 1 mM dNTP mix (Takara Korea Biomedical, Seoul, Republic of Korea) with specific primer pairs—and was amplified as follows: 94 °C for 5 min, then 25–40 cycles at 94 °C for 45 s, 56 °C for 45 s, and 72 °C for 1 min, followed by a final extension of 7 min at 72 °C using GeneAmp PCR system 2700 (Applied Biosystems, Foster City, CA). PCR primers were designed using the Primer3 program and were purchased from Bioneer (Daejeon, Korea). The sequences of used primers were as follows: PRDM1 forward, 5′-ATGCGGATATGACTCTGTGGA-3′, reverse, 5′-CTCGGTTGCTTTAGACTGCTC-3′; c-Myc forward, 5′-TCCACCTCCAGCTTGTACCT-3′, reverse, 5′-CCTGCCTCTTTTCCACAGAA-3′; p21 forward, 5′-CCAAGAGGAAGCCCTAATCC-3′, reverse, 5′-CCCTAGGCTGTGCTCACTTC-3′; and GAPDH forward, 5′-CCATCACCATCTTCCAGGAG-3′, reverse, 5′-ACAGTCTTCTGGGTGGCAGT-3′. The PCR products were separated on 1.5% agarose gel, stained with ethidium bromide, visualized with a Gel Doc 2000 UV trans-illuminator (Bio-Rad Laboratories, Hercules, CA), and analyzed using Quantity One software (Bio-Rad Laboratories). Each sample was tested more than three times, and representative data were shown.
WESTERN BLOT ANALYSIS
Soluble lysates from the cultured cells were extracted in ice-cold SDS-lysis buffer (50 mM HEPES, 150 mM NaCl, 0.2 mM EDTA, 0.5% NP-40, 0.1% SDS, 1 mM Na3VO4, 10 mM NaF, and complete protein inhibitor cocktail) for 30 min on ice. Thirty to fifty micrograms of the cell lysate were resolved by SDS-PAGE on 10% or 12% gels and transferred to PVDF membranes (Millipore, Billerica, MA). The membranes were incubated with primary antibodies followed by peroxidase-conjugated anti-rabbit or anti-mouse immunoglobulin (Ig) G secondary antibodies (Calbiochem, EMD Chemicals Inc., San Diego, CA) and ECL reagent (Millipore) for band visualization. To verify equal loading and adequate transfer, the membranes were probed with anti-GAPDH and anti-ß-actin antibodies (Santa Cruz Biotechnology). The primary antibodies were anti-PRDM1, anti-c-Myc, anti-p21/WAF1 (Santa Cruz Biotechnology), anti-phospho-PKD1, and anti-PKD1 (Cell Signaling Technology).
CONFOCAL MICROSCOPY
Cells were cultured on gelatin-coated coverslips, rinsed three times in cold PBS, and fixed and permeabilized using a BD cytofix/cytoperm fixation/permeabilization kit (BD Biosciences). The cells were blocked with 1% BSA in PBS for 30 min and then stained with anti-PRDM1 and c-Myc mAbs (1:200 dilution, Santa Cruz Biotechnology) for 2 h. Finally, the coverslips were incubated with fluorescent Alexa Fluor 594 anti-rabbit antibody (1:200 dilution, Life Technologies, Karlsruhe, Germany) in the dark for 1 h, and the nuclei were stained with 0.1 μg/ml DAPI for 5 min. The coverslips containing the cells were mounted on glass slides using VectaShield mounting medium (Vector Laboratories, Burlingame, CA). Confocal images were captured using a Zeiss LSM510META system (Carl Zeiss, Jena, Germany) at 60× magnification with the Zeiss LSM Image Browser program. The images were processed and analyzed by the same software.
CELL PROLIFERATION ASSAY
Cell proliferation was measured using the WST-1 reagent (Roche, Mennheim, Germany) according to the manufacturer's protocol. SW620 and K562 cells were seeded with about 1 × 103 cells per well on a 96-well flat-bottom plate. After seeding the cells, the wells were treated with the indicated concentration of GD-1 for 5 days. Plates were read on a microplate reader at 450 nm before and 45 min after adding the WST-1 reagent. The reference wavelength was 650 nm. Cell proliferation rates were calculated according to the manufacturer's protocol.
STATISTICAL ANALYSIS
All data in this report are means plus standard deviations (SDs) of at least three independent experiments. Statistical analyses were performed by student's t-test for data involving two groups or by analysis of variance for data involving more than two groups. P < 0.05 was considered statistically significant.
RESULTS
PRDM1 WAS EXPRESSED IN GD-1-TREATED HUMAN CELL LINES
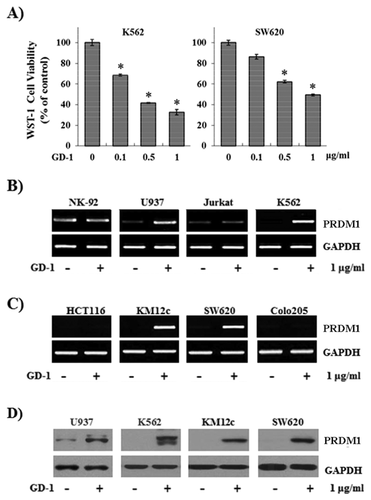
To study the tumor growth-arrest potential of the extracts, the K562 human leukemia cell line and the SW620 human colon cancer cell line were treated with the fractions from the flower bud extracts, and the cell proliferation was measured with WST-1. The proliferation of K562 and SW620 cells was reduced by GD-1 treatment in a dose-dependent manner (Fig. 1A). To further explore whether these effects are regulated through the transcriptional activity of genes involved in cellular proliferation, the authors evaluated the mRNA levels of PRDM1 following GD-1 treatment in lymphoid (Fig. 1B) and colon cancer cell lines (Fig. 1C). The mRNA level of PRDM1 was increased in the U937, K562, KM12C, and SW620 cell lines. These protein levels were achieved by GD-1 treatment. Because the mRNAs encoded by PRDM1 have two transcript isoforms, PRDM1alpha and PRDM1beta, a double band was detected. PRDM1 expressions were also stimulated by GD-1 treatment (Fig. 1D). Thus, these data indicate that GD-1 induces the mRNA and protein expressions of PRDM1.
MODULATION OF c-Myc AND p21 EXPRESSION BY GD-1 WAS DEPENDENT ON PRDM1 VIA PKD1 AND MEK ACTIVATION
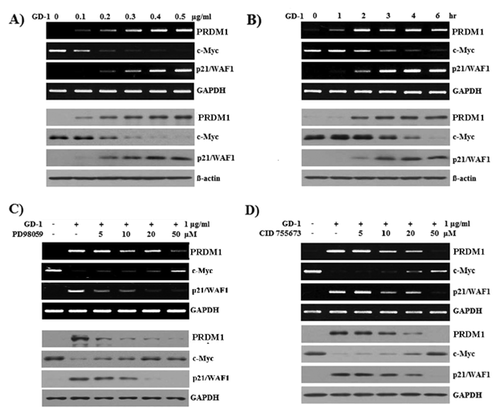
It is well known that PRDM1 binds to the c-Myc gene promoter and the decreased levels of c-Myc affect p21 transcript levels [Lin et al., 1997]. To determine whether GD-1 has an effect on the inverse correlation between c-Myc and p21 expressions, SW620 colon cancer cells were treated with 0–0.5 ug/ml of GD-1 for 6 h. The mRNA (upper panel) and protein (lower panel) levels of PRDM1, c-Myc, and p21/WAF1 were analyzed. GAPDH and β-actin were used as internal controls. GD-1 induced the suppression of c-Myc expression and lead to the up-regulation of p21/WAF1 (Fig. 2A). Following 1 ug/ml of GD-1 treatment for 0–6 h, c-Myc expression was down-regulated and p21/WAF1 was up-regulated (Fig. 2B). These results show that GD-1-induced PRDM1 affected c-Myc and p21/WAF1 expressions in a dose- and time-dependent manner, indicating that GD-1 controls the expression of PRDM1 through the activation of PKD1 and MEK in SW620 cells.
The phosphorylation of PKD1 and ERK1/2 in the GD-1-treated SW620 cells was determined by western blot analysis using anti-phospho-PKD1 (Fig. S1A and C) and anti-phospho-ERK1/2 (Fig. S1B and D), respectively. Anti-PKD1 and anti-ERK1/2 were used as internal controls. PKD1 and ERK1/2 phosphorylation started immediately after GD-1 treatment and increased for 5 min (Fig. S1B and D). These results suggest that GD-1 controls the phosphorylation of PKD1 and MEK in SW620 cells in a dose- and time-dependent manner.
To further confirm the mechanism involving PKD1 and MEK signaling, PRDM1 transcript (Fig. 2C and D, upper panel) and expressed PRDM1 protein (Fig. 2C and D, lower panel) were determined with a PKD1 inhibitor (CID 755763) and MEK inhibitor (PD98059). The reduced expression of c-Myc transcript and protein in the GD-1-treated SW620 cells was recovered by the MEK inhibitor (PD98059) (Fig. 2C) and PKD1 inhibitor (CID 755763) (Fig. 2D) in a dose-dependent manner. These observations suggest that the suppression of c-Myc in SW620 cells is controlled by PKD1 and MEK signaling, which are activated by GD-1.
As shown in Figure 2A and B, p21 was up-regulated by GD-1 in SW620 cells, which suggested that growth arrest in SW620 cells induced by GD-1 could be mediated by enhanced p21 expression. The induced expression of PRDM1 and p21 transcript and protein was inhibited dose-dependently by the PKD1 inhibitor (CID 755763), and the reduced expression of c-Myc transcript and protein in the GD-1-treated SW620 cells was recovered by the PKD1 inhibitor (CID 755763) in a dose-dependent manner (Fig. 2D). These results indicate that GD-1 controls the expression of PRDM1 through the activation of PKD1, and PRDM1 down-regulates the expression of c-Myc in SW620 cells. Therefore, negatively modulated p21 expression by c-Myc is enhanced by GD-1 treatment. These findings provide evidence that GD-1 inhibits SW620 cell proliferation through cell-cycle arrest by enhancing p21 expression.
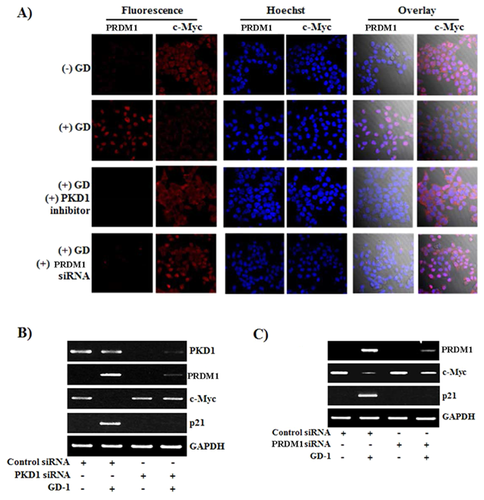
PKD1 KNOCKDOWN INDUCED DECREASE OF PRDM1 EXPRESSION IN SW620 HUMAN COLON CANCER CELLS
To determine whether GD-1 has an effect on c-Myc through PRDM1 expression and PKD1 signaling, cells were pretreated with PKD1 inhibitor or were transfected with PRDM1 siRNA. GD-1-treated SW620 cells were shown to induce PRDM1 and decrease c-Myc (Fig. 3A). Following treatment with the PKD1 inhibitor (CID 755763) or PRDM1 siRNA transfection, PRDM1 could not be induced and c-Myc was not decreased by GD-1. After the transfection of PKD1 siRNA to the SW620 cells, PRDM1 was not induced by GD-1, c-Myc was not decreased, and eventually, p21 was not induced by GD-1 (Fig. 3B). When cells were transfected with PRDM1 siRNA, c-Myc was not decreased, and eventually, p21 was not induced by GD-1 (Fig. 3C). Taken together, these data suggest that because p21 is a well-known cell-cycle inhibitor involved in cell-cycle regulation, the effect of GD-1 on induced p21 expression demonstrates cell-growth arrest in SW620, as shown in Figure 1A.
DISCUSSION
In this study, the authors found that GD-1 has anti-cancer activity (Fig. 1A) and increases tumor suppressor-gene PRDM1 expression in human colorectal cancer cells through the activation of PKD1, which is a key molecule in PRDM1 induction (Fig. 3B). Increased PRDM1 represses c-Myc expression and modulates the c-Myc target gene, p21/WAF1, following anti-proliferating activity, including the arrest of cell growth.
The function and biological role of GD-1 in epithelial cells have not been previously reported on in detail. Here, the authors identified GD-1 as a PRDM1 inducer in several cultured tumor-cell lines. GD-1 originating from natural products has biological effects on PKD1 activation. PKD1 is a serine-threonine kinase that regulates various functions within the cell, including cell proliferation, apoptosis, adhesion, and cell motility [Sundram et al., 2011]. PKD1 is activated by a number of different agents, including pharmacological agents, phorbol esters, and/or physiological stimuli, such as tumor necrosis factor (TNF), neuropeptides, angiotensin II, and platelet-derived growth factors (PDGF) [Van Lint et al., 2002; Rozengurt et al., 2005]. The authors previously reported that GD-1 and yuanhuacine in NK-92 cells serve as inducers of IFN-γ via the PKD1/NF-κB/STAT1-dependent pathway [Kang and Ahn, 2014].
PRDM1 is well known as a differentiation factor of lymphocytes [Crotty et al., 2010]. PRDM1 is a zinc finger containing a transcriptional repressor that is necessary for the terminal differentiation of B cells into antibody-secreting plasma cells. Recent work indicated that STAT5 activation by IL-2 and IL-5 is critical for the expression of PRDM1 in B cell differentiation [Turner et al., 1994; Nurieva et al., 2012]. STAT5 regulated the expression of T follicular helper (Tfh) cell-suppressor factor PRDM1, and STAT5 deficiency impaired PRDM1 expression and resulted in the elevated expression of Tfh-specific genes. Similarly, the inhibition of IL-2-potentiated Tfh generation was associated with dampened PRDM1 expression, and PRDM1 overexpression inhibited Tfh gene expression in STAT5-deficient T cells, suggesting that the IL-2/STAT5 axis functions to regulate PRDM1 expression. In vivo, deletion of STAT5 in CD4(+) T cells resulted in the enhanced development of Tfh cells and germinal center B cells and led to the impairment of B cell tolerance in a well-defined mouse tolerance model [Nurieva et al., 2012]. Activating the CD8(+) T cells with antigen and IL-2 causes the expression of the core Hippo pathway components, including the pivotal transcriptional cofactor Yap. Contact between activated CD8(+) T cells induces Hippo pathway-mediated Yap degradation and PRDM1 expression, and a Hippo-resistant, stable form of Yap suppresses PRDM1 expression. The CTLA-4/Hippo pathway/PRDM1 system may couple the terminal differentiation of CD8(+) T cells with the magnitude of clonal expansion [Thaventhiran et al., 2012]. PRDM1 is also induced during the differentiation of U937 and HL-60 cells treated with PMA into macrophages or granulocytes [Chang et al., 2000].
In addition to B cells, PRDM1 is expressed by several different cell lineages, including memory T cells (but not naïve T cells), myeloid lineage cells, and epithelial cells. According to several recent studies, PRDM1 expression has also been reported in epithelial cells and is the most important factor for keratinocyte differentiation [Magnusdottir et al., 2007]. In the present study, GD-1-mediated PRDM1 expression was associated with the phosphorylation of PKD1 in a human SW620 colon cancer cell line. PRDM1 induced by GD-1 was attenuated by the PKD1 inhibitor (CID 755763) in a dose-dependent manner (Fig. 2D). When SW620 cells were transfected with PKD1 siRNA, PRDM1 was not induced by GD-1 treatment (Fig. 3B). Although further studies are needed to determine the expression of PRDM1 in the presence of GD-1 with and without adding a specific inhibitor of PKD1 phosphorylation, these data clearly demonstrate that PKD1 activation is crucial for GD-1-mediated PRDM1 expression.
PRDM1 inhibited c-Myc transcription [Yu et al., 2000], which explains the cessation of the cell cycle in plasma cells, as c-Myc is required for proliferation and growth [Chang et al., 2000; Shaffer et al., 2002]. PRDM1 repressed its known target gene, c-Myc [Lin et al., 1997], as well as genes that are transcriptionally activated by c-Myc. The mechanism of reciprocal regulation of c-Myc by PRDM1 expression caused by GD-1 was revealed through the PKD1-signaling pathway. In the signal blocking assay by several kinase inhibitors, MEK inhibitor (PD98059) (Fig. 2C) and PKD1 inhibitor (CID 755763) (Fig. 2D) dose-dependently restored c-Myc expression. Our data show that GD-1 activates the PKD1 and MEK signals, induces PRDM1 expression, and subsequently reduces c-Myc expression in SW620 cells.
As mentioned above, treatment with the daphnane diterpene esters GD-1 and yuanhuacine from the dried flower buds of Daphne genkwa resulted in the up-regulation of p21 expression and growth arrest in cancer cell lines [Hong et al., 2011; Zhang et al., 2014]. Our study found that PKD1 was activated by GD-1 treatment and that GD-1-induced phosphorylated PKD1 induced PRDM1 expression and subsequently repressed c-Myc expression. The mechanism of transcriptional repression of p21 by c-Myc has been a subject of intensive study for several years, as it may explain how c-Myc promotes cell-cycle progression [Jung and Hermeking, 2009]. In our study, the GD-1 treatment of SW620 colon cells suppressed cell proliferation, and it was characterized by the down-regulation of c-Myc followed by the up-regulation of p21. Growth arrest by p21 can promote cellular differentiation, thereby preventing cell proliferation [Abbas and Dutta, 2009]. PRDM1 regulated cell growth through the repression of p53 transcription, and the authors observed that GD-1 also down-regulated p53 transcription (data not shown) [Yan et al., 2007]. It remains unclear whether GD-1 mediates a direct effect on the PKD1 pathway and PRDM1 expression, and thus, further studies are needed.
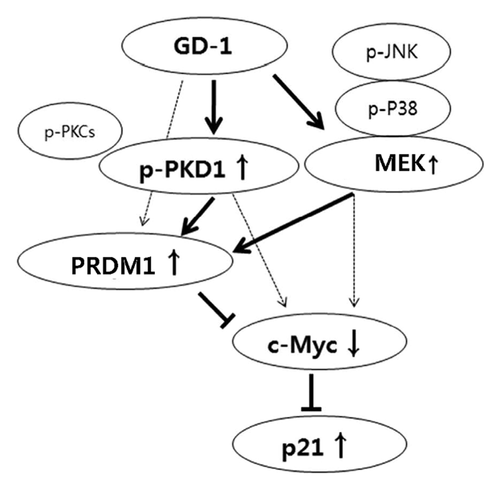
In this report, the authors revealed that GD-1, a purified natural product from Genkwadaphnin plant extracts, has potential activity in PRDM1 expression in SW620 cell lines. In summary, based on the data presented here, the authors propose a model (Fig. 4) in which PRDM1 expression by GD-1 is mediated by the activity of PKD1 and MEK and, subsequently, regulates the expression of c-Myc and p21. These results suggest important new insights into the anti-tumor activity of GD-1 and provide helpful information for the treatment of cancer.
ACKNOWLEDGMENTS
This work was supported by the KRIBB Research Initiative Program (KGM4701511), the National Research Foundation of Korea (NRF-2012R1A1A2043799), and Small & Medium Business Administration of Korea (SCE0101312).