Down-regulation of c-fos by shRNA sensitizes adriamycin-resistant MCF-7/ADR cells to chemotherapeutic agents via P-glycoprotein inhibition and apoptosis augmentation†‡
Conflict of Interest Statement: The authors report no conflicts of interest.
Ruizan Shi and Hongwei Peng contributed equally to this work.
Abstract
Multidrug resistance (MDR) is a major hurdle in the treatment of cancer. Research indicated that the main mechanisms of most cancers included so-called “pump” (P-glycoprotein, P-gp) and “non-pump” (apoptosis) resistance. Identification of novel signaling molecules associated with both P-gp and apoptosis will facilitate the development of more effective strategies to overcome MDR in tumor cells. Since the proto-oncogene c-fos has been implicated in cell adaptation to environmental changes, we analyzed its role in mediating “pump” and “non-pump” resistance in MCF-7/ADR, an adriamycin (ADR)-selected human breast cancer cell line with the MDR phenotype. Elevated expression of c-fos in MCF-7/ADR cells and induction of c-fos by ADR in the parental drug-sensitive MCF-7 cells suggested a link between c-fos and MDR phenotype. Down-regulation of c-fos expression via shRNA resulted in sensitization of MCF-7/ADR cells to chemotherapeutic agents, including both P-gp and non-P-gp substrates. Further results proved that c-fos down-regulation in MCF-7/ADR cells resulted in decreased P-gp expression and activity, enhanced apoptosis, and altered expression of apoptosis-associated proteins (i.e., Bax, Bcl-2, p53, and PUMA). All above facts indicate that c-fos is involved in both P-gp- and anti-apoptosis-mediated MDR of MCF-7/ADR cells. Based on these results, we propose that c-fos may represent a potential molecular target for resistant cancer therapy, and suppressing c-fos gene expression may therefore be an effective means to temper breast cancer cell's MDR to cytotoxic chemotherapy. J. Cell. Biochem. 114: 1890–1900, 2013. © 2013 Wiley Periodicals, Inc.
Breast cancer is a major cause of cancer deaths in women, second only to lung cancer [Sledge, 2004]. Chemotherapy plays a key role in the management of patients with advanced breast cancer [Moulder and Hortobagyi, 2008]. Some chemotherapeutic drugs, such as adriamycin (ADR), docetaxel, and paclitaxel, have been shown to be effective as first- or second-line anti-cancer drugs in the management of breast cancer [Vanhoefer et al., 1997]. However, the clinical usefulness of these drugs is severely restricted because of the development of acquired multidrug resistance (MDR) [Guo et al., 2004]. MDR is a major hurdle to effective chemotherapy, and patients with MDR tumor types are often left with few options but exceptionally high doses. Therefore, improving the effectiveness of these cytotoxic agents and reducing their side effects is an important area of cancer research.
There are a number of mechanisms that may contribute to the MDR phenomenon. The primary mechanisms of MDR can be broken down into two major aspects: “pump” and “non-pump” resistance. The best characterized form of pump resistance in human cells is due to the overexpression of P-glycoprotein (P-gp), the MDR1 gene product [Sharom, 2011]. P-gp is a transmembrane protein and functions as an ATP-dependent drug transporter which unilaterally transports the intracellular drugs out of the cells, as such, the intracellular drugs in the MDR cancer cells were kept at sub-lethal level, by which cancer cells circumvent the effective attack by the anti-cancer drugs. The non-pump resistance is the result of anti-death effect, which mainly improves survival and suppresses apoptosis [Lo et al., 2008]. Resistance to anticancer treatment is usually correlated with a low propensity to apoptosis [Kong et al., 2006]. Some of the more heavily studied proteins of importance for the control of apoptosis are Bcl-2, Bax, p53, and PUMA (p53 up-regulated modulator of apoptosis), and alternations in the expression of these proteins in tumor cells have been shown to confer the drug-resistant phenotype in various types of cancer [Kang and Reynolds, 2009].
In the past two decades, a number of strategies have been implemented to overcome cancer cell MDR. Numerous targeted molecules and pharmacologic compounds that modulate P-gp-mediated MDR have been identified. However, P-gp inhibition has had, to date, little or no clinically significant effect in multidrug-resistant cancer [Kannan et al., 2009; Palmeira et al., 2012]. Additionally, multiple approaches have been developed to target Bcl-2 family members in cancer cells, but few of these have so far proved to be clinically useful for reversing the drug resistance in cancer patients [Bajwa et al., 2012]. The fact that drug resistance could not be completely reversed by specific chemical inhibitors or gene-specific siRNAs against individual drug-resistant mechanism indicates that the MDR phenotype represents a complex, multifactorial process, with at least two or more resistance mechanisms [Jabr-Milane et al., 2008]. Therefore, the studies on the identification of novel approaches or molecules targeting multiple drug-resistant mechanisms are therefore an area of high priority in cancer research.
A class of proto-oncogenes referred to as immediate early response genes is crucial for cell adaptation to chemotherapy [Okuma et al., 2003; Zhao et al., 2011]. Among potential proto-oncogenes would be c-fos. The c-fos gene encodes a nuclear transcription factor, c-fos, that dimerizes with the gene product of c-Jun to form the transcription factor activating protein 1 (AP-1). As a member of AP-1, c-fos protein has been implicated mainly in signal transduction, cell differentiation, proliferation, apoptosis, and tumor progression [Chiu et al., 1988]. One of the earliest responses of cells upon exposure to DNA-damaging agents (including ADR) is the induction of c-fos [Kaina et al., 1997], suggesting a functional involvement of c-fos protein in cellular protection against genotoxic agents.
The main aim of this study is to investigate whether c-fos expression was associated with the MDR phenotype in breast cancer cells. If so, whether reducing c-fos expression was an effective method for increasing the sensitivity of resistant breast caner cells to chemotherapeutic agents. The functional involvement of c-fos in MDR was then investigated in MCF-7/ADR, an ADR-selected human breast cancer cell line with the MDR phenotype. Our findings indicated that c-fos and MDR of MCF-7/ADR cells were closely associated, and c-fos down-regulation via shRNA resulted in decreased P-gp activity and expression, increased apoptosis and alterations of apoptosis associated proteins in MCF-7/ADR cells. The totality of these results suggests that c-fos may play an important role in MDR of breast cancers and highlights a potential role for c-fos as a molecular target in resistant cancer therapy.
MATERIALS AND METHODS
Materials
ADR, paclitaxel, VP-16 (etoposide), and vincristine were purchased from Wanle Pharmaceutical (Shenzhen, China). 5-fluorouracil (5-Fu) was obtained commercially from Tianjin Jinyao Group Company (Tianjin, China). Cisplatin (CDDP) was purchased from Shandong Qilu Pharmaceutical Company (Shandong, China). MTT [3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di-phenytetrazoliumromide] and G418 were purchased from Sigma–Aldrich (St. Louis, MO).
Cell Culture
All cells were grown in a humidified atmosphere incubator with 5% CO2 and 95% air at 37°C. MCF-7 cells were cultured in RPMI-1640 medium containing 100 U/ml penicillin, 100 mg/ml streptomycin, and 10% fetal bovine serum (FBS, GIBCO, Grand Island, NY). MCF-7/ADR cells were maintained in an RPMI-1640 medium containing 10% FBS and 1 µg/ml ADR to retain their MDR phenotype in daily culture. The cells were cultured for 2 weeks in drug-free medium prior to their use in the experiments.
Cytotoxicity Assay
Cells were plated at a density of 5,000 cells per well in a 96-well plate. After 24 h, cells were treated with different concentrations of anti-cancer drugs. After 68 h, 20 µl of MTT solution (5 mg/ml in PBS) was added to each well and the plates were incubated at 37°C for an additional 4 h. At the end of the incubation, 0.1 ml DMSO was added to each well to dissolve the formazan crystals. Absorbance in each well was read by 546 nm in a microplate reader (Bio-Rad). Drug concentrations producing 50% cell growth inhibition (IC50) were determined by curvefitting analyses using Prism software (GraphPad Software, San Diego, CA). The resistance fold (RF) was calculated using the following formula: RF = IC50 drug-resistant cells/IC50 drug-sensitive cells. The experiments were performed three times in triplicates.
Reverse Transcriptase PCR and Quantitative Real-Time PCR
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA) and the first strand of cDNA was obtained by reverse transcription (Invitrogen) according to the manufacturer's instructions. cDNA was used to detect human c-fos and MDR1 gene expression by conventional reverse transcriptase PCR and real-time PCR. The following PCR primers (Invitrogen) were used for the conventional RT-PCR: 5′-GTCTCCAGTGCCAACTTCAT-3′ (sense) and 5′-CAGCCATCTTATTCCTTTCC-3′ (antisense) for the c-fos gene (to generate a 289-bp fragment); 5′-ATAATGCGACAGGAGATAGG-3′ (sense) and 5′-TTGCCATTGACTGAAAGAAC-3′ (antisense) for the MDR1 gene (to generate a 133-bp fragment); 5′-CTACAATGAGCTGCGTGTGGC-3′ (sense) and 5′-CAGGTCCAGACGCAGGATGGC-3′ (antisense) for the β-actin gene (to generate a 271-bp fragment). The reaction conditions were set as follows: 94°C for 5 min, 30 cycles at 94°C for 50 s, 55°C for 30 s, and 72°C for 50 s; followed by extension at 72°C for 10 min. PCR product is analyzed in the following way: MDR1 and β-actin PCR product from the same sample were observed with 1.8% agarose gel electrophoresis. Fix the images with gel imaging system and measure the target gene expression level with semi-quantitative analysis. Relative content of mRNA = accumulative photon value of target gene band/accumulative photon value of internal standard β-actin band.
Real-time PCR was performed with SYBR Green Master Mix (Applied Biosystems, CA), using a 7500 Real-Time PCR System (Applied Biosystems). The quantitative measurement of each gene was normalized to the amount of GAPDH cDNA. Primers used in real-time PCR are as follows: 5′-AGCGAGCAACTGAGAAGC-3′ (sense) and 5′-CGCTGTGAAGCAGAGCTGG-3′ (antisense) for the c-fos gene (to generate an 83-bp fragment); 5′-AGTCAACGGATTTGGTCGTA-3′ (sense) and 5′-GGAACATGTAAACCATGTAG-3′ (antisense) for the GAPDH gene (to generate a 122-bp fragment). Primers of MDR1 used for real-time PCR were the same as that for RT-PCR. The GAPDH gene was used as an endogenous control to normalize the mRNA values in each sample. The relative values were determined by the comparative computed tomography analysis method.
Western Bolt Analysis
Cells were harvested and rinsed twice with PBS. Cell lysates were prepared in modified RIPA buffer (Beyotime, China) with protease inhibitor cocktail (Sigma, USA). Protein concentrations were determined using the BCA™ Protein Assay Kit (Pierce, Rockford, IL). Cell lysates containing 50 µg of total protein were resolved on 10% or 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to nitrocellulose membranes (Millipore, Billerica, MA), and blocked overnight at 4°C with 5% skim milk. The membrane was incubated with various primary antibodies at room temperature, followed by HRP-conjugated secondary antibodies. After rinsing the membrane three times, the immunoreactive bands were detected by using enhanced chemiluminescent (ECL) plus reagent kit (Beyotime, China) and exposed to film according to the manufacturer's protocol. The band density for the target protein in each sample was quantified by densitometry and normalized to GAPDH expression. The following antibodies were used for immunoblot analysis: c-fos (sc-52, 1:500); and GAPDH (6C5, 1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA); Bcl-2 (#2870, 1:1,000); Bax (#2772, 1:1,000); and p53 (#2527, 1:1,000; Cell Signaling Technology, Inc.); PUMA (#3041, 1:1,000; ProSci Incorporated, CA).
Plasmids and Stable Transfection
Expression vector for anti-c-fos shRNA Psilencer 3.1/c-fos was prepared by ligating the annealed synthetic oligonucleotides into the RNAi plasmid Psilencer 3.1 vector (Ambion) with BamHI and HindIII restriction sites downstream of the H1 promoter, and confirmed by DNA sequencing. MCF-7/ADR cells were transfected with Psilencer 3.1/c-fos using Lipofectamine 2000 as recommended by the manufacturer (Invitrogen). Stable clone cells were selected for about 4 weeks in medium containing 500 µg/ml G418, and then maintained in medium containing 200 µg/ml G418 and transferred to G418-free medium at least 1 week before the experiments.
The oligonucleotide sequence used for c-fos RNAi experiments was: 5′-gatccgTCCGAAGGGAAAGGAATAAttcaagagaTTATTCCTTTCCCTTCGGAtttttt-3′ (Invitrogen). The siRNA oligonucleotide sequence used for negative control was 5′-gatccgCTTACAATCAGACTGGCGAttcaagagaTCGCCAGTCTGATTGTAAGtttttt-3′ (Invitrogen). The human c-fos and negative control (NC) target sequences were shown in capital letters.
Radiation Treatment
Cells were seeded overnight in six-well plate; the medium was removed and replaced with fresh medium. Irradiation of cells in vitro was carried out using 133Cs gamma rays at a dose rate of 1.47 Gy/min. For radiation experiments, cells were either left untreated or treated with different doses of radiation (10 or 20 Gy), and then incubated for another 24 h for apoptosis detection using a flow cytometry.
Cell Death and Apoptosis Assessments by Annexin V-FITC and PI Staining
Phosphatidylserine (PS) translocation from the inner to the outer leaflet of the plasma membrane is one of the earliest apoptotic features. The PS exposure in cells was detected with an annexin V-FITC/PI apoptosis detection kit I (BD Biosciences Pharmingen, San Diego, CA). Briefly, the cells (5 × 105/well) were seeded into six-well plates and then treated with different types of anti-cancer drugs or radiation. After 24 h, cells were harvested and washed with ice-cold PBS. The cell pellets were resuspended in ice-cold binding buffer to 1 × 106 cells/ml. Five microliter of annexin V-FITC solution and 5 µl of dissolved PI were added to 100 µl of the cell suspensions. The samples were mixed gently and incubated at room temperature for 15 min in the dark. Then 400 µl of ice-cold binding buffer was added and mixed gently before the cell preparations were examined by flow cytometry (FACSCalibur, Becton–Dickinson, San Jose, CA). The data were analyzed with CellQuest software. Annexin V-positive, PI-negative cells were scored as apoptotic. Double-stained cells were considered either as necrotic or as late apoptotic [Wang et al., 2006].
Flow Cytometric Analysis of P-GP Expression
The P-gp protein expression was determined by labeling cells with 5 µl of a PE-conjugated mouse anti-human monoclonal antibody against P-gp (Becton–Dickinson) for 30 min at 4°C, washed twice in PBS and the fluorescent intensity on cells was analyzed using FACS Caliber (Becton–Dickinson). Appropriate isotypic controls were used. Results were expressed as the relative percentage of positive cells. The P-gp expression in MCF-7/ADR was used as control to normalize the protein content in each sample. Duplicate experiments with triplicate samples were performed.
Flow Cytometric Analysis of Intracellular Rhodamine 123 (RH-123) Accumulation
P-gp pump function was assessed by measuring intracellular accumulation of Rh-123 [Ji and He, 2007]. MCF-7, MCF-7/ADR, MCF-7/ADR/siNC, and MCF-7/ADR/sic-fos cells were incubated with or without 5 µM of Rh-123 in culture medium in dark at 37°C in 5% CO2 for 2 h. The cells were washed twice with ice-cold PBS. The median fluorescence intensities (MFI) associated with Rh-123 was determined by measuring the cell-associated fluorescence emission (480 nm) using flow cytometry (Becton–Dickinson).
Statistical Analysis
All of the results obtained represent the mean ± SD from triplicate experiments performed in a parallel manner unless otherwise indicated. All the data were processed with the software SPSS13.0. One-factor variance analysis (ANOVA) was used in the comparison of several groups, while Student's t-test was used to compare two groups. Probability values of P < 0.05 were considered to be statistically significant.
RESULTS
Determination of MDR of MCF-7/ADR
The MCF-7/ADR cell line was generated by in vitro incubation of MCF-7 cells with increasing concentrations of ADR. When MTT assay was performed, it was found that MCF-7/ADR cells were resistant not only to ADR but also to multiple anti-cancer drugs. Among them, we have tested VP-16, vincristine, paclitaxel, 5-Fu, and CDDP (Tables I and II). The IC50 of these drugs to MCF-7/ADR cells increased significantly when compared with non-resistant MCF-7 cells. MCF-7/ADR cells in these experiments were about 40-fold resistant to ADR in comparison with the parental drug-sensitive MCF-7 cells.
| Anti-cancer drugs | IC50 (µM) ± SDa | Resistant fold | |
|---|---|---|---|
| MCF-7 | MCF-7/ADR | ||
| ADR | 3.45 ± 0.05 | 137.80 ± 12.77 | 39.94 |
| Paclitaxel | 0.26 ± 0.07 | 27.11 ± 1.98 | 102.34 |
| VP-16 | 217.10 ± 31.96 | 2,640 ± 391.7 | 12.16 |
| Vincristine | 2.69 ± 0.64 | 207.6 ± 8.89 | 77.26 |
- The resistance of MCF-7/ADR cells to different chemotherapeutic agents was detected by using the MTT assay as described in Materials and Methods Section.
- a IC50 values (mean ± SD) were calculated from three independent experiments performed in triplicates.
| Cells | IC50a | ||
|---|---|---|---|
| Adriamycin (µM) | 5-Fluorouracil (µM) | Cisplatin (µM) | |
| MCF-7 | 3.45 ± 0.05 | 9.59 ± 1.03 | 2.31 ± 0.97 |
| MCF-7/ADR | 137.80 ± 12.77 | 149.54 ± 7.98 | 28.69 ± 4.86 |
| MCF-7/ADR/siNC | 142.60 ± 26.67 | 147.99 ± 1.86 | 24.60 ± 0.22 |
| MCF-7/ADR/sic-fos-8B | 34.34 ± 4.38* | 105.93 ± 3.31* | 17.73 ± 2.81* |
| MCF-7/ADR/sic-fos-3D | 15.46 ± 0.80* | 72.96 ± 0.55* | 14.85 ± 1.81* |
- Effects of c-fos interference on the sensitivity of MCF-7/ADR cells toward chemotherapeutic agents were examined by MTT method as described in Materials and Methods Section.
- a IC50 values (mean ± SD) were calculated from three independent experiments performed in triplicates.
- * P < 0.05 compared to the control MCF-7/ADR cells group.
Both Pump and Non-Pump Resistance Are Involved in MDR Mechanisms in MCF-7/ADR Cells
Overexpression of P-gp is thought to be one of the most important mechanisms of pump resistance [Sharom, 2011]. As shown in Figure 1A, both MDR1 gene transcripts and P-gp were pronounced in MDR MCF-7/ADR cells compared to the drug-sensitive parental MCF-7 cell line. P-gp overexpression in multidrug-resistant cell line was further confirmed by flow cytometry using the fluorescent P-gp-PE antibody, and higher fluorescence intensity was observed in the MCF-7/ADR than that in MCF-7 cells (Fig. 1B). There was a good correlation between P-gp up-regulation and the change in P-gp function, and fluorescent dye Rh-123 is a substrate for P-gp and widely used as an indicator for testing the activities of P-gp, so Rh-123 efflux assay was used to detect P-gp function. As expected, MFI in MCF-7 cells was obviously higher than that in MCF-7/ADR cells (Fig. 1C). Based on the above results, it was concluded that the overexpression of drug efflux protein P-gp mediated the pump resistance in MCF-7/ADR cells.
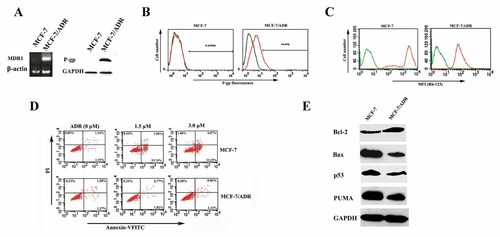
Pump and non-pump resistance in MCF-7/ADR cells. A: The RT-PCR and Western blot analysis were performed to determine the MDR1 and P-gp expression, respectively. B: P-gp expression in MCF-7 and MCF-7/ADR cells was determined by flow cytometry using PE-conjugated mouse anti-human monoclonal antibody against P-gp, and the non-specific fluorescent labeling was corrected by the isotype control as described in Materials and Methods Section. Green line: isotype control; orange line: PE-conjugated mouse anti-human monoclonal antibody against P-gp. C: The MFI associated with Rh-123 in MCF-7 and MCF-7/ADR cells was determined by flow cytometry. Cells were treated with medium alone (green line) or with 5 µM Rh-123 (orange line) for 1 h. D: MCF-7 and MCF-7/ADR cells were incubated with or without 1.5 and 3.0 µM ADR for 24 h, then the rate of apoptosis induced by ADR was measured by Annexin V-FITC and PI staining analysis, as described in Materials and Methods Section. ADR induced significant necrosis in MCF-7 but not in MCF-7/ADR cells. E: Western blot analysis was performed to determine the levels of Bcl-2, Bax, p53, and PUMA in MCF-7 and MCF-7/ADR cells. The protein levels of GAPDH were determined as control.
In addition, resistant cells often show characteristic cross-resistance to apoptosis (non-pump resistance) [Hsu et al., 2008]. As expected, treatment of MCF-7 cells with ADR resulted in a significant apoptosis and necrosis. When exposed to 1.5 and 3.0 µM of ADR, the mean percentage of apoptotic MCF-7 cells (Annexin V-FITC positive, PI negative) was 19.69 ± 0.88% and 35.84 ± 2.69%, respectively (Fig. 1D, top panel). However, ADR of 3.0 µM treatment resulted in only 2.54 ± 0.36% of apoptosis of MCF-7/ADR cells (Fig. 1D, bottom panel). Moreover, the results of Western blotting proved that MCF-7/ADR cells had increased Bcl-2 expression, decreased expression of Bax, p53, and PUMA (Fig. 1E). These results suggested that non-pump drug resistance in MCF-7/ADR cells was attributed primarily to the mechanisms responsible for the activation of anti-apoptotic cellular defense and apoptosis regulatory proteins were the key players in this defense.
Overexpression of c-fos in MCF-7/ADR Cells
The expression of c-fos in MCF-7/ADR and MCF-7 cells was detected by RT-PCR and Western blot analysis. As shown in Figure 2A, the c-fos mRNA and protein levels are higher in MCF-7/ADR than in MCF-7 cells. To test the synchronization of the up-regulation of c-fos and the acquirement of drug resistance, we observed the alteration of c-fos expression after ADR treatment in MCF-7 cells. Incubation of MCF-7 cells with 3 µM ADR led to a time-dependent increase in c-fos mRNA (eight-fold after treatment with 3 µM ADR for 36 h), as demonstrated by RT-PCR (Fig. 2B, left panel). Induction of c-fos by ADR was also observed at a protein level, and a significant six-fold increased c-fos expression after treatment with 3 µM ADR for 36 h was detected compared to untreated cells (Fig. 2B, right panel). These results indicated that overexpression of c-fos might be related to the drug-resistant phenotype of breast cancer cells.
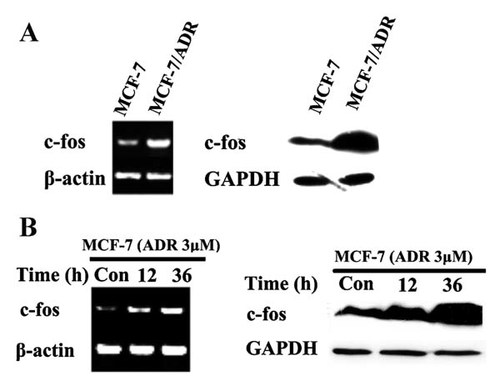
Overexpression of c-fos in multidrug resistant MCF-7/ADR cells and the induction by ADR in parental drug-sensitive MCF-7 cells. A: RT-PCR and Western blot were performed to determine the c-fos mRNA (left panel) and protein expression (right panel) in sensitive MCF-7 and drug resistant MCF-7/ADR cells. B: MCF-7 cells were treated with or without 3 µM ADR for the indicated times, then RT-PCR and Western blot were performed to determine the level of c-fos mRNA (left panel) and protein (right panel) in MCF-7 and MCF-7/ADR cells, respectively. β-actin mRNA and GAPDH protein expression were used as loading controls.
Down-Regulation of c-fos Expression by shRNA in MCF-7/ADR Cells
To further evaluate the roles of c-fos in the MDR of breast cancer cells, we performed the stable transfection of MCF-7/ADR cells with the Psilencer 3.1-sic-fos construct, generating MCF-7/ADR/sic-fos cells. Two clones, MCF-7/ADR/sic-fos-8B and MCF-7/ADR/sic-fos-3D, were selected for further expansion and characterization. Total RNA was isolated from MCF-7/ADR, MCF-7/ADR/sic-fos-8B, MCF-7/ADR/sic-fos-3D, and negative control MCF-7/ADR/siNC cells and examined for c-fos gene expression. The lower levels of c-fos mRNA were detected in MCF-7/ADR/sic-fos-8B and MCF-7/ADR/sic-fos-3D cells by conventional RT-PCR (Fig. 3A). Very low levels were detected in MCF-7/ADR/sic-fos-3D cells by quantitative real-time PCR, and the percentage of c-fos mRNA levels of MCF-7/ADR/sic-fos-8B and MCF-7/ADR/sic-fos-3D was 35.75% and 22.67%, respectively, compared to MCF-7/ADR cells (Fig. 3B). To confirm that the expression of sic-fos in MCF-7/ADR/sic-fos cells abolishes not only c-fos mRNA expression but also protein expression, we examined endogenous c-fos levels by Western blotting using the anti-c-fos pAb. The expression of c-fos was pronouced in MCF-7/ADR and MCF-7/ADR/siNC cells, but it was low in MCF-7/ADR/sic-fos-8B and MCF-7/ADR/sic-fos-3D cells (Fig. 3C). These data indicated that c-fos mRNA and protein expression were abolished in MCF-7/sic-fos cells.
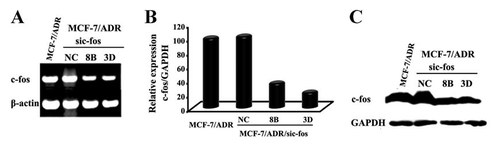
Transfection and interference of c-fos in MCF-7/ADR cells. MCF-7/ADR cells were transfected with the Psilencer 3.1-sic-fos construct. The vector containing random sequences was used as a negative control plasmid (Psilencer 3.1-siNC). Two representative stable clones, MCF-7/ADR-sic-fos-8B and -3D, were selected for further expansion and characterization. A: RT-PCR analysis of c-fos gene levels. Agarose gel electrophoresis of RT-PCR products revealed the specific transcripts for c-fos, and β-actin was used to normalize the amount of DNA template in each PCR reaction. B: The expression of the c-fos gene relative to that of the endogenous control gene in cells was examined by quantitative real-time PCR. The gene expression levels in MCF-7/ADR cells were set at 100%. The relative values were determined by the comparative computed tomography analysis method. C: Western blot analysis of c-fos protein levels. Cell extracts separated by SDS–PAGE and analyzed by immune-blotting with anti-c-fos antibody. Equivalent loading was verified with anti-GAPDH antibody.
Silencing of c-fos Strengthens the Potency of Chemotherapeutic Agents
We performed the MTT assay in MCF-7/ADR/sic-fos clones to confirm the effect of c-fos down-regulation on the sensitivity of resistant breast cell line against chemotherapeutic agents. P-gp substrate ADR, non-P-gp substrates 5-Fu and CDDP were chosen for the sensitivity tests. As shown in Table II, down-regulation of c-fos expression by shRNA significantly increased drug sensitivity of MCF-7/ADR cells (P < 0.05). Compared to MCF-7/ADR cells, the resistance of MCF-7/ADR/sic-fos-8B to ADR, 5-Fu and CDDP was reduced by 75.1%, 29.2% and 38.2%, respectively; while MCF-7/ADR/sic-fos-3D by 88.8%, 51.2%, and 48.2%, respectively. Thus, the down-regulation of c-fos expression increased the sensitivity of MCF-7/ADR cells against both P-gp and non-P-gp substrates, suggesting that both pump- and non-pump-mediated mechanisms might be involved in the effects of c-fos on MDR.
c-fos Down-Regulation Affects MDR by Regulation of P-gp Activity and Expression
Rh-123 is a substrate for P-gp and widely used as an indicator for testing the activities of P-gp. The stronger intensity of fluorescence associated with Rh-123 could be detected in MCF-7/ADR/sic-fos-8B and MCF-7/ADR/sic-fos-3D cells, while MCF-7/ADR/siNC and MCF-7/ADR cells showed approximately equal fluorescence intensity (Fig. 4A), suggesting that anti-c-fos shRNA transfection increased the intracellular accumulation of P-gp substrate Rh-123 in MCF-7/ADR cells. Specifically, MCF-7/ADR/sic-fos-8B and MCF-7/ADR/sic-fos-3D cells showed 1.68- and 2.49-fold of increase in the MFI of Rh-123, respectively, compared to MCF-7/ADR cells (Fig. 4B). Next, we tested whether c-fos down-regulation influenced, besides P-gp function, the MDR1 mRNA and P-gp expression. MCF-7/ADR/siNC cells displayed same amount of MDR1 levels as MCF-7/ADR cells, while MCF-7/ADR/sic-fos-8B and MCF-7/ADR/sic-fos-3D cells displayed lower MDR1 levels (Fig. 4C), which was further confirmed by relative quantification of the MDR1 gene (Fig. 4D). The expression levels of P-gp were analyzed by flow cytometry. Consistent with MDR1 expression, P-gp was down-regulated in MCF-7/ADR/sic-fos cells (Fig. 4E). Quantification results in Figure 4F showed that the expression levels of P-gp in MCF-7/ADR/sic-fos-8B and MCF-7/ADR/sic-fos-3D cells were decreased by 34.86 ± 2.41% and 38.34 ± 2.22%, respectively, in comparison with MCF-7/ADR cells. Based on the results obtained above, we conclude that down-regulation of c-fos expression sensitizes MCF-7/ADR cells to P-gp substrates mainly by inhibiting MDR1/P-gp activity and expression.
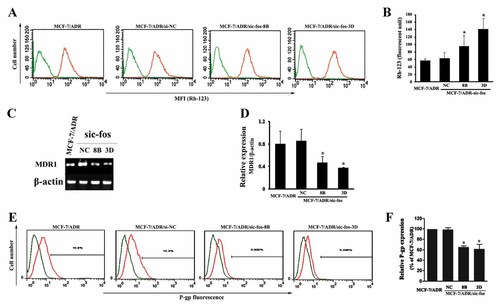
The effects of c-fos down-regulation on the efflux activity and expression of P-gp in MCF-7/ADR cells. A: The MFI associated with Rh-123 in MCF-7, MCF-7/ADR, MCF-7/ADR-siNC, MCF-7/ADR-sic-fos-8B, and -3D cells was determined by flow cytometry. Cells were treated with medium alone (green lines) or 5 µM Rh-123 (orange lines) for 1 h. B: A graph representing the average values of the MFI of Rh123 obtained from three independent experiments. Each bar represents the mean ± SD from three independent experiments. *P < 0.05 versus MCF-7/ADR cells. C: Total RNA from MCF-7/ADR, MCF-7/ADR-siNC, MCF-7/ADR-sic-fos-8B, and -3D cells was extracted and used to examine MDR1 and β-actin gene expression by RT-PCR analysis. D: Bar diagram showing densitometry quantified data of MDR1/β-actin mRNA ratios. Each bar represents the mean ± SD from three independent experiments. *P < 0.05 versus MCF-7/ADR cells group. E: P-gp expression in MCF-7/ADR, MCF-7/ADR-siNC, MCF-7/ADR-sic-fos-8B, and -3D cells. P-gp was measured by flow cytometry using PE-conjugated mouse anti-human monoclonal antibody and the non-specific fluorescent labeling was corrected by the isotype control as described in Materials and Methods Section. Green line: isotype control; orange line: PE-conjugated mouse anti-human monoclonal antibody against P-gp. F: A graph representing the analysis of P-gp fluorescence. Each bar represents the mean ± SD values from three independent experiments. *P < 0.05 versus MCF-7/ADR cells group.
c-fos Down-Regulation Affects MDR by Enhancing Apoptosis Induced by Chemotherapeutic Agents and Radiation
Apoptosis resistance is another common mechanism that might contribute to MDR of tumor cells [Lo et al., 2008]. We attempted to determine whether c-fos down-regulation affects the sensitivity of MCF-7/ADR cells to apoptosis. Since P-gp has been reported to counteract apoptosis, non-P-gp substrate 5-Fu and CDDP were chosen as apoptosis-inducing agent. As shown in Figure 5A, MCF-7/ADR cells also showed the resistance to apoptosis induced by 5-Fu, and the treatment of 160 µM 5-Fu induced only 5.98 ± 2.88% of early apoptotic cells. However, the mean apoptotic amount for MCF-7/ADR/sic-fos-8B and MCF-7/ADR/sic-fos-3D induced by the same concentration of 5-Fu (160 µM) was 26.40 ± 1.92% and 32.31 ± 1.57%. The difference is significant when compared with that from MCF-7/ADR cells (P < 0.05 or 0.01). Similar findings were observed for CDDP-induced apoptosis (Fig. 5B). In all CDDP doses tested, MCF-7/ADR and MCF-7/ADR/siNC cells contained an approximately equal percentage of apoptotic cells, whereas MCF-7/ADR/sic-fos cells exhibited higher percentage of apoptotic cells. Specifically, the mean percentage of apoptotic cells in MCF-7/ADR/sic-fos-8B cells was 9.03 ± 0.88%, 16.47 ± 1.71% and 39.58 ± 2.30%, respectively, when exposed to 10, 20, and 30 µM of CDDP, and only high-concentration CDDP treatment induced a significant increase in apoptosis compared to MCF-7/ADR cells (P < 0.05). Whereas CDDP in all three treated concentrations induced significantly increased apoptosis in MCF-7/ADR/sic-fos-3D cells compared to MCF-7/ADR cells (P < 0.01), and the mean apoptotic percentage was 27.00 ± 1.69%, 37.93 ± 0.47%, and 57.60 ± 11.88%, respectively. In summary, these results indicated that c-fos down-regualtion raised the 5-Fu and CDDP-induced apoptosis percentage of MCF-7/ADR cells.
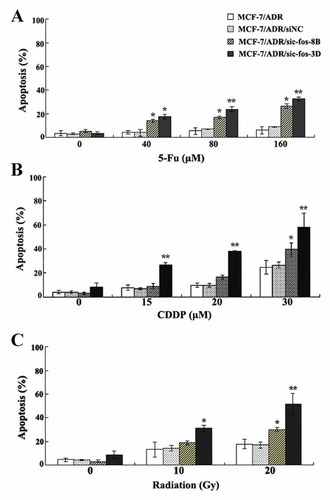
The effects of c-fos down-regulation on apoptosis induced by chemotherapeutic agents and radiation in MCF-7/ADR cells. Cells were incubated with different doses of 5-Fu (A), or CDDP (B), or radiation (C) for 24 h, and the rate of apoptosis was measured by flow cytometry as described in Materials and Methods Section. c-fos down-regulation enhanced 5-Fu-, CDDP-, and radiation-induced apoptosis. Data were presented as the mean ± SD of three independent experiments (*P < 0.05, **P < 0.01 compared with MCF-7/ADR cells).
In addition, the acquisition of resistance to radiation remains the major obstacle in the successful treatment of cancer patients, so we examined the effect of c-fos down-regulation on the radiation-induced apoptosis. Exposure of MCF-7/ADR cells to radiation (10 and 20 Gy) resulted in induction of apoptosis, whereas increased apoptosis was observed in MCF-7/ADR/sic-fos cells (Fig. 5C). Specifically, 10 and 20 Gy radiation resulted in the apoptotic percentages of 18.82 ± 1.58% and 29.97 ± 1.60%, respectively, in MCF-7/ADR/sic-fos-8B cells, and only high-dose radiation (20 Gy) induced a significant increase in apoptosis compared to MCF-7/ADR cells (P < 0.05). Whereas both 10 and 20 Gy radiation induced a significant increase in apoptosis in MCF-7/ADR/sic-fos-3D cells compared to MCF-7/ADR cells (P < 0.05 or 0.01), and the mean apoptotic percentage was 30.80 ± 2.74% and 51.40 ± 9.05%, respectively. These results suggested that the increased apoptosis of MCF-7/ADR/sic-fos cells induced by radiation in was achieved by the inhibition of c-fos.
Subsequently, several important proteins regulating apoptosis, Bcl-2, Bax, p53, and PUMA, were investigated for possible involvement. As observed in Figure 6A, up-regulation of Bax and down-regulation of Bcl-2 were observed in MCF-7/ADR/sic-fos-8B and MCF-7/ADR/sic-fos-3D cells, suggesting that down-regulation of c-fos expression may increase the ratio of Bax/Bcl-2. Meanwhile, p53 and PUMA accumulation was detected in both cell lines upon c-fos down-regulation. The densitometric analysis showed 1.2- and 2.2-fold decrease of Bcl-2, 1.6- and 1.7-fold increase of Bax, 2.0- and 5.1-fold increase of p53, 3.6- and 4.8-fold increase of PUMA in MCF-7/ADR/sic-fos-8B and MCF-7/ADR/sic-fos-3D cells, respectively, when compared to MCF-7/ADR cells (Fig. 6B). Significant changes in Bcl-2, Bax, p53 and PUMA expression were observed in MCF-7/ADR/sic-fos-3D cells compared to MCF-7/ADR cells (P < 0.05). However, only the change of Bax and PUMA expression was significant in MCF-7/ADR/sic-fos-8B cells (P < 0.05), which might explain why the percentage values of apoptosis induced by CDDP and radiation were insignificant in MCF-7/ADR/sic-fos-8B compared to MCF-7/ADR cells (P > 0.05; Fig. 5B,C). All of the above results suggesting that down-regulation of c-fos expression may promote the multiple anticancer drugs-induced apoptosis via influencing the expression of apoptosis-associated proteins.
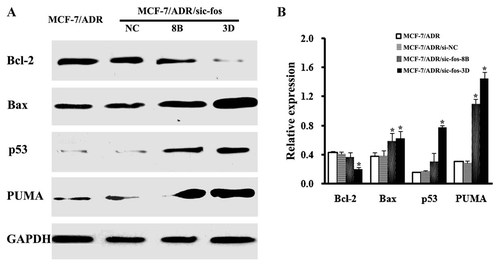
The effects of c-fos down-regulation on the expression of apoptosis-associated proteins in MCF-7/ADR cells. A: Western blot analysis of protein expression of Bcl-2, Bax, p53, and PUMA. Lysates from cells were separated by SDS–PAGE and analyzed by Western blotting using the antibody against Bcl-2, Bax, p53, and PUMA. Sequential incubation of the sheet with anti-GAPDH antibody confirmed equal protein loading. B: Band intensities of Bcl-2, Bax, p53, and PUMA protein bands were quantified by densitometry to assess the relative abundance of the target protein against GAPDH determined in control. Data presented are the mean ± SD values from three independent experiments. *P < 0.05 versus MCF-7/ADR cells group.
DISCUSSION
ADR is one of the most effective cytotoxic agents for treating breast cancer. However, the resistance of tumor cells to ADR remains a major cause of treatment failure in patients with breast cancer. Development of resistance to ADR is accompanied by myriad genetic alterations that affect membrane transporters, tumor suppressors, and regulators of cell cycle and cell death [Kudoh et al., 2000; Watts et al., 2001]. Hence, identification of novel signaling molecules will facilitate the development of more effective strategies to overcome ADR resistance in breast cancer.
In the presented study, the proto-oncogene c-fos was confirmed to be up-regulated in MCF-7/ADR (Fig. 2A), an ADR-selected human breast cell line with the MDR phenotype mediated by P-gp overexpression and apoptosis resistance (Fig. 1, Table I). Further results proved that ADR treatment induces the up-regulation of c-fos (Fig. 2B). Previous studies have defined MDR is encountered when cancer cells are exposed to chemotherapeutic agents, so the induction of c-fos by ADR led us to hypothesize that c-fos is involved in the acquirement and development of MDR. To test this hypothese, we then performed shRNA-mediated c-fos knock-down in MCF-7/ADR cells (Fig. 3). The increased sensitivity of MCF-7/ADR cells to chemotherapeutic drugs after silencing c-fos further proved that c-fos could be an important mediator of ADR-mediated resistance (Table II). Since c-fos down-regulation affects the sensitivity against both P-gp and non-P-gp substrates (Table II), we mainly focus the mechanisms of c-fos involved in MDR on “pump” and “non-pump” pathways.
The main mechanism of pump resistance is that efflux membrane proteins can pump out the anti-cancer agents through the cell membrane and reduce the intracellular drug accumulation. The MDR1 gene product P-gp is the best characterized drug efflux pump, and it acts as a representative efflux pump for a number of commonly used cytotoxic agents, for example, ADR, vincristine, vinblastine, paclitaxel, colchicine, actinomycin D, and mitomycin C [Dean et al., 2001]. P-gp plays a central role in the anticancer drug efflux to augment cell survival against the drug, and its up-regulation is one of the most important mechanisms by which cancer cells resist the cytotoxic effects of anticancer agents [Ueda et al., 1987; Giai et al., 1991]. One of the strategies to overcome P-gp-mediated MDR is to develop agents that block P-gp pump function, leading to accumulation of cytotoxic drugs in tumor cells. In this study, increased intracellular concentration of Rh-123 in MCF-7/ADR/si-c-fos cells proved that c-fos might modulate P-gp transport function (Fig. 4A,B). Consistent with decreased P-gp pumping activity, RT-PCR and Western blot analyses revealed that c-fos down-regulation effectively diminished the expression of MDR1/P-gp (Fig. 4C–F). All these data indicate that silencing of c-fos could inhibit the expression of P-gp which resulted in increased intracellular accumulation of P-gp substrate.
The main mechanism of non-pump resistance is the activation of cellular anti-apoptotic defense. The resistance to apoptosis is one of major cause of failure in the treatment of malignancies, which can be acquired by cancer cells through a variety of strategies. The proto-oncogene c-fos has been proved to be implicated in the regulation of apoptosis [Takeuchi et al., 2006]. Elevated c-fos levels may activate anti-apoptotic pathways and stimulate the repair of ADR induced DNA lesions that counteract pro-apoptotic processes initiated by anti-cancer agents [Teng, 2000]. Cells lacking the c-fos gene are more sensitive than the corresponding wild-type cells as to the induction of chromosomal aberrations and apoptosis [McGill and Fisher, 1997]. In the present study, the hypothesis of c-fos involvement in ADR-induced apoptosis was supported by the fact that c-fos down-regulation enhanced apoptosis induced by both chemotherapeutic agents and radiation (Fig. 5). Cell apoptosis is a defined program of cell death that is markedly influenced both positively and negatively by a variety of genes. Among the most important of these are members of the bcl-2 gene family and the tumor suppressor gene p53 [Reed et al., 1996]. The Bcl-2 family of proteins plays a central regulatory role via its interacting pro- and antiapoptotic members, which integrate a wide array of diverse upstream survival and distress signals to decide the fate of the cells [Adams and Cory, 2001; Cory and Adams, 2002]. The Bax and Bcl-2 are the key players of this family. Cells apoptosis may be turned on and off by the pro-apoptotic proteins and anti-apoptotic proteins of the Bcl-2 family because the formation of heterodimers among these proteins [Danial and Korsmeyer, 2004]. Therefore, the balance between the expression levels of the protein units is critical for cell survival or death. The result in this study showed inhibition of c-fos in MCF-7/ADR cells up-regulated Bax and concomitantly down-regulated Bcl-2 levels (Fig. 6), and then cell apoptosis induced by chemotherapeutic agents was enhanced. Down-regulation of c-fos expression in MCF-7/ADR cells also resulted in the up-regulation of p53 and PUMA expression (Fig. 6). The p53 protein exhibits a number of activities that contribute to the induction of apoptosis, and disruption of this process can promote tumor progression and chemoresistance [Fridman and Lowe, 2003]. More recent studies have indicated a surprisingly strong dependence of p53- mediated apoptosis on the presence of PUMA, a key mediator of apoptosis induced by a wide variety of stimuli [Vousden, 2005]. After activation, PUMA interacts with antiapoptotic Bcl-2 family members, thus freeing Bax and/or Bak which are then able to signal apoptosis to the mitochondria [Mustata et al., 2011]. All these data suggest that c-fos is a pleiotropic gene and could regulate apoptosis primarily through a p53-dependent pathway involving its target proteins of the Bcl-2 family (i.e., Bax and Bcl-2).
Our study is the first report describing a shRNA-mediated c-fos silencing strategy to enhance drug cytotoxicity in breast cancer. Molecular targeted therapy has been proposed as a strategy to enhance drug activity and reducing drug doses in advanced or/and resistant cancers [Türk and Szakács, 2009; Johnson and Brown, 2010]. Although development of resistance to ADR is accompanied by myriad genetic alterations that affect membrane transporters, tumor suppressors, and regulators of cell cycle and cell death, several lines of evidence lead us to choose c-fos as molecular target for multidrug resistant breast cancer: First, c-fos plays an important role in development and acquirement of MDR phenotype in breast cancer cells, and is associated with both P-gp overexpression and apoptosis resistance which are two most important mechanisms to cause MDR; Second, distinct from other genes involved in drug resistance, c-fos as a transcription factor located at a more distal point in the cascade, where many mitogenic signals converge, so inhibition of c-fos could cause blockade of multiple signal transduction pathways [Liu et al., 2002]. Hence, c-fos could be good molecular target for new therapeutic agents. Thus, suppressing c-fos gene expression by antisense gene transfection and RNA interference may therefore be an effective means to temper a cancer cell's resistance to cytotoxic chemotherapy.
In conclusion, our study demonstrates that c-fos is a critical regulator of apoptosis and P-gp expression in resistant breast cancer MCF-7/ADR cells. These results will enhance our current understanding of how c-fos functions in breast cancer cells, and provide a firm rationale for developing selective c-fos inhibitors or gene therapy targeted on c-fos for the treatment and prevention of resistant breast cancer. We here suggest a model for the presence of the c-fos as an anti-apoptotic and P-gp-associated factor that could be identified as a promising molecular target of resistant breast cancer, as shown in Figure 7. Further studies to characterize the signal transduction pathways that are involved in c-fos-mediated drug resistance may provide an approach to prevent anthracycline resistance.
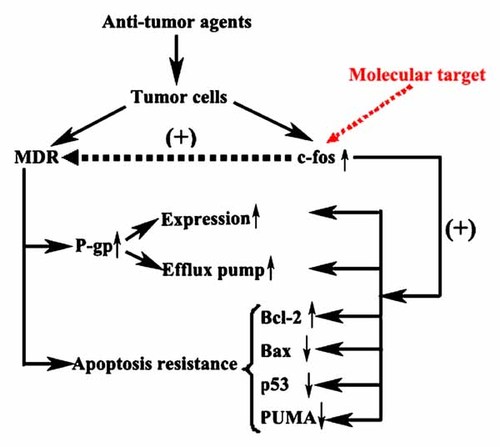
A model for the role of c-fos in MDR.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Nos. 30873091, 30971291). In addition, the authors thank Dr. Kenneth H. Cowan (National Cancer Institute, NIH. Bethesda, MD) for the generous gifts of the human breast cancer cell line MCF-7 and the adriamycin-selected MDR cell line MCF-7/ADR.




