Numb Protects Human Renal Tubular Epithelial Cells From Bovine Serum Albumin-Induced Apoptosis Through Antagonizing CHOP/PERK Pathway
ABSTRACT
In recent studies, we found that Numb is involved in oxidative stress-induced apoptosis of renal proximal tubular cells; however, its function on ER stress-induced apoptosis in proteinuric kidney disease remains unknown. The objective of the present study is to explore the role of Numb in urinary albumin-induced apoptosis of human renal tubular epithelial cells (HKCs). In this study, we demonstrate that incubation of HKCs with bovine serum albumin (BSA) resulted in caspase three-dependent cell death. Numb expression was down-regulated by BSA in a time- and dose-dependent manner. Knockdown of Numb by siRNA sensitized HKCs to BSA-induced apoptosis, whereas overexpression of Numb protected HKCs from BSA-induced apoptosis. Moreover, BSA activated CHOP/PERK signaling pathway in a time- and dose-dependent manner as indicated by increased expression of CHOP, PERK, and P-PERK. Furthermore, knockdown of CHOP or PERK significantly attenuated the promoting effect of Numb on BSA-induced apoptosis, while overexpression of CHOP impaired the protective effect of Numb on BSA-induced apoptosis. Taken together, our findings demonstrate that Numb plays a protective role on BSA-induced apoptosis through inhibiting CHOP/PERK signaling pathway in human renal tubular epithelial cells. Therefore, the results from this study provides evidence that Numb is a new target of ER-associated apoptotic signaling networks and Numb may serve as a promising therapeutic target for proteinuric diseases. J. Cell. Biochem. 117: 163–171, 2016. © 2015 Wiley Periodicals, Inc.
Many progressive kidney diseases contributing to loss of kidney function are characterized by proteinuria, which is involved in activation of proinflammatory and profibrotic molecules [Gekle, 2005; Gorriz and Martinez-Castelao, 2012; Lawson et al., 2015]. Therefore, proteinuria reduction has become the goal of therapy for proteinuric kidney disease, and the degree of urinary albumin is widely used as a parameter to monitor the response to therapy [Cravedi and Remuzzi, 2013]. Excessive urinary albumin reabsorption stimulates inflammatory cytokine recruitment and apoptotic regulators activation in tubulointerstitial, resulting in proximal tubule cells injury [Erkan, 2013; Hodgkins and Schnaper, 2012]. Recent urinary albumin related injury studies mainly focus on apoptosis suppression, which is shown to be an effective approach to ameliorate the proximal tubule cells injury and urinary albumin-induced inflammation. Activation of extrinsic and intrinsic signaling pathways leads to tubular cell apoptosis in proteinuric kidney diseases [Chen et al., 2013]. Accumulating data demonstrate the involvement of ER stress in the apoptosis of both proteinuric rat kidney proximal tubular cells and proteinuric kidney biopsy [Ohse et al., 2006; Cybulsky, 2010]. It is well known that endoplasmic reticulum (ER) stress is one of the key mechanisms involved in response to deleterious stimuli in tubular cells. Excessive and persistent ER stress might eventually trigger apoptosis via activating ER transmembrane protein PERK [Cunard and Sharma, 2011], which induces translation of its target genes such as CHOP [Nishitoh, 2012].
Numb was originally discovered as an evolutionarily conserved adapter protein regulating cell differentiation in Drosophila [Verdi et al., 1996]. Many findings, including our own, suggest that Numb plays a role in apoptosis. Short PTB domain Numb isoforms regulate sensitivity of the PC12 cells to neurotrophic factor withdrawal-induced death [Pedersen et al., 2002]. In the serotonin lineage of drosophila, Numb influences neuronal differentiation by inhibiting apoptosis initiated by Notch signaling pathway [Lundell et al., 2003]. Overexpression of Numb increases p53 stability and enhances p53-mediated apoptosis [Colaluca et al., 2008]. Our own recent investigation demonstrates that in renal proximal tubular cells, Numb functions as a protective molecule on oxidative stress agent puromycin aminonucleoside (PA)-induced apoptosis through antagonizing Notch signaling activity [Ding et al., 2011].
As there are virtually no data on the role of Numb in proteinuria induced tubule cells apoptosis, the aim of the present study is to explore the role of Numb in the regulation of renal proximal tubular cell apoptosis induced by bovine serum albumin (BSA) and the participation of ER stress related CHOP/PERK pathway.
MATERIALS AND METHODS
REAGENTS AND ANTIBODIES
BSA and Annexin V-FITC Apoptosis Detection Kit were purchased from Sigma–Aldrich (St. Louis, MO). Dulbecco's Modified Eagle Medium Ham's Nutrient Mixture F-12 (DMEM/F-12), fetal bovine serum (FBS) was purchased from Invitrogen (Carlsbad, CA). Numb and cleaved caspase 3 antibodies were obtained from Cell Signaling (Danvers, MA); CHOP, PERK, and P-PERK antibodies were acquired from Santa Cruz Biotechnology (Santa Cruz, CA).
CELL CULTURE AND TREATMENTS
Human renal proximal tubular cell line (HKCs) was obtained from the American Type Culture Collection (Manassas, VA) and cultured in DMEM/F-12 supplemented with 10% FBS at 37°C in a 5% CO2 incubator. After reaching 80% confluence, cells were seeded into six-well plates at a density of 1 × 105 cells per well to grow again to 80% confluence for treatments. To detect Numb expression induced by BSA in HKCs, cells were cultured in serum free medium for 24 h, then incubated with 0, 5, 10, 20, and 30 mg/ml BSA for 36 h or treated with 30 mg/ml BSA for 0, 24, 32, 40, and 48 h.
PLASMID TRANSFECTION
pcDNA-Numb plasmid was kindly provided by Dr. Jane McGlade (University of Toronto, Canada). When the HKCs density was up to 80%, we transiently transfected HKCs with siRNA by Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Briefly, the pcDNA-Numb and Lipofectamine 2000 were premixed in OPTI-medium (Gibco, Carlsbad, CA) for 10 min and then applied to the cells. After transfection for 6 h, Lipofectamine 2000 containing medium was replaced with fresh DMEM/F-12 medium containing 10% FBS, and cells were treated for another 18 h and harvested.
siRNA TRANSFECTION
Numb siRNA was synthesized by Shanghai Gene Pharma (Shanghai, China). siRNA sequence was 5′-GGACCTCATAGTTGACCAG-3′. CHOP siRNA (sc-35437) and P-PERK siRNA (sc-36213) were purchased from Santa Cruz Biotechnology. The transfection of siRNA in HKCs was carried out with Lipofectamine 2000, according to the manufacturer's instructions.
WESTERN BLOT ANALYSIS
Cells with indicated treatment were homogenized in lysis buffer (150 mM NaCl,10 mM Tris-HCl, 5 mM EDTA, 1 mM EGTA, and 1% TritonX-100) containing a protease inhibitor cocktail (Roche, Mannheim, Germany). Protein concentration was determined by the Bradford assay (Bio-Rad, Hercules, CA), equal amount of protein were separated on SDS-polyacrylamide gels, and transferred to PVDF membranes (Amersham Biosciences, Piscataway, NJ). After blocking in 5% skim milk for 0.5 h at room temperature, membranes were incubated with indicated primary antibody at 4°C overnight followed by horseradish peroxidase-conjugated second antibody (Amersham Biosciences) and detected by chemiluminescence. All Western blot in this study was subjected to different exposures: from 5 s to 8 min, and the best exposures were selected for data presentation. Quantification of the Western blot data was performed by measuring the intensity of the hybridization signals using NIH Image analysis program.
FLOW CYTOMETRIC ANALYSIS
After cells were subjected to the designated treatments, 1 × 106 cells were collected and resuspended in 500 ml binding buffer. For apoptosis determination, cells were double-stained with Annexin V-FITC and propidium iodide (PI) using Annexin V-FITC Apoptosis Detection Kit according to the manufacturer's instruction. The stained cells were analyzed by Cytomics FC 500 Flow Cytometer (Beckman Coulter, Fullerton, CA) and Cell Quest TM (BD Biosciences, San Jose, CA) software. Annexin V+/PI− cells were identified as apoptotic cells.
STATISTICAL ANALYSIS
Data were expressed as mean ± SD. Standard statistical software (SPSS for Windows, version 13.0) was used for data analysis. Statistical significance among mean values was evaluated using one-way ANOVA tests or the Student's t-test. A value of P < 0.05 was considered statistically significant.
RESULTS
BSA INDUCES HKCs APOPTOSIS IN A CASPASE 3-DEPENDENT MANNER
To mimic the in vivo conditions of HKCs exposed to proteinuria, a fatty acid-free BSA overload approach was employed. HKCs were incubated with various concentrations of BSA for different times. Apoptosis was detected with Annexin V-FITC/PI double staining for flow cytometric assay. As shown in Figure 1A, percentages of apoptosis were at 13.1%, 28.4%, 43.8%, and 45.9% after incubation of 30 mg/ml BSA for 24, 32, 40, and 48 h, respectively, whereas less than 3.4% apoptotic cells were examined in control cells. Based on these results, HKCs were then incubated with incremental concentrations of BSA for 36 h. As shown in Figure 1B, 10.0%, 19.2%, 24.8%, and 37.4% apoptosis were detected after 5, 10, 20, and 30 mg/ml BSA treatment, respectively, compared to the control group containing 3.6% apoptotic cells. These data reveal that BSA induces HKCs apoptosis in a time- and dose- dependent manner.
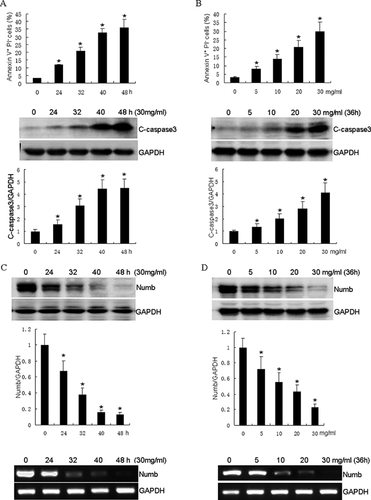
Caspase 3 is involved in both extrinsic and intrinsic pathway of apoptosis [Elmore, 2007]. To determine whether BSA-induced apoptosis of HKCs was associated with caspase 3 activation, the protein level of cleaved caspase 3 in the HKCs was examined after BSA treatment. As shown in Figure 1A, significant caspase activation was detected at the concentration of 30 mg/ml BSA from 24 h incubation. These results indicate that BSA induces caspase 3-dependent apoptosis in HKCs.
NUMB PROTECTS HKCs FROM BSA-INDUCED APOPTOSIS
In order to determine whether Numb is involved in BSA-induced apoptosis in HKCs, we examined the expression of Numb in response to BSA treatment. As shown in Figure 1C, BSA induced Numb down-regulation in a dose-dependent manner in HKCs. A significant decrease of Numb expression was observed in cells exposed to 30 mg/ml BSA from 24 h, and Numb expression was almost completely eliminated after treatment for 48 h. Subsequent experiments revealed that the expression of Numb decreased gradually with increased concentrations of BSA. At 30 mg/ml of BSA treatment, Numb was decreased to approximately 23% of that in control cells (Figure 1D). To determine whether BSA could regulate Numb level at the mRNA level or post-translationally, we next examined the Numb transcript by real time PCR. As shown in Figure 1C and D, Numb mRNA was gradually decreased after BSA treatment in HKCs, indicating that BSA regulates Numb expression at the transcription level. Thus, we demonstrate that BSA suppressed Numb expression in a time- and dose- dependent manner.
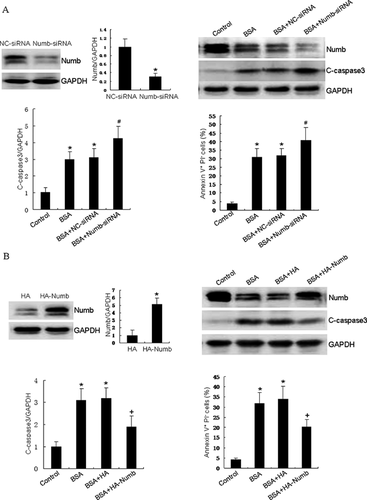
To explore whether expression of Numb is involved in HKCs apoptosis by BSA overloading, we first knocked down endogenous Numb by siRNA before BSA incubation. As shown in Figure 2A, BSA treatment induced approximately 31.4% apoptotic cells. Furthermore, knockdown of Numb enhanced BSA-induced apoptosis to 41.6%. Moreover, the down-regulation of Numb expression significantly increased the activation of caspase 3. To further confirm the role of Numb in BSA-induced cell apoptosis, we overexpressed Numb by transfecting HKCs with HA-Numb plasmid before BSA incubation. As shown in Figure 2B, Numb overexpression markedly attenuated BSA-induced apoptosis to approximately 20.3%, compared to vector transfected HKCs 34.6%. These results provide the first evidence for a protective role of Numb in BSA-induced apoptosis in HKCs.
NUMB SUPPRESSES BSA-INDUCED CHOP/PERK PATHWAY ACTIVITY IN HKCs
Accumulated evidence suggests that the ER stress pathway significantly contributes to apoptosis of renal tubular cells both in vitro and in vivo models of albuminuria [Li et al., 2010; Fang et al., 2013]. Therefore, we hypothesized that Numb increases BSA-induced apoptosis in HKCs via activating ER stress pathway. To test our hypothesis, we first examined whether BSA induces ER stress in HKCs, we then detected CHOP expression and ER stress markers after treatment with BSA in HKCs. As shown in Figure 3A and B, BSA treatment increased CHOP expression in HKCs in a time- and dose- dependent manner. PERK has been reported to play an important role as an essential molecule in CHOP protein expression. Therefore, we evaluated whether PERK is upregulated or activated by BSA overload. Western blot analysis demonstrates that BSA overload significantly upregulated the total protein levels and the active form of PERK in HKCs in a time- and dose- dependent manner. These results indicate that ER stress-related CHOP/PERK pathway is induced in BSA-exposed HKCs.
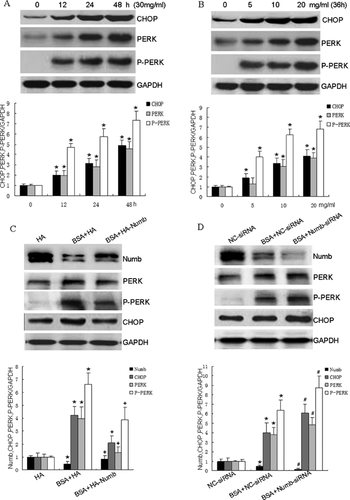
We next investigated whether Numb expression is involved in CHOP/PERK pathway activation in BSA-exposed HKCs apoptosis. As shown in Figure 3C, up-regulation of Numb expression using HA-Numb plasmid, but not the vector control, counteracted BSA-induced CHOP, PERK expression and PERK phosphorylation in HKCs. Moreover, knockdown of Numb by its specific siRNA, but not in negative control siRNA, increased BSA-induced CHOP, PERK expression and PERK phosphorylation in HKCs (Figure 3D). These data demonstrate that knockdown of Numb is associated with CHOP/PERK pathway activation in BSA-exposed HKCs, contributing to subsequent apoptosis.
NUMB PROTECTS HKCs FROM BSA-INDUCED APOPTOSIS BY INHIBITING CHOP/PERK PATHWAY ACTIVATION
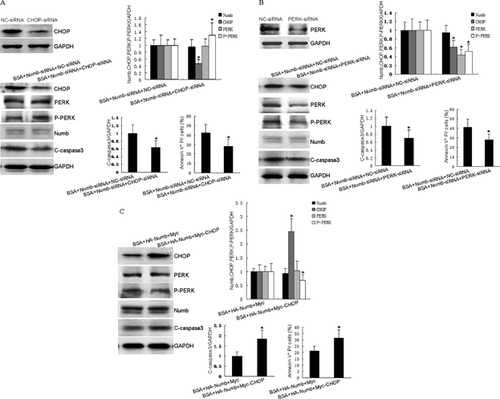
To further determine the roles of PERK/CHOP in Numb's protective effect on BSA-exposed HKCs apoptosis, the siRNA approach was used to reduce levels of PERK and CHOP expression. The efficiency of CHOP siRNA or PERK siRNA on inhibiting protein expression was demonstrated by Western blotting (Figure 4A and B). As shown in Figure 4A and B, CHOP siRNA transfection resulted in down-regulation of CHOP expression and up-regulation of P-PERK expression. Meanwhile, CHOP siRNA transfection led to 14.4% decrease of Numb knockdown-augmented apoptosis in BSA-exposed HKCs compared to negative control siRNA. Moreover, PERK siRNA transfection resulted in down-regulation of CHOP, PERK, and P-PERK expression. Meanwhile, PERK siRNA transfection led to 12.5% decrease of Numb knockdown-augmented apoptosis in BSA-exposed HKCs compared to negative control siRNA. Furthermore, Myc-CHOP plasmid was used to increase CHOP expression level. As shown in Figure 4C, Myc-CHOP transfection resulted in up-regulation of CHOP expression and down-regulation of P-PERK expression. Meanwhile, Myc-CHOP transfection led to 10.2% increase of Numb-overexpression-attenuated apoptosis in BSA-exposed HKCs compared to vector control. Taken together, these data provide evidence that Numb protects HKCs from BSA-induced apoptosis via inhibiting CHOP/PERK pathway activation.
DISCUSSION
It has been reported that proteinuria contributes to tubulointerstitial fibrosis of progressive kidney diseases [Erkan, 2013; Hodgkins and Schnaper, 2012]. In this context, it has been suggested that urinary albumin overload means a direct injury for renal proximal tubular cells (PTCs) [Ohse et al., 2006]. In the present study, we employed the fatty acid-free albumin to establish an albumin overload model. We found that the albumin induces HKCs apoptosis in a time- and dose- dependent manner and the function of Numb on BSA-induced apoptosis is via suppressing CHOP/PERK signaling pathway.
PTCs reabsorpting albumin is a process of endolysosomal phagocytosis, therefore, albumin internalization is closely related to endocytic protein [Comper, 2014; Dickson et al., 2014]. Numb protein, involved in protein endocytosis [Dho et al., 2006], selectively binds to certain transmembrane receptors and links them to endocytosis for receptors internalization [Nishimura and Kaibuchi, 2007]. Our recent study suggests that overexpression of Numb enhanced integrin endocytosis in PTCs [Ding et al., 2015]. Taken together, these results suggest that Numb plays a role on albumin endocytosis in PTCs. In our earlier study, we found that Numb also acts as a protective molecule on PA-induced apoptosis through antagonizing Notch signaling activity [Lundell et al., 2003]. Our current work characterizes a novel function of Numb as a survival factor in HKCs and its up-regulation protects HKCs from apoptosis caused by albumin overload.
Although both extrinsic and intrinsic pathways of apoptosis have been shown to contribute to tubular apoptosis in response to proteinuria stimulating [Ohse et al., 2006], recent studies further suggest the involvement of ER stress [Cybulsky, 2010; Chen et al., 2013]. ER-stress-induced apoptosis is mainly mediated by CHOP. CHOP is a transcription factor which induces several proapoptotic factors, and is downstream of the PERK and ATF6 pathway in proapoptotic UPR [Liu and Kaufman, 2003]. Wu et al. found that there was a positive correlation between the enhanced expression of CHOP and cell apoptosis in the tubule epithelial cells of NS patients [Wu et al., 2010]. It has been reported that the activation of PERK may be the key to regulate CHOP expression [Wan et al., 2014]. In the present study, we demonstrate that HKCs exposed to albumin underwent the state of ER stress and the exposure of HKCs to BSA upregulated CHOP and PERK expression, as well as PERK phosphorylation.
In mammals, it has been demonstrated that Numb interacts with Notch NICD domain and promotes its degradation [Wakamatsu et al., 1999]. Numb and Notch signaling interact with each other in modulating the balance of cell proliferation, differentiation, and apoptosis, affecting the development and function of many organs [McGill and McGlade, 2003]. It has also been found that MAPK/PERK pathway positively regulates activation of Notch in a variety of cell-fate specification processes [Yousef et al., 2014]. In this study, we show that up-regulation of Numb expression suppressed BSA-induced CHOP, PERK expression, and PERK phosphorylation, while down-regulation of Numb expression increased BSA-induced CHOP, PERK expression, and PERK phosphorylation in HKCs. We also demonstrate that the knockdown of CHOP by siRNA had no influence on BSA-induced PERK levels, and knockdown of PERK by RNAi inhibited the BSA-induced increase of CHOP protein, suggesting that CHOP is a downstream signal of PERK. Moreover, we further show that the p-PERK level was considerably inhibited by overexpression of CHOP, implying that the level of CHOP may feedback to regulate the activation of PERK by BSA overload. Furthermore, we display that CHOP/PERK pathway is down-regulated in Numb modulating BSA-induced apoptosis in HKCs as knockdown of CHOP and PERK could reverse Numb siRNA augmented HKCs apoptosis induced by BSA. In contrast, the protective effect of Numb overexpression on BSA-induced apoptosis in HKCs was inhibited by Myc-CHOP overexpression. These results indicate that Numb attenuates BSA-induced apoptosis through antagonizing CHOP/PERK signaling activity in HKCs.
In summary, our study, for the first time, provides evidence that Numb has a protective effect on BSA-induced HKCs apoptosis and the effect of Numb on BSA-induced apoptosis is through down-regulating ER stress-related CHOP/PERK signaling pathway. Therefore, this work proffers a new insight for exploring the signaling networks of ER related apoptosis, and Numb may serve as a novel therapeutic target for proteinuric diseases.
ACKNOWLEDGMENTS
This work was supported by project grants from the National Natural Science Foundation of China (No. 81471307, 81301086, 81100881, 81100949) and the Youth Innovation Fund of The First Affiliated Hospital of Zhengzhou University (Xuejing Wang and Xuebing Ding) and the 5451 Project of Health Department of Henan Province (Xuejing Wang, Xuebing Ding).