Zap70 inhibits Syk-mediated osteoclast function†
Conflicts of interest: Nothing to declare.
Abstract
The αvβ3 integrin stimulates the resorptive capacity of the differentiated osteoclast (OC) by organizing its cytoskeleton via the tyrosine kinase, Syk. Thus, Syk-deficient OCs fails to spread or form actin rings, in vitro and in vivo. The Syk family of tyrosine kinases consists of Syk itself and Zap70 which are expressed by different cell types. Because of their structural similarity, and its compensatory properties in other cells, we asked if Zap70 can substitute for absence of Syk in OCs. While expression of Syk, as expected, normalizes the cytoskeletal abnormalities of Syk−/− OCs, Zap70 fails do so. In keeping with this observation, Syk, but not Zap70, rescues αvβ3 integrin-induced SLP76 phosphorylation in Syk−/− OCs. Furthermore the kinase sequence of Syk partially rescues the Syk−/− phenotype but full normalization also requires its SH2 domains. Surprisingly, expression of Zap70 inhibits WT OC spreading, actin ring formation and bone resorptive activity, but not differentiation. In keeping with arrested cytoskeletal organization, Zap70 blocks integrin-activated endogenous Syk and Vav3, SLP76 phosphorylation. Such inhibition requires Zap70 kinase activity, as it is abolished by mutation of the Zap70 kinase domain. Thus, while the kinase domain of Syk is uniquely required for OC function that of Zap70 inhibits it. J. Cell. Biochem. 114: 1871–1878, 2013. © 2013 Wiley Periodicals, Inc.
Integrins are transmembrane α/β heterodimers that mediate cell-cell and cell-matrix interactions and generate intracellular signals when occupied by ligand [Hynes, 2002]. The integrin, αvβ3, is expressed by macrophages as they commit to the osteoclast phenotype and is a marker of the cell's differentiation. Binding of this heterodimeric matrix receptor to bone is also pivotal to the resorptive process [Teitelbaum, 2007]. The integrin assumes its high affinity conformation under the aegis of macrophage colony-stimulating factor (M-CSF) and when in contact with bone, activates a canonical signaling pathway required for osteoclast cytoskeleton polarization and efficient resorption. This cytoskeleton-organizing signaling sequence involves c-Src and Syk phosphorylation and recruitment of the co-stimulatory ITAM protein, Dap12 [Zou et al., 2009]. The relatively osteoclast-specific guanine nucleotide exchange factor, Vav3, is phosphorylated by Syk leading to transit of cytoskeleton-organizing Rac to its active, GTP-associated state [Faccio et al., 2005].
The Syk family of nonreceptor tyrosine kinase includes two members, Syk and Zap70 [van Oers and Weiss, 1995]. Both contain a carboxy-terminal kinase motif and two tandemly arranged Src homology 2 (SH2) domains with comparable avidities for the double phosphorylated T-cell receptor (TCR)-encoded ITAM. Zap70, however, is confined to T- and natural killer (NK) cells. Syk is also present in T-cells although its temporal activity differs somewhat than that of Zap-70 [Palacios and Weiss, 2007]. On the other hand, Syk is more promiscuous than Zap-70, appearing in most myeloid cells, B-lymphocytes, mast cells, platelets and osteoclasts. In keeping with their homology, Zap70 and Syk are functionally interchangeable in B and T-cells. For example, expression of Syk in Zap70−/− lymphocytes restores TCR-mediated signals activated by the deleted kinase [Gong et al., 1997]. Alternatively, Syk-deficient B cells have a nonfunctional B cell receptor that can be rescued by Zap70 [Takata et al., 1994; Kong et al., 1995].
These studies support the notion of overlapping functions of Syk and Zap70 in receptors utilizing ITAM. Given their similarities and their interchangeability in other cells we reasoned Zap70 may compensate for Syk when absent in osteoclasts. On the other hand, differences exist in the properties of the two kinases. For example, Syk's enzymatic activity is greater than that of Zap70 [Latour et al., 1996]. Unlike Zap70, Syk may be activated in an src kinase-independent fashion and its capacity to promote TCR-mediated calcium flux is greater [Noraz et al., 2000]. Thus, while Syk and Zap70 have functional similarities, their properties are also distinct raising the question as to whether Zap70 can restore normal function in osteoclasts lacking Syk.
MATERIALS AND METHODS
Mice
Syk+/− mice were described previously [Turner et al., 1995]. Because of perinatal lethality of Syk−/− mice, we generated bone marrow chimeras by transplanting Syk−/− fetal liver cells into lethally irradiated WT recipients [Zou et al., 2007]. Chimeras were used as a source of BMMs 4–8 weeks after bone marrow transplantation.
All mice used in these experiments were 6–8 weeks old and housed in the animal care unit of Washington University School of Medicine, where they were maintained according to guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care. All animal experimentation was approved by the Animal Studies Committee of Washington University School of Medicine.
Reagents
Recombinant murine M-CSF was obtained from R&D Systems (Minneapolis, MN). Glutathione S-transferase-RANKL was expressed in our laboratory as described [Lam et al., 2000]. The source of antibodies is as follows: mouse anti-Syk monoclonal antibody from Abcam (Cambridge, MA); rabbit anti-Syk (N-19) from Santa Cruz Biotechology (Santa Cruz, CA); anti-phosphotyrosine mAb 4G10 and rabbit anti-Vav3 from Millipore (Charlottesville VA); mAb 327, directed against the c-Src protein were gifts of Dr. A. Shaw (Department of Pathology, Washington University School of Medicine, St. Louis, MO); anti Src p-Y416 antibody and rabbit anti-SLP76 antibody from Cell Signaling (Beverly MA); Anti-Zap70 antibody from eBioscience (San Diego, CA). Anti-HA antibody was got from Invitrogen and anti-Flag and Anti-Myc antibodies were from Sigma (St. Louis, MO). All other chemicals were obtained from Sigma.
Macrophage Isolation and Osteoclast Culture
Primary BMMs were prepared as described previously [Faccio et al., 2003] with slight modification. Marrow was extracted from femora and tibiae of 6- to 8-week-old mice with α-MEM and cultured in α-MEM containing 10% inactivated fetal bovine serum, 100 IU/ml penicillin, and 100 µg/ml streptomycin (α-10 medium) with 1:10 CMG condition media [Takeshita et al., 2000] in Petri plastic dishes. Cells were incubated at 37°C in 6% CO2 for 3 days and then washed with PBS and lifted with 1× trypsin/EDTA (Invitrogen, Carlsbad, CA) in PBS. A total of 5 × 103 cells were cultured in 200 µl α-MEM containing 10% heat inactivated FBS with GST-RANKL and 30 ng/ml of mouse recombinant M-CSF in 96-well tissue culture plates, some containing sterile bone slices. Cells were fixed and stained for TRAP activity after 5 days in culture, using a commercial kit (Sigma 387-A, St. Louis, MO). For pre-osteoclast generation, 1.5 × 106 BMMs were plated per 10 cm tissues culture dish and cultured in 30 ng/ml M-CSF and 100 ng/ml GST-RANKL for 3 days.
Actin Ring Staining and Bone Resorption Assay
Osteoclasts were generated on bone slices from bone marrow-derived macrophages by exposure to 100 ng/ml RANKL and 30 ng/ml MCSF. For actin ring staining, the cells were fixed in 4% paraformaldehyde and permeablized in 0.1% Triton X-100, rinsed in PBS, and immunostained with AlexaFluor 488-phalloidin (Invitrogen). For bone resorption assay, osteoclasts were removed and resorption pits were visualized by incubation of the specimen with 20 µg/ml peroxidase-conjugated wheat germ agglutinin and stained with 3,3′-diaminobenzidine (Sigma).
Plasmids and Retroviral Transduction
Wild type Syk and Zap70, were sub-cloned into the BamH1 and Xho1 sites of a pMX retroviral vector in which the puromycin resistance sequence was replaced with one coding for blastocidin resistance. Kinase inactive Zap70 (K369A) mutant [Brdicka et al., 2005] was generated using the QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). We used standard molecular biological methods to construct Syk-Zap70 and Zap70-Syk chimeras. Syk and Zap70 were transiently transfected into Plat-E packaging cells using calcium phosphate precipitation. Virus was collected 48 h after transfection. BMMs were infected with virus for 24 h in the presence of 100 ng/ml M-CSF and 4 µg/ml polybrene (Sigma). Cells were selected in the presence of M-CSF and 1 µg/ml blasticidin (Calbiochem) for 3 days prior to use as osteoclast precursors.
Western Blotting and Immunoprecipitation
Cultured cells were washed twice with ice-cold PBS and lysed in RIPA buffer containing 20 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 mM NaF, and 1× protease inhibitor mixture (Roche). After incubation on ice for 10 min, cell lysates were clarified by centrifugation at 15,000 rpm for 10 min. Forty micrograms of total lysates were subjected to 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto PVDF membranes. Filters were blocked in 0.1% casein in PBS for 1 h and incubated with primary antibodies at 4°C overnight followed by probing with fluorescence-labeled secondary antibodies (Jackson Lab). Proteins were detected with the Odyssey Infrared Imaging System (LI-COR Biosciences).
Dynamic Imaging of Osteoclasts on Bone
WT BMMs transduced with GFP-Actin and WT Syk, WT Zap70 or empty vector were maintained using standard culture conditions (37°C and 5% CO2, 95% air atmosphere) with RANKL and M-CSF for 5 days on Bioptechs (non-liquid perfused) Delta T culture system, consisting of a heated, indium-tin-oxide-coated glass dish attached to a calibrated Bioptechs micro-perfusion peristaltic pump. All cultures were observed with the 20× objective (NA, 0.4) of an inverted automated wide-field epifluorescence DIC microscope (Leica DMIRE2, Leica Microsystems, Wetzlar, Germany). An objective lens heater was used to improve temperature homogeneity. Images (608 × 512 pixels spatial and 12-bit intensity resolution) were recorded with a cooled Retiga 1300 camera (Qimaging, Burnaby, BC, Canada) every 2 min in 2 × 2 binned acquisition mode, using 100–300 ms exposures. Dynamic images were composed using ImageJ.
RESULTS
Zap70 Cannot Substitute for Syk in Osteoclasts
Syk−/− bone marrow macrophages (BMMs) differentiate normally into osteoclasts, but the mutant polykaryons are dysfunctional as they fail to organize their cytoskeleton and optimally resorb bone [Zou et al., 2007]. Neither WT BMMs nor osteoclasts express Zap70 (Fig. 1A). To determine if Zap70 can substitute for Syk in the bone resorptive cell, we retrovirally transduced WT Syk or Zap70 into Syk−/− BMMs which were differentiated into osteoclasts by exposing them to M-CSF and RANKL for 5 days. Mirroring our previous data [Zou et al., 2007], WT Syk rescues the cytoskeletal abnormalities of plastic-residing Syk−/− TRAP-expressing polykaryons as the transduced cells form sheets of characteristic osteoclasts with podosome belts (Fig. 1B, upper panels); Syk deficient cells bearing Zap70, however, do not spread nor do they organize their podosomes (Fig. 1B, lower panels).
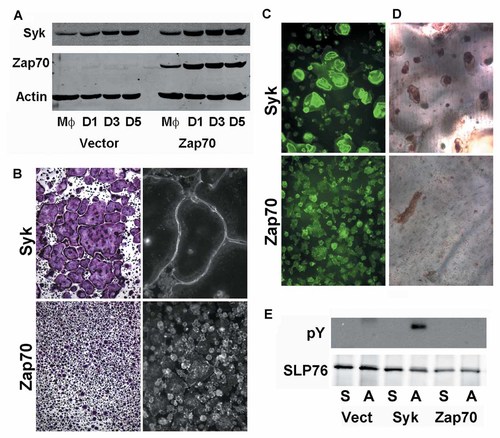
Zap70 cannot rescue Syk−/− OCs function. A: WT macrophages, transduced with WT Zap70 or empty vector, were cultured with RANKL+ MCSF with time. Syk and Zap70 expression was determined by immunoblot. B: Syk−/− BMMs, transduced with Syk or Zap70, were cultured with RANKL and MCSF on plastic for 5 days. Cells were stained with TRAP activity (left panel) or FITC-phalloidin (right panel). C: Syk−/− BMMs, transduced with Syk or Zap70, were cultured with RANKL + MCSF, on bone, for 6 days. Cells were stained with FITC-phalloidin. D: Bone slices, described in C, were stained with peroxidase-labeled wheat germ agglutinin to visualize resorption lacunae after removal of osteoclasts. E: Syk−/− preosteoclasts, transduced with empty vector, Syk or Zap70, were maintained in suspension (S) or plated on vitronectin (A) for 30 min. SLP76 immunoprecipitates were immunoblotted for phosphorylated tyrosine (pY) and SLP76.
Physiologically relevant cytoskeletal organization of osteoclasts requires their residence on mineralized substrate. Thus, we visualized the actin cytoskeleton of Syk−/− osteoclasts expressing Syk or Zap70, generated on bovine bone, by FITC-phalloidin staining. Reflecting cytoskeletal derangement, bone-residing Syk−/− osteoclasts bearing Zap70, are incapable of forming actin rings (Fig. 1C) nor can they degrade bone as shown by absence of resorptive lacunae (Fig. 1D). In keeping with its failure to rescue function of Syk−/− osteoclasts, Zap70 is also incapable of restoring integrin-activated phosphorylation of the cytoskeleton-organizing adaptor protein, SLP76 [Reeve et al., 2009], (Fig. 1E). Thus, Zap70 cannot compensate for absence of Syk in osteoclasts.
Syk, but Not Zap70, Kinase Domain Is Essential for OC Function
Syk and Zap70 contain homologs SH2 and kinase domains and we next asked which are functional in the context of the osteoclast. To this end we generated chimeric constructs consisting of combinations of Syk and Zap70 SH2 and kinase domains (Fig. 2A) and transduced them into Syk−/− BMMs which were differentiated into osteoclasts. The chimeric structure Syk-Zap70, consisting of the Syk SH2 regions and Zap70 kinase motif, fails to impact the abnormal appearance of Syk−/− osteoclasts or their inability to form actin rings and resorb bone (Fig. 2B–D). In contrast, hybridization of the Syk kinase and Zap70 SH2 domains partially rescues spreading, actin ring formation and bone resorptive capacity of the mutant cells (Fig. 2B–D). Thus, while the Syk, but not Zap70, kinase domain regulates the osteoclast cytoskeleton, optimal organization also requires the Syk SH2 motifs.
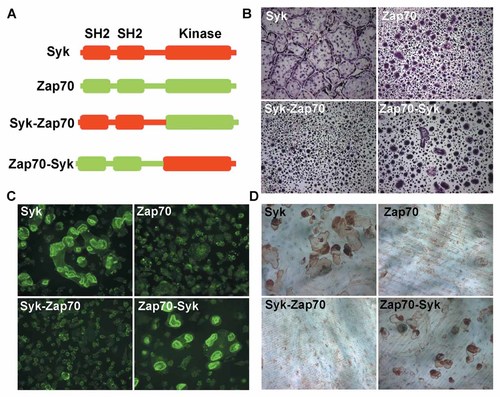
Syk kinase activity is essential for OC function. A: Scheme of Syk and Zap70 chimeric constructs. B: Syk−/− BMMs, transduced with Syk, Zap70 or chimeras, were cultured with RANKL and MCSF on plastic 5 days. Cells were stained with TRAP activity. C,D: Syk−/− BMMs, transduced with Syk, Zap70 or chimeras, were cultured with RANKL and MCSF on bone for 6 days. The cells were stained with FITC-phalloidin to visualize actin rings (C) or removed and the bone slices stained with peroxidase-labeled wheat germ agglutinin to delineate resorption lacunae (D).
Zap70 Exerts a Dominant-Negative Effect on the Osteoclast Cytoskeleton
Having determined that Zap70 fails to rescue Syk−/− osteoclasts, we explored the effects of overexpression of the two kinases in WT cells. While excess Syk has no apparent impact on WT osteoclasts, Zap70 restricts spreading and virtually eliminates actin ring formation and bone resorption, features characteristic of cytoskeletal disorganization (Fig. 3A). In keeping with this conclusion, living Zap70-, but not Syk- overexpressing osteoclasts fail to generate podosome belts (see Supplementary Movie 1). Confirming these effects do not represent arrested differentiation, β3 integrin and c-Src expression is similar in all transductants (Fig. 3B). Finally, we asked if the dominant-negative effects of Zap70 are kinase domain specific. To this end, we transduced WT cells with kinase-inactive Zap70K369A. Absence of Zap70 kinase activity in face of intact SH2 domains fails to impact WT osteoclast morphology confirming the molecule's disruptive effects on osteoclasts is mediated by its kinase function (Fig. 3C).
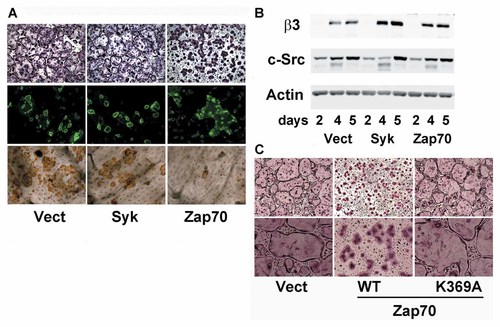
Zap70 inhibits OC function via its kinase activity. A: WT BMMs, transduced with WT Syk, Zap70 or empty vector, were cultured with RANKL and MCSF on plastic (top panels) or bone slice (middle and lower panels) for 5−6 days. Cells were stained with TRAP activity (top panels) or FITC-phalloidin (middle panels). Resorption lacunae were visualized by peroxidase-labeled wheat germ agglutinin (lower panels). B: WT BMMs, transduced with WT Syk, Zap70 or empty vector, were cultured with RANKL and MCSF with time. Osteoclast differentiation markers were determined by immunoblot. C: WT BMMs, transduced with WT or kinase inactive (K369A) Zap70, were cultured with RANKL and MCSF on plastic for 5 days and stained for TRAP activity.
Zap70 Exerts its Dominant-Negative Effects by Arresting Activation of αvβ3-Activated Cytoskeleton-Organizing Molecules
Syk exerts its effects in osteoclasts as a consequence of αvβ3 integrin activation. Thus, upon liganding, αvβ3 induces a signaling complex in which Rac is the most distal established cytoskeleton-organizing component. This sequence of events requires the ITAM adaptor, Dap12, which when phosphorylated by c-Src, serves as a docking protein for Syk. A reasonable hypothesis therefore holds that the dominant-negative properties of Zap70 reflect competition with Syk for Dap12. As seen in Figure 4A, Dap12 binds as effectively to kinase-inactive Zap70K369A as to WT. Moreover, neither Zap70 constructs blunt Syk/Dap12 association (Fig. 4B). Thus, the dominant-negative effects of Zap70 do not reflect competition for Dap12 or impaired interaction of the ITAM protein with Syk.
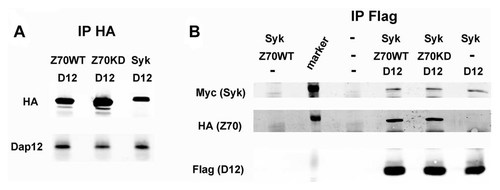
Zap70 does not affect Syk and DAP12 association. A: 293T cells were co-transfected with Dap12 and HA tagged Syk, WT or KD Zap70. HA immunoprecipitates were immunoblotted for DAP12 and HA. B: 293T cells were co-transfected with Flag-Dap12, Myc-Syk, and HA-WT or -KD Zap70. Flag immunoprecipitates were immunoblotted for Myc, HA and Flag.
Syk is essential for osteoclast function and must be phosphorylated to activate its downstream targets. We find integrin-induced Syk phosphorylation arrested in osteoclasts overexpressing Zap70 (Fig. 5A). In consequence, the kinase's effector molecules, SLP76 and Vav3, also are also hypo-phosphorylated in these transduced cells upon αvβ3 liganding (Fig. 5B,C). This inhibition is reflective of Zap70 kinase activity as integrin-induced Syk phosphorylation is robust in cells bearing the kinase-inactive mutant (Fig. 5D). Interestingly, integrin activation of c-Src, which phosphorylates Syk in osteoclasts, is unimpaired in Zap70 transductants (Fig. 5E). Thus, Zap70 targets distal components of the osteoclast cytoskeleton-organizing complex, whose activity it arrests in a c-Src-independent manner.
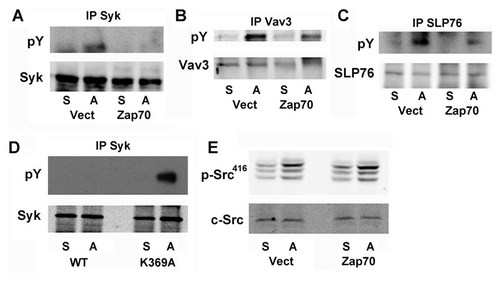
Zap70 inhibits integrin-induced Syk activation. A−C: WT preosteoclasts, transduced with empty vector or WT Zap70, were maintained in suspension (S) or plated on vitronectin (A) for 30 min. A: Syk immunoprecipitates were immunoblotted for phosphorylated tyrosine (pY) and Syk. B: Vav3 immunoprecipitates were immunoblotted for phosphorylated tyrosine (pY) and Vav3. C: SLP76 immunoprecipitates were immunoblotted for phosphorylated tyrosine (pY) and SLP76. D: WT preosteoclasts, transduced with WT or kinase inactive Zap70 (K369A), were maintained in suspension (S) or plated on vitronectin (A) for 30 min. Syk immunoprecipitates were immunoblotted for phosphorylated tyrosine (pY) and Syk. E: WT preosteoclasts, transduced with empty vector, or WT Zap70, were maintained in suspension (S) or plated on vitronectin (A) for 30 min. Phospho-Src (p416) and c-Src were determined by immunoblot.
DISCUSSION
Osteoclasts are myeloid lineage polykaryons. They are the principal if not exclusive bone resorptive cells and the targets of anti-resorptive therapies used in the treatment of osteoporosis and other osteolytic disorders [Teitelbaum, 2000]. Enhanced bone degradation often reflects a combination of increased osteoclast abundance and stimulated activity of the mature polykaryon. Both events are mediated by cytokines such as RANKL and M-CSF [Boyle et al., 2003], but activation of the individual cell is also influenced by signals derived from contact with mineralized matrix. In this circumstance, the osteoclast polarizes fibrillar actin to the cell-bone interface to form a sealing zone or actin ring [Saltel et al., 2008]. This gasket-like structure isolates the resorptive microenvironment from the general extracellular space. Matrix-derived signals induce transport of lysosomal vesicles to the actin ring-encompassed plasma membrane into which they insert to form the osteoclast-unique ruffled border, the cell's bone-degrading organelle. Bone degradation occurs by exocytic transport of HCl and cathepsin K into the resorptive space, via the ruffled border, to mobilize the mineral and organic phases of bone, respectively [Vaananen et al., 2000]. This scenario underscores the importance of osteoclast polarization via cytoskeletal organization in the resorptive process. Current evidence indicates that the matrix derived signals initiating this event is mediated by integrins, principally αvβ3 [McHugh et al., 2000].
The signaling capacity of αvβ3, in the osteoclast, depends upon its affinity for RGD-containing ligands present in bone-residing proteins such as osteopontin and bone sialoprotein. The high affinity state of the integrin is induced by M-CSF or contact with matrix, per se. These events permit activation of a canonical, signaling complex, of which c-Src and Syk are major components and Rac is the most distal established effector molecule [Soriano et al., 1991; Zou et al., 2007; Croke et al., 2011]. An important confirmation of the biological relevance of this pathway is the fact that deletion of any member yields a relatively similar osteoclast phenotype wherein the cells fail to spread, leading to a “crenated” appearance and ineffective bone resorption. Also indicative of cytoskeleton dysfunction, absence of many of these signaling molecules, including c-Src and Syk, does not prevent osteoclastogenesis but obviates normal actin ring and ruffled border formation [Soriano et al., 1991; Zou et al., 2007; Croke et al., 2011]. Because Syk is an essential tyrosine kinase in osteoclast cytoskeletal organization, we have explored its various components in the bone resorptive process. For example we find SykY317 negatively regulates osteoclast function upon interaction with the ubiquitin ligase Cbl [Zou et al., 2009].
In the present study, we continue our exploration of the means by which Syk organizes the osteoclast cytoskeleton by comparing its properties to those of homologous Zap70 despite the fact the latter enzyme is not expressed in the polykaryon or precursor BMMs. Because the two tyrosine kinases have similar molecular structures and Syk can substitute for Zap70 in TCR mediated signals in T cells we suspected the same would obtain in the context of the osteoclast cytoskeleton. Exogenous expression of Zap70, however, fails to normalize the disorganized cytoskeleton or resorptive capacity of Syk−/− cells.
This observation prompted us to construct chimeras of the SH2 and kinase domains of the two proteins to identify the components of Syk which, unlike Zap70, endow it with the capacity to organize the osteoclast cytoskeleton. While the Syk SH2/Zap70 kinase construct does not modify the Syk−/− osteoclast phenotype, the reciprocal Zap70 SH2/Syk kinase chimera partially restores spreading and resorption by the mutant cells. This observation of substantial but incomplete rescue indicates that the Syk kinase motif is essential, but not sufficient, for optimal osteoclast function and the SH2 domains likely contribute.
In other cells, the kinase activity of Syk supersedes that of Zap70. For example, antibody-mediated aggregation of a CD16-CD7-Syk construct, but not a homologous Zap70 chimera, is sufficient to induce cytolytic activity in human T cells [Kolanus et al., 1993]. In this regard, the function of Zap70, but not of Syk, may require activation by a Src family kinase. Interestingly, we find osteoclastic Syk is phosphorylated by c-Src in the context of αvβ3-mediated outside-in signaling [Zou et al., 2007]. In contrast, M-CSF stimulates Syk autophosphorylation in the same cell [Zou et al., 2008]. Our data establish that failure of Zap70 to rescue Syk−/− osteoclast does not reflect dampened c-Src activity which is unaltered in the mutant cells.
Overexpressed Zap70 has dominant-negative effects in other cells and we find the same obtains in osteoclasts. The mechanism by which the kinase exerts its dominant-negative properties in the bone resorptive polykaryon differs, however, from that previously reported. The inhibitory effects of Zap70 on TCR signaling, for example, is achieved by inactivation of its kinase activity or a construct consisting only of the tandem SH2 domains [Qian et al., 1996; Gladue et al., 1997]. The dominant-negative properties of these plasmids likely reflect competition for ITAM-bearing co-stimulatory molecules such as Dap12.
In contrast to Zap70's SH2-mediated dominant-negative effects in other cells, it is the kinase domain, which disorganizes the osteoclast cytoskeleton. Specifically, a point mutation of this region, which eliminates enzymatic activity, completely lacks dominant-negative properties. The arrested spreading of WT cells transduced with Zap70 does not reflect competition for Dap12, an essential component of the αvβ3-activated signaling complex nor does it involve dampened activity of c-Src. On the other hand, activation of more distal components of the cytoskeleton-organizing signaling complex, namely Syk, itself, the adaptor protein SLP76 and the guanine nucleotide exchange factor, Vav3, which activates Rac, are hypophosphorylated in Zap70 bearing WT osteoclasts. Moreover, the fact that Syk overexpression in WT cells does not impair the cytoskeleton indicates that it and Zap70 probably have distinct kinase targets in the osteoclast.
Acknowledgements
This work was supported by National Institutes of Health grants HL53325 and HL74138 (RPM), AR032788, AR046523 and AR057037 (SLT) and P30 AR057235 Pilot Grant (WZ). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. We would also like to acknowledge funding from the Barnes-Jewish Hospital Foundation that supports the live cell imaging facility.




